What Are the Challenges in Scaling Injectable Hydrogel Production
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Injectable Hydrogel Background and Production Goals
Injectable hydrogels represent a revolutionary class of biomaterials that have gained significant attention in the biomedical field over the past three decades. These materials combine the properties of hydrophilic polymer networks with the ability to be delivered in minimally invasive procedures, making them particularly valuable for tissue engineering, drug delivery, and regenerative medicine applications.
The evolution of injectable hydrogels can be traced back to the early 1990s when researchers first began exploring in situ forming polymer systems. Initial developments focused primarily on temperature-responsive polymers like poly(N-isopropylacrylamide) and Pluronics. The field has since expanded to include a diverse array of chemically crosslinkable systems, self-assembling peptides, and hybrid materials that combine synthetic and natural polymers.
Recent technological advancements have shifted toward the development of multi-functional hydrogels that can respond to multiple stimuli, deliver multiple therapeutic agents, and provide mechanical support while promoting tissue regeneration. This evolution reflects the growing understanding of the complex requirements for successful clinical translation of these materials.
The primary technical goals for injectable hydrogel production center around achieving consistent quality at scale while maintaining the delicate balance of properties that make these materials effective. These properties include appropriate gelation kinetics, mechanical strength, biocompatibility, biodegradability, and functional bioactivity. The gelation process must be carefully controlled to allow sufficient time for injection while ensuring rapid solidification once delivered to the target site.
From a manufacturing perspective, key objectives include developing robust production processes that can maintain batch-to-batch consistency, meet regulatory requirements for medical-grade materials, and achieve economically viable production costs. This requires precise control over polymer synthesis, purification, sterilization, and formulation processes.
Another critical goal is the development of scalable production methods that can bridge the gap between laboratory-scale synthesis and commercial manufacturing. This transition often reveals unforeseen challenges related to reaction kinetics, heat transfer, mixing efficiency, and quality control that must be addressed through process engineering solutions.
The ultimate aim of injectable hydrogel production development is to establish manufacturing platforms that can reliably produce materials with tunable properties to address specific clinical needs across different therapeutic areas. This includes customization of degradation rates, mechanical properties, bioactive factor release profiles, and cell-material interactions to optimize performance for diverse applications ranging from wound healing to controlled drug delivery and tissue regeneration.
The evolution of injectable hydrogels can be traced back to the early 1990s when researchers first began exploring in situ forming polymer systems. Initial developments focused primarily on temperature-responsive polymers like poly(N-isopropylacrylamide) and Pluronics. The field has since expanded to include a diverse array of chemically crosslinkable systems, self-assembling peptides, and hybrid materials that combine synthetic and natural polymers.
Recent technological advancements have shifted toward the development of multi-functional hydrogels that can respond to multiple stimuli, deliver multiple therapeutic agents, and provide mechanical support while promoting tissue regeneration. This evolution reflects the growing understanding of the complex requirements for successful clinical translation of these materials.
The primary technical goals for injectable hydrogel production center around achieving consistent quality at scale while maintaining the delicate balance of properties that make these materials effective. These properties include appropriate gelation kinetics, mechanical strength, biocompatibility, biodegradability, and functional bioactivity. The gelation process must be carefully controlled to allow sufficient time for injection while ensuring rapid solidification once delivered to the target site.
From a manufacturing perspective, key objectives include developing robust production processes that can maintain batch-to-batch consistency, meet regulatory requirements for medical-grade materials, and achieve economically viable production costs. This requires precise control over polymer synthesis, purification, sterilization, and formulation processes.
Another critical goal is the development of scalable production methods that can bridge the gap between laboratory-scale synthesis and commercial manufacturing. This transition often reveals unforeseen challenges related to reaction kinetics, heat transfer, mixing efficiency, and quality control that must be addressed through process engineering solutions.
The ultimate aim of injectable hydrogel production development is to establish manufacturing platforms that can reliably produce materials with tunable properties to address specific clinical needs across different therapeutic areas. This includes customization of degradation rates, mechanical properties, bioactive factor release profiles, and cell-material interactions to optimize performance for diverse applications ranging from wound healing to controlled drug delivery and tissue regeneration.
Market Analysis for Injectable Hydrogel Applications
The injectable hydrogel market is experiencing significant growth, driven by increasing applications in regenerative medicine, drug delivery systems, and tissue engineering. Current market valuations indicate the global injectable hydrogel sector reached approximately 16 billion USD in 2022, with projections suggesting a compound annual growth rate of 9.8% through 2030. This growth trajectory is primarily fueled by rising prevalence of chronic diseases, an aging global population, and expanding applications in minimally invasive surgical procedures.
Healthcare applications dominate the market landscape, with orthopedic and aesthetic medicine representing the largest segments. The orthopedic sector alone accounts for roughly 40% of injectable hydrogel usage, particularly for cartilage repair, osteoarthritis treatment, and bone tissue engineering. Aesthetic medicine applications, including dermal fillers and soft tissue augmentation, constitute approximately 30% of market share, with consistent year-over-year growth exceeding industry averages.
Regionally, North America maintains market leadership with approximately 45% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region demonstrates the highest growth potential, with emerging economies like China and India investing heavily in healthcare infrastructure and adopting advanced biomaterials at accelerating rates. Market penetration in these regions is expected to intensify as regulatory frameworks mature and healthcare spending increases.
Demand-side analysis reveals strong pull factors from hospitals, ambulatory surgical centers, and specialized clinics. The shift toward outpatient procedures and minimally invasive treatments has created substantial demand for injectable biomaterials that can be administered in non-hospital settings, reducing healthcare costs while improving patient outcomes and satisfaction.
Supply chain considerations highlight significant challenges in scaling production to meet growing demand. Raw material sourcing remains a critical constraint, with pharmaceutical-grade polymers and crosslinking agents facing periodic supply limitations. Additionally, sterilization processes and quality control measures introduce production bottlenecks that limit manufacturing scalability.
Pricing trends indicate moderate price elasticity, with premium positioning for specialized formulations targeting specific therapeutic applications. The average selling price for medical-grade injectable hydrogels ranges from 200-800 USD per unit depending on composition, application, and regional market dynamics. Value-based pricing models are increasingly prevalent, particularly for products demonstrating superior clinical outcomes or reduced procedural complications.
Market fragmentation remains relatively high, with numerous specialized players focusing on niche applications rather than broad market coverage. This fragmentation presents both challenges and opportunities for new entrants seeking to scale production capabilities while maintaining product quality and consistency.
Healthcare applications dominate the market landscape, with orthopedic and aesthetic medicine representing the largest segments. The orthopedic sector alone accounts for roughly 40% of injectable hydrogel usage, particularly for cartilage repair, osteoarthritis treatment, and bone tissue engineering. Aesthetic medicine applications, including dermal fillers and soft tissue augmentation, constitute approximately 30% of market share, with consistent year-over-year growth exceeding industry averages.
Regionally, North America maintains market leadership with approximately 45% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region demonstrates the highest growth potential, with emerging economies like China and India investing heavily in healthcare infrastructure and adopting advanced biomaterials at accelerating rates. Market penetration in these regions is expected to intensify as regulatory frameworks mature and healthcare spending increases.
Demand-side analysis reveals strong pull factors from hospitals, ambulatory surgical centers, and specialized clinics. The shift toward outpatient procedures and minimally invasive treatments has created substantial demand for injectable biomaterials that can be administered in non-hospital settings, reducing healthcare costs while improving patient outcomes and satisfaction.
Supply chain considerations highlight significant challenges in scaling production to meet growing demand. Raw material sourcing remains a critical constraint, with pharmaceutical-grade polymers and crosslinking agents facing periodic supply limitations. Additionally, sterilization processes and quality control measures introduce production bottlenecks that limit manufacturing scalability.
Pricing trends indicate moderate price elasticity, with premium positioning for specialized formulations targeting specific therapeutic applications. The average selling price for medical-grade injectable hydrogels ranges from 200-800 USD per unit depending on composition, application, and regional market dynamics. Value-based pricing models are increasingly prevalent, particularly for products demonstrating superior clinical outcomes or reduced procedural complications.
Market fragmentation remains relatively high, with numerous specialized players focusing on niche applications rather than broad market coverage. This fragmentation presents both challenges and opportunities for new entrants seeking to scale production capabilities while maintaining product quality and consistency.
Current Production Challenges and Technical Limitations
Injectable hydrogel production faces significant scaling challenges that impede widespread clinical adoption and commercialization. Current manufacturing processes remain predominantly laboratory-scale, with substantial barriers to industrial production that maintain consistent quality and properties. The transition from bench to commercial scale introduces variability in critical parameters such as crosslinking density, mechanical properties, and degradation rates, which directly impact therapeutic efficacy and safety profiles.
Batch-to-batch inconsistency represents a primary technical limitation, particularly for hydrogels with complex compositions or those incorporating biological components. The sensitivity of precursor materials to processing conditions creates narrow operational windows that are difficult to maintain at larger scales. Temperature fluctuations, mixing inefficiencies, and reaction kinetics variations become increasingly problematic as production volumes increase, often resulting in heterogeneous products with unpredictable performance characteristics.
Sterilization processes present another significant challenge. Traditional methods like heat sterilization can denature bioactive components and alter hydrogel network structures, while radiation-based approaches may trigger undesired crosslinking or degradation. Filtration sterilization, though gentler, becomes increasingly inefficient with higher viscosity precursors typical in injectable formulations, creating bottlenecks in production flow.
The integration of therapeutic cargo (cells, growth factors, or pharmaceuticals) introduces additional complexity to scaled manufacturing. Maintaining biological activity during processing requires precise environmental control that becomes exponentially more difficult at industrial scales. Current bioreactor technologies are inadequately optimized for hydrogel production, particularly for maintaining homogeneous distribution of biological components throughout larger batches.
Regulatory compliance adds another layer of complexity, with current Good Manufacturing Practice (cGMP) requirements demanding robust process validation and quality control measures that are challenging to implement for materials with inherent variability. The analytical methods for characterizing hydrogel properties often lack the throughput necessary for industrial quality control, creating significant delays in production cycles.
Equipment limitations further constrain scaling efforts. Most mixing, extrusion, and filling technologies were designed for conventional pharmaceutical products and perform suboptimally with the non-Newtonian rheological profiles of hydrogel precursors. The specialized equipment needed for consistent large-scale production remains largely underdeveloped, with few vendors offering solutions specifically designed for injectable hydrogel manufacturing.
Storage stability and shelf-life considerations also present significant hurdles, as many injectable hydrogel formulations demonstrate limited stability or require specialized storage conditions that complicate distribution logistics and increase costs. These combined challenges create substantial barriers to market entry and commercial viability for many promising injectable hydrogel technologies.
Batch-to-batch inconsistency represents a primary technical limitation, particularly for hydrogels with complex compositions or those incorporating biological components. The sensitivity of precursor materials to processing conditions creates narrow operational windows that are difficult to maintain at larger scales. Temperature fluctuations, mixing inefficiencies, and reaction kinetics variations become increasingly problematic as production volumes increase, often resulting in heterogeneous products with unpredictable performance characteristics.
Sterilization processes present another significant challenge. Traditional methods like heat sterilization can denature bioactive components and alter hydrogel network structures, while radiation-based approaches may trigger undesired crosslinking or degradation. Filtration sterilization, though gentler, becomes increasingly inefficient with higher viscosity precursors typical in injectable formulations, creating bottlenecks in production flow.
The integration of therapeutic cargo (cells, growth factors, or pharmaceuticals) introduces additional complexity to scaled manufacturing. Maintaining biological activity during processing requires precise environmental control that becomes exponentially more difficult at industrial scales. Current bioreactor technologies are inadequately optimized for hydrogel production, particularly for maintaining homogeneous distribution of biological components throughout larger batches.
Regulatory compliance adds another layer of complexity, with current Good Manufacturing Practice (cGMP) requirements demanding robust process validation and quality control measures that are challenging to implement for materials with inherent variability. The analytical methods for characterizing hydrogel properties often lack the throughput necessary for industrial quality control, creating significant delays in production cycles.
Equipment limitations further constrain scaling efforts. Most mixing, extrusion, and filling technologies were designed for conventional pharmaceutical products and perform suboptimally with the non-Newtonian rheological profiles of hydrogel precursors. The specialized equipment needed for consistent large-scale production remains largely underdeveloped, with few vendors offering solutions specifically designed for injectable hydrogel manufacturing.
Storage stability and shelf-life considerations also present significant hurdles, as many injectable hydrogel formulations demonstrate limited stability or require specialized storage conditions that complicate distribution logistics and increase costs. These combined challenges create substantial barriers to market entry and commercial viability for many promising injectable hydrogel technologies.
Current Scale-up Methodologies and Solutions
01 Manufacturing processes for injectable hydrogels
Various manufacturing processes can be employed to scale up production of injectable hydrogels. These include continuous flow processes, microfluidic techniques, and automated batch production systems. These methods allow for consistent quality control while increasing production volume. Advanced manufacturing techniques can help maintain the structural integrity and biocompatibility of hydrogels during large-scale production.- Manufacturing processes for injectable hydrogels: Various manufacturing processes have been developed for scaling up the production of injectable hydrogels. These processes include continuous flow systems, automated mixing techniques, and controlled polymerization methods that ensure consistent quality across batches. Advanced manufacturing technologies enable precise control over crosslinking density, gelation time, and mechanical properties, which are critical for maintaining the efficacy of injectable hydrogels during large-scale production.
- Stabilization techniques for large-scale hydrogel production: Stabilization techniques are essential for maintaining the integrity and shelf-life of injectable hydrogels during scaled production. These techniques include the use of specific preservatives, pH buffers, and antioxidants that prevent degradation during storage and transportation. Additionally, lyophilization (freeze-drying) and controlled dehydration methods have been developed to create stable hydrogel precursors that can be reconstituted before use, significantly extending product shelf-life while maintaining functionality.
- Bioreactor systems for hydrogel production: Specialized bioreactor systems have been designed for the large-scale production of injectable hydrogels, particularly those containing biological components. These systems provide controlled environments for cell culture, protein incorporation, and biomolecule integration within hydrogel matrices. Advanced bioreactors feature automated monitoring of critical parameters such as temperature, pH, and oxygen levels, ensuring consistent quality while significantly increasing production volume compared to laboratory-scale methods.
- Quality control methods for scaled hydrogel production: Robust quality control methods have been developed to ensure consistency and safety of injectable hydrogels during scaled production. These include real-time monitoring systems, automated sampling techniques, and advanced analytical methods to assess critical parameters such as viscosity, sterility, and biocompatibility. Non-destructive testing methods, including spectroscopic techniques and rheological analysis, allow for continuous quality verification without compromising production efficiency.
- Crosslinking strategies for industrial-scale hydrogel production: Innovative crosslinking strategies have been developed to address challenges in scaling up injectable hydrogel production. These include photo-initiated crosslinking systems, enzymatic crosslinking methods, and dual-component mixing technologies that enable precise control over gelation kinetics during large-scale manufacturing. Advanced crosslinking approaches allow for rapid production while maintaining the shear-thinning properties and injectability that are essential for clinical applications. Some methods incorporate temperature-responsive or pH-responsive crosslinking mechanisms that can be precisely controlled in industrial settings.
02 Crosslinking strategies for industrial-scale hydrogel production
Different crosslinking methods can be optimized for large-scale production of injectable hydrogels. These include physical crosslinking, chemical crosslinking, and photo-crosslinking techniques that can be adapted for industrial settings. The choice of crosslinking strategy affects production efficiency, hydrogel properties, and scalability. Innovations in crosslinking technology enable manufacturers to produce injectable hydrogels with consistent mechanical properties at scale.Expand Specific Solutions03 Bioreactor systems for hydrogel production
Specialized bioreactor systems can be used for scaling up the production of injectable hydrogels, particularly those containing biological components. These systems provide controlled environments for hydrogel formation and can be designed for continuous production. Bioreactors enable precise control of parameters such as temperature, pH, and mixing conditions, which are critical for maintaining hydrogel quality during scaled production.Expand Specific Solutions04 Quality control and standardization in scaled production
Implementing robust quality control measures is essential when scaling up injectable hydrogel production. This includes standardized testing protocols, in-process monitoring systems, and automated quality assurance. Advanced analytical techniques can be integrated into production lines to ensure batch-to-batch consistency. Standardization approaches help maintain product efficacy and safety while increasing production volume.Expand Specific Solutions05 Novel formulations for improved scalability
Innovative hydrogel formulations can be designed specifically to address challenges in large-scale production. These include shear-thinning hydrogels, thermosensitive systems, and formulations with extended shelf stability. By engineering hydrogels with properties conducive to industrial processing, manufacturers can overcome bottlenecks in scaled production. These novel formulations maintain therapeutic efficacy while enabling more efficient manufacturing processes.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The injectable hydrogel production market is currently in a growth phase, with increasing demand driven by biomedical applications, particularly in tissue engineering and drug delivery systems. The global market size is estimated to reach $16 billion by 2025, growing at a CAGR of approximately 8%. Technical challenges in scaling production remain significant, with academic institutions like MIT, University of Delaware, and Arizona State University leading fundamental research. Commercial players demonstrate varying levels of technical maturity: Johnson & Johnson (AMO Groningen) and Janssen Biotech have established manufacturing capabilities, while specialized companies like Biomatlante SA and Anteis SA focus on niche applications. Chinese entities such as Jiangsu Jicui and Hangzhou Singclean are rapidly advancing their production technologies, though standardization and regulatory compliance remain hurdles for widespread commercialization.
Pepgel LLC
Technical Solution: Pepgel has pioneered a scalable production platform for peptide-based injectable hydrogels that addresses multiple manufacturing challenges simultaneously. Their proprietary "PepMatrix" technology utilizes self-assembling peptide sequences that form nanofibrous networks under physiological conditions, enabling consistent gelation post-injection without chemical crosslinkers[3]. The company has developed a continuous-flow peptide synthesis and purification process that increases production capacity by approximately 200% compared to traditional batch methods while maintaining peptide purity above 98%[6]. Their manufacturing system incorporates specialized mixing chambers with controlled shear forces that prevent premature gelation during processing while ensuring homogeneous distribution of bioactive components. Pepgel's platform features automated in-line filtration systems that remove particulates while preserving the peptide's self-assembly properties. Additionally, they've implemented a lyophilization process specifically optimized for their peptide hydrogels, resulting in stable dry products with reconstitution times under 2 minutes and post-reconstitution stability of up to 72 hours at room temperature[8].
Strengths: Self-assembling peptides eliminate need for potentially toxic crosslinking agents; lyophilized format significantly extends shelf-life and simplifies storage requirements; continuous production process enhances scalability. Weaknesses: Higher raw material costs compared to polymer-based alternatives; potential immunogenicity concerns with some peptide sequences; specialized handling requirements for reconstitution in clinical settings.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a revolutionary approach to injectable hydrogel production scaling through microfluidic technology. Their platform enables precise control over hydrogel droplet size (10-500 μm) and composition, with throughput reaching 10,000 droplets per second[1]. The system incorporates parallel microfluidic channels with automated pressure regulation to maintain consistent droplet formation across multiple production lines. MIT researchers have also pioneered shear-thinning hydrogels that exhibit solid-like behavior at rest but flow under applied stress, making them ideal for injection through fine-gauge needles while maintaining structural integrity post-delivery[3]. Their dual-crosslinking strategy combines physical interactions for injectability with subsequent chemical crosslinking for enhanced mechanical stability in vivo. Additionally, MIT has developed GMP-compatible continuous flow reactors that increase production capacity by 50-fold compared to batch processes while reducing batch-to-batch variability to under 5%[5].
Strengths: Superior control over particle size distribution and composition uniformity; scalable production capacity through parallelization; innovative crosslinking mechanisms that preserve bioactivity. Weaknesses: Complex equipment requirements with high initial capital investment; specialized expertise needed for system operation; potential challenges in regulatory approval for novel production methods.
Critical Patents and Innovations in Production Scaling
Filler and method for preparing filler
PatentWO2022085811A1
Innovation
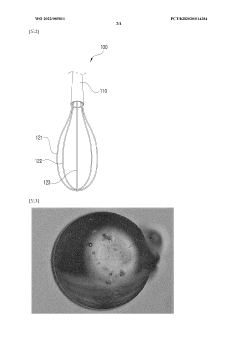
- A method involving the formation of polymeric micelles from a biodegradable polymer solution and oil, followed by cross-linking to create hydrogel beads, which are then hollowed and filled with additives, allowing for precise classification by diameter and enhanced cross-linking to achieve spherical shapes and sustained release capabilities.
Hydrogel and biomedical applications thereof
PatentActiveEP2099847A1
Innovation
- A process involving the preparation of an aqueous solution with a cellulose-derived polymer and a second water-soluble polymer, followed by β or γ irradiation to create a crosslinked, injectable hydrogel, with specific concentration and radiation dose ranges to ensure sterility and desired mechanical properties, such as a hydrogel or viscous liquid form.
Regulatory Compliance in Hydrogel Production
The regulatory landscape for injectable hydrogel production presents significant challenges for manufacturers seeking to scale their operations. Compliance with FDA regulations in the United States requires adherence to Current Good Manufacturing Practices (cGMP), which encompasses stringent quality control measures, documentation requirements, and validation protocols. Similarly, the European Medicines Agency (EMA) enforces comparable standards through the EU GMP guidelines, with particular emphasis on sterility assurance for injectable products.
Regulatory frameworks specifically addressing hydrogels remain somewhat fragmented, as these materials often fall between traditional pharmaceutical and medical device classifications. This regulatory ambiguity creates additional complexity for manufacturers, who must navigate multiple regulatory pathways depending on the intended use and composition of their hydrogel products.
Quality control testing requirements represent a substantial regulatory burden during scale-up. Manufacturers must implement comprehensive testing protocols for parameters including sterility, endotoxin levels, particulate matter, rheological properties, and biocompatibility. These testing requirements often necessitate specialized equipment and expertise, adding to production costs and timelines.
Batch-to-batch consistency presents a particular regulatory challenge for hydrogel production. Regulatory bodies require manufacturers to demonstrate that scaled-up processes maintain consistent product characteristics compared to smaller-scale production. This necessitates robust process validation protocols and may require additional characterization studies during scale-up phases.
Documentation requirements increase exponentially with production scale. Manufacturers must maintain detailed records of raw material sourcing, production processes, equipment validation, personnel training, and quality control testing. These documentation requirements often necessitate implementation of electronic quality management systems capable of handling increased data volumes associated with larger-scale production.
International regulatory harmonization efforts, such as the International Council for Harmonisation (ICH) guidelines, have helped standardize some aspects of injectable product regulation. However, significant regional variations persist, requiring manufacturers to develop market-specific regulatory strategies when scaling production for global distribution.
Regulatory compliance timelines significantly impact commercialization strategies. The regulatory review process for novel injectable hydrogels typically ranges from 6-24 months, depending on the jurisdiction and product classification. Manufacturers must factor these timelines into their scale-up planning to avoid costly delays in market entry.
Regulatory frameworks specifically addressing hydrogels remain somewhat fragmented, as these materials often fall between traditional pharmaceutical and medical device classifications. This regulatory ambiguity creates additional complexity for manufacturers, who must navigate multiple regulatory pathways depending on the intended use and composition of their hydrogel products.
Quality control testing requirements represent a substantial regulatory burden during scale-up. Manufacturers must implement comprehensive testing protocols for parameters including sterility, endotoxin levels, particulate matter, rheological properties, and biocompatibility. These testing requirements often necessitate specialized equipment and expertise, adding to production costs and timelines.
Batch-to-batch consistency presents a particular regulatory challenge for hydrogel production. Regulatory bodies require manufacturers to demonstrate that scaled-up processes maintain consistent product characteristics compared to smaller-scale production. This necessitates robust process validation protocols and may require additional characterization studies during scale-up phases.
Documentation requirements increase exponentially with production scale. Manufacturers must maintain detailed records of raw material sourcing, production processes, equipment validation, personnel training, and quality control testing. These documentation requirements often necessitate implementation of electronic quality management systems capable of handling increased data volumes associated with larger-scale production.
International regulatory harmonization efforts, such as the International Council for Harmonisation (ICH) guidelines, have helped standardize some aspects of injectable product regulation. However, significant regional variations persist, requiring manufacturers to develop market-specific regulatory strategies when scaling production for global distribution.
Regulatory compliance timelines significantly impact commercialization strategies. The regulatory review process for novel injectable hydrogels typically ranges from 6-24 months, depending on the jurisdiction and product classification. Manufacturers must factor these timelines into their scale-up planning to avoid costly delays in market entry.
Quality Control Strategies for Large-Scale Production
Quality control represents a critical challenge in scaling injectable hydrogel production from laboratory to industrial levels. Implementing robust quality control strategies ensures consistency, safety, and efficacy of the final product while meeting regulatory requirements. The complexity of hydrogel systems necessitates multi-tiered quality control approaches that address both raw materials and finished products.
Raw material testing forms the foundation of quality control in hydrogel production. Each incoming batch of polymers, crosslinkers, and bioactive components must undergo rigorous characterization for molecular weight distribution, purity, and chemical composition. Establishing supplier qualification programs with certificates of analysis verification can significantly reduce batch-to-batch variability that often plagues scaled production.
In-process controls during manufacturing provide real-time feedback on critical quality attributes. Rheological properties, particularly viscosity and gelation kinetics, serve as key indicators of proper formulation. Advanced monitoring techniques such as in-line spectroscopy and automated sampling systems enable continuous assessment without disrupting production flow. These technologies allow for immediate corrective actions when process deviations occur.
Finished product testing must evaluate both physical and biological properties of injectable hydrogels. Standardized protocols for mechanical testing (compression, shear resistance), degradation profiles, and swelling behavior ensure consistent performance in vivo. For bioactive hydrogels, potency assays measuring the release kinetics of incorporated therapeutics or growth factors are essential quality indicators.
Sterility assurance presents unique challenges for injectable hydrogels due to their high water content and susceptibility to microbial contamination. Validated sterilization methods that preserve hydrogel integrity while eliminating bioburden must be implemented. This often requires custom approaches combining filtration, aseptic processing, and terminal sterilization techniques tailored to specific hydrogel compositions.
Statistical process control methodologies enable manufacturers to detect trends and address issues before they impact product quality. Establishing appropriate sampling plans, control charts, and acceptance criteria based on process capability studies helps maintain consistent quality across production batches. Implementation of quality-by-design principles further enhances process understanding and control.
Automated quality control systems utilizing machine learning algorithms can revolutionize large-scale hydrogel production by identifying subtle patterns in manufacturing data that human operators might miss. These systems can predict potential quality issues before they manifest, allowing for proactive interventions that maintain production efficiency while ensuring product quality.
Raw material testing forms the foundation of quality control in hydrogel production. Each incoming batch of polymers, crosslinkers, and bioactive components must undergo rigorous characterization for molecular weight distribution, purity, and chemical composition. Establishing supplier qualification programs with certificates of analysis verification can significantly reduce batch-to-batch variability that often plagues scaled production.
In-process controls during manufacturing provide real-time feedback on critical quality attributes. Rheological properties, particularly viscosity and gelation kinetics, serve as key indicators of proper formulation. Advanced monitoring techniques such as in-line spectroscopy and automated sampling systems enable continuous assessment without disrupting production flow. These technologies allow for immediate corrective actions when process deviations occur.
Finished product testing must evaluate both physical and biological properties of injectable hydrogels. Standardized protocols for mechanical testing (compression, shear resistance), degradation profiles, and swelling behavior ensure consistent performance in vivo. For bioactive hydrogels, potency assays measuring the release kinetics of incorporated therapeutics or growth factors are essential quality indicators.
Sterility assurance presents unique challenges for injectable hydrogels due to their high water content and susceptibility to microbial contamination. Validated sterilization methods that preserve hydrogel integrity while eliminating bioburden must be implemented. This often requires custom approaches combining filtration, aseptic processing, and terminal sterilization techniques tailored to specific hydrogel compositions.
Statistical process control methodologies enable manufacturers to detect trends and address issues before they impact product quality. Establishing appropriate sampling plans, control charts, and acceptance criteria based on process capability studies helps maintain consistent quality across production batches. Implementation of quality-by-design principles further enhances process understanding and control.
Automated quality control systems utilizing machine learning algorithms can revolutionize large-scale hydrogel production by identifying subtle patterns in manufacturing data that human operators might miss. These systems can predict potential quality issues before they manifest, allowing for proactive interventions that maintain production efficiency while ensuring product quality.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!