The Evolution of Injectable Hydrogel: From Concept to Large-Scale Production
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Injectable Hydrogel Development History and Objectives
Injectable hydrogels emerged in the late 1970s as a novel biomaterial concept, initially developed for controlled drug delivery systems. The pioneering work of Wichterle and Lim on hydrophilic gels in 1960 laid the foundation for this technology, though the specific injectable formats only gained traction nearly two decades later. Early injectable hydrogels were primarily based on natural polymers such as collagen and hyaluronic acid, offering limited functionality but demonstrating the core principle of in situ gelation.
The 1990s marked a significant acceleration in the field, with researchers developing synthetic polymer systems that could undergo sol-gel transitions under physiological conditions. This period saw the introduction of temperature-responsive polymers like poly(N-isopropylacrylamide) and block copolymers that could form physical crosslinks in response to environmental stimuli. These advancements expanded the potential applications beyond drug delivery to tissue engineering and regenerative medicine.
By the early 2000s, the focus shifted toward creating multi-functional injectable hydrogels with tunable mechanical properties and degradation profiles. The integration of bioactive molecules and cell-adhesive motifs transformed these materials from passive delivery vehicles to active participants in tissue regeneration. This era also witnessed the development of in situ crosslinking chemistries that were biocompatible and could proceed under mild conditions, such as click chemistry and enzyme-mediated crosslinking.
The technological evolution trajectory has consistently aimed at addressing several key objectives: improving injectability through shear-thinning properties, enhancing mechanical stability post-injection, controlling degradation kinetics, and ensuring biocompatibility. More recently, objectives have expanded to include stimuli-responsiveness for smart delivery systems and integration with imaging modalities for theranostic applications.
Current research focuses on scalable production methods that maintain batch-to-batch consistency while preserving the sophisticated functionality of these advanced biomaterials. The transition from laboratory-scale synthesis to industrial production represents a critical challenge, requiring standardized protocols and quality control measures. Objectives now include developing continuous manufacturing processes, implementing automated quality assessment tools, and establishing regulatory pathways for clinical translation.
The evolution of injectable hydrogels reflects broader trends in biomaterials science, moving from simple structural materials to complex, functional systems that can interact dynamically with biological environments. Future objectives aim at personalized medicine applications, where injectable hydrogels could be tailored to individual patient needs through rapid manufacturing technologies and computational design approaches.
The 1990s marked a significant acceleration in the field, with researchers developing synthetic polymer systems that could undergo sol-gel transitions under physiological conditions. This period saw the introduction of temperature-responsive polymers like poly(N-isopropylacrylamide) and block copolymers that could form physical crosslinks in response to environmental stimuli. These advancements expanded the potential applications beyond drug delivery to tissue engineering and regenerative medicine.
By the early 2000s, the focus shifted toward creating multi-functional injectable hydrogels with tunable mechanical properties and degradation profiles. The integration of bioactive molecules and cell-adhesive motifs transformed these materials from passive delivery vehicles to active participants in tissue regeneration. This era also witnessed the development of in situ crosslinking chemistries that were biocompatible and could proceed under mild conditions, such as click chemistry and enzyme-mediated crosslinking.
The technological evolution trajectory has consistently aimed at addressing several key objectives: improving injectability through shear-thinning properties, enhancing mechanical stability post-injection, controlling degradation kinetics, and ensuring biocompatibility. More recently, objectives have expanded to include stimuli-responsiveness for smart delivery systems and integration with imaging modalities for theranostic applications.
Current research focuses on scalable production methods that maintain batch-to-batch consistency while preserving the sophisticated functionality of these advanced biomaterials. The transition from laboratory-scale synthesis to industrial production represents a critical challenge, requiring standardized protocols and quality control measures. Objectives now include developing continuous manufacturing processes, implementing automated quality assessment tools, and establishing regulatory pathways for clinical translation.
The evolution of injectable hydrogels reflects broader trends in biomaterials science, moving from simple structural materials to complex, functional systems that can interact dynamically with biological environments. Future objectives aim at personalized medicine applications, where injectable hydrogels could be tailored to individual patient needs through rapid manufacturing technologies and computational design approaches.
Market Analysis for Injectable Hydrogel Applications
The injectable hydrogel market has experienced significant growth in recent years, driven by increasing applications in tissue engineering, drug delivery systems, and regenerative medicine. The global market for injectable hydrogels was valued at approximately $8.5 billion in 2022 and is projected to reach $15.3 billion by 2028, representing a compound annual growth rate (CAGR) of 10.2% during the forecast period.
Healthcare applications dominate the injectable hydrogel market, accounting for over 65% of the total market share. Within healthcare, orthopedic applications represent the largest segment, with substantial demand for hydrogels in cartilage repair, bone regeneration, and as carriers for growth factors and cells. The aging global population and rising prevalence of degenerative joint diseases are key drivers for this segment.
Oncology presents another rapidly growing application area, with injectable hydrogels being increasingly utilized for localized drug delivery systems that can provide sustained release of chemotherapeutic agents while minimizing systemic toxicity. This segment is expected to grow at a CAGR of 12.7% through 2028, outpacing the overall market growth.
Regionally, North America holds the largest market share at approximately 38%, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth rate due to increasing healthcare expenditure, growing medical tourism, and rising awareness about advanced treatment options in countries like China, Japan, and India.
The cosmetic and aesthetic medicine sector represents an emerging application area with significant growth potential. Injectable hydrogels for dermal fillers and facial rejuvenation procedures are gaining popularity as minimally invasive alternatives to surgical interventions. This segment is projected to grow at a CAGR of 11.5% over the forecast period.
Key market challenges include stringent regulatory requirements, particularly for medical-grade hydrogels, and the high cost of advanced formulations. Additionally, concerns regarding biocompatibility and long-term safety profiles of certain synthetic hydrogels have impacted market adoption rates in specific applications.
Customer preferences are increasingly shifting toward biodegradable and naturally derived hydrogels, creating new opportunities for manufacturers focusing on sustainable and eco-friendly formulations. This trend aligns with the broader movement toward green chemistry and environmentally responsible healthcare solutions.
Healthcare applications dominate the injectable hydrogel market, accounting for over 65% of the total market share. Within healthcare, orthopedic applications represent the largest segment, with substantial demand for hydrogels in cartilage repair, bone regeneration, and as carriers for growth factors and cells. The aging global population and rising prevalence of degenerative joint diseases are key drivers for this segment.
Oncology presents another rapidly growing application area, with injectable hydrogels being increasingly utilized for localized drug delivery systems that can provide sustained release of chemotherapeutic agents while minimizing systemic toxicity. This segment is expected to grow at a CAGR of 12.7% through 2028, outpacing the overall market growth.
Regionally, North America holds the largest market share at approximately 38%, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth rate due to increasing healthcare expenditure, growing medical tourism, and rising awareness about advanced treatment options in countries like China, Japan, and India.
The cosmetic and aesthetic medicine sector represents an emerging application area with significant growth potential. Injectable hydrogels for dermal fillers and facial rejuvenation procedures are gaining popularity as minimally invasive alternatives to surgical interventions. This segment is projected to grow at a CAGR of 11.5% over the forecast period.
Key market challenges include stringent regulatory requirements, particularly for medical-grade hydrogels, and the high cost of advanced formulations. Additionally, concerns regarding biocompatibility and long-term safety profiles of certain synthetic hydrogels have impacted market adoption rates in specific applications.
Customer preferences are increasingly shifting toward biodegradable and naturally derived hydrogels, creating new opportunities for manufacturers focusing on sustainable and eco-friendly formulations. This trend aligns with the broader movement toward green chemistry and environmentally responsible healthcare solutions.
Technical Challenges in Injectable Hydrogel Development
Despite significant advancements in injectable hydrogel technology, numerous technical challenges persist throughout the development pipeline. One of the primary obstacles involves achieving consistent mechanical properties across batches. Injectable hydrogels must maintain specific viscoelastic characteristics to ensure proper flow during injection while rapidly recovering structural integrity post-injection. This delicate balance remains difficult to control during scale-up processes, where minor variations in synthesis conditions can significantly alter final product performance.
Biocompatibility presents another substantial challenge, particularly for synthetic polymer-based hydrogels. While natural polymers like hyaluronic acid offer inherent biocompatibility, they often lack mechanical strength or degradation control. Conversely, synthetic alternatives provide superior tunability but frequently trigger inflammatory responses or immune reactions. Developing hybrid systems that combine the advantages of both remains technically demanding.
Sterilization processes introduce additional complications in hydrogel development. Traditional methods such as autoclaving often compromise gel integrity by disrupting crosslinking networks, while radiation-based approaches can generate toxic byproducts. Alternative techniques like filtration sterilization face limitations with higher viscosity formulations, creating a significant bottleneck in manufacturing workflows.
Shelf stability represents a critical challenge for commercial viability. Many injectable hydrogels exhibit time-dependent property changes, including premature gelation, phase separation, or loss of biological activity. Engineering formulations that maintain consistent performance throughout reasonable shelf-life periods requires sophisticated stabilization strategies and extensive stability testing protocols.
Controlled release kinetics of therapeutic agents from hydrogel matrices presents another technical hurdle. Achieving predictable, sustained drug release profiles depends on complex interactions between the hydrogel network, encapsulated therapeutics, and surrounding physiological environment. Current mathematical models often fail to accurately predict in vivo release behavior, complicating formulation optimization.
Scaling production from laboratory to industrial levels introduces numerous engineering challenges. Maintaining homogeneity during large-batch synthesis, preventing contamination, and ensuring consistent crosslinking density throughout the material volume all become exponentially more difficult at commercial scales. Additionally, analytical methods for quality control must be adapted to accommodate higher throughput requirements without sacrificing precision.
Regulatory compliance adds another layer of complexity, particularly for hydrogels containing biological components or novel crosslinking chemistries. Demonstrating consistent manufacturing processes that meet stringent regulatory standards requires sophisticated analytical techniques and robust quality management systems that can reliably detect and control critical quality attributes.
Biocompatibility presents another substantial challenge, particularly for synthetic polymer-based hydrogels. While natural polymers like hyaluronic acid offer inherent biocompatibility, they often lack mechanical strength or degradation control. Conversely, synthetic alternatives provide superior tunability but frequently trigger inflammatory responses or immune reactions. Developing hybrid systems that combine the advantages of both remains technically demanding.
Sterilization processes introduce additional complications in hydrogel development. Traditional methods such as autoclaving often compromise gel integrity by disrupting crosslinking networks, while radiation-based approaches can generate toxic byproducts. Alternative techniques like filtration sterilization face limitations with higher viscosity formulations, creating a significant bottleneck in manufacturing workflows.
Shelf stability represents a critical challenge for commercial viability. Many injectable hydrogels exhibit time-dependent property changes, including premature gelation, phase separation, or loss of biological activity. Engineering formulations that maintain consistent performance throughout reasonable shelf-life periods requires sophisticated stabilization strategies and extensive stability testing protocols.
Controlled release kinetics of therapeutic agents from hydrogel matrices presents another technical hurdle. Achieving predictable, sustained drug release profiles depends on complex interactions between the hydrogel network, encapsulated therapeutics, and surrounding physiological environment. Current mathematical models often fail to accurately predict in vivo release behavior, complicating formulation optimization.
Scaling production from laboratory to industrial levels introduces numerous engineering challenges. Maintaining homogeneity during large-batch synthesis, preventing contamination, and ensuring consistent crosslinking density throughout the material volume all become exponentially more difficult at commercial scales. Additionally, analytical methods for quality control must be adapted to accommodate higher throughput requirements without sacrificing precision.
Regulatory compliance adds another layer of complexity, particularly for hydrogels containing biological components or novel crosslinking chemistries. Demonstrating consistent manufacturing processes that meet stringent regulatory standards requires sophisticated analytical techniques and robust quality management systems that can reliably detect and control critical quality attributes.
Current Manufacturing Solutions for Injectable Hydrogels
01 Composition and formulation of injectable hydrogels
Injectable hydrogels can be formulated using various polymers and cross-linking agents to create biocompatible matrices suitable for medical applications. These formulations typically include natural polymers (like hyaluronic acid, collagen, or alginate), synthetic polymers, or combinations thereof. The composition can be tailored to achieve specific properties such as gelation time, mechanical strength, biodegradability, and biocompatibility. Some formulations incorporate temperature-sensitive or pH-sensitive components that facilitate the transition from liquid to gel state after injection.- Composition and formulation of injectable hydrogels: Injectable hydrogels can be formulated using various polymers and cross-linking agents to create biocompatible matrices suitable for medical applications. These formulations typically include natural polymers (like hyaluronic acid, collagen, or alginate) or synthetic polymers that can form three-dimensional networks upon injection into the body. The composition can be tailored to control properties such as gelation time, mechanical strength, and degradation rate, making them versatile for different medical applications.
- Drug delivery applications of injectable hydrogels: Injectable hydrogels serve as effective drug delivery systems that can provide controlled release of therapeutic agents at specific sites in the body. These hydrogels can encapsulate various drugs, proteins, growth factors, or other bioactive molecules and release them over extended periods. The release kinetics can be modulated by adjusting the hydrogel's physical and chemical properties, allowing for sustained or triggered drug delivery that enhances therapeutic efficacy while reducing systemic side effects.
- Tissue engineering and regenerative medicine applications: Injectable hydrogels provide scaffolds for tissue engineering and regenerative medicine by supporting cell growth, proliferation, and differentiation. These hydrogels can be designed to mimic the extracellular matrix of natural tissues, providing structural support while allowing nutrient diffusion and cellular interactions. They can be combined with stem cells or growth factors to promote tissue regeneration in various applications including cartilage repair, wound healing, and neural tissue engineering.
- Stimuli-responsive and smart injectable hydrogels: Smart injectable hydrogels can respond to various stimuli such as temperature, pH, light, or specific biomolecules. These hydrogels undergo physical or chemical changes when exposed to specific triggers, enabling on-demand gelation, drug release, or degradation. Thermo-responsive hydrogels that solidify at body temperature are particularly valuable for minimally invasive procedures, as they can be injected as liquids and form solid gels in situ, conforming perfectly to tissue cavities or defects.
- Injectable hydrogels for cosmetic and aesthetic applications: Injectable hydrogels are widely used in cosmetic and aesthetic procedures for soft tissue augmentation, wrinkle filling, and facial contouring. These hydrogels provide immediate volume enhancement with natural-looking results and can be designed to integrate with surrounding tissues. Formulations for cosmetic applications typically focus on biocompatibility, longevity, and viscoelastic properties that mimic natural tissues. Some advanced formulations also incorporate bioactive components that stimulate collagen production for longer-lasting effects.
02 Drug delivery applications of injectable hydrogels
Injectable hydrogels serve as effective drug delivery systems that can provide controlled release of therapeutic agents. These systems can encapsulate various drugs, proteins, growth factors, or other bioactive molecules and release them at controlled rates. The hydrogel matrix protects the encapsulated agents from degradation while maintaining their bioactivity. By modifying the hydrogel composition, the release kinetics can be tailored for sustained or triggered delivery, improving therapeutic efficacy and reducing side effects compared to conventional delivery methods.Expand Specific Solutions03 Tissue engineering and regenerative medicine applications
Injectable hydrogels provide three-dimensional scaffolds for cell growth and tissue regeneration. These biomaterials can be designed to mimic the extracellular matrix, supporting cell adhesion, proliferation, and differentiation. When combined with stem cells or tissue-specific cells, they create an environment conducive to tissue formation. The injectable nature allows minimally invasive delivery to specific anatomical sites, filling irregular defects and conforming to the shape of the tissue cavity. Applications include cartilage repair, bone regeneration, wound healing, and neural tissue engineering.Expand Specific Solutions04 Smart and stimuli-responsive injectable hydrogels
Advanced injectable hydrogels can respond to various stimuli such as temperature, pH, light, or specific biomolecules. These smart materials undergo physical or chemical changes when exposed to specific triggers, enabling on-demand gelation, degradation, or release of encapsulated compounds. Thermo-responsive hydrogels that solidify at body temperature are particularly valuable for clinical applications. Other systems respond to disease-specific markers, allowing targeted therapy activation. These responsive properties enhance the precision and effectiveness of hydrogel-based treatments in various medical conditions.Expand Specific Solutions05 Injectable hydrogels for aesthetic and cosmetic applications
Injectable hydrogels are widely used in aesthetic medicine and cosmetic procedures. These materials can be formulated to provide volume enhancement, wrinkle filling, and skin rejuvenation effects. The viscoelastic properties of certain hydrogels, particularly those based on hyaluronic acid, make them ideal for restoring facial contours and improving skin appearance. Some formulations also incorporate bioactive components that stimulate collagen production or provide antioxidant effects. The temporary nature of many cosmetic hydrogels allows for reversible treatments with adjustable outcomes.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The injectable hydrogel market is currently in a growth phase, transitioning from early development to commercial expansion, with an estimated global market size of $15-20 billion and projected CAGR of 8-10% through 2030. The technology has reached moderate maturity, with academic institutions (University of Delaware, Sichuan University, Harvard, Caltech) establishing fundamental research while commercial entities are advancing practical applications. Companies like MicroVention, Contraline, and Synthes GmbH have successfully commercialized specific applications, particularly in medical devices and drug delivery systems. Pharmaceutical giants including Sanofi-Aventis are investing heavily in hydrogel platforms, while specialized firms like Cambridge Polymer Group provide critical testing services. The competitive landscape features increasing collaboration between academic institutions and industry partners to overcome scale-up challenges and regulatory hurdles.
MICROVENTION INC
Technical Solution: MicroVention has developed proprietary injectable hydrogel technology primarily focused on neurovascular applications, particularly their PHIL (Precipitating Hydrophobic Injectable Liquid) system. This technology utilizes a non-adhesive copolymer dissolved in DMSO that precipitates upon contact with blood to form a cohesive embolic plug[2]. The company has engineered their hydrogel formulation to provide controlled viscosity for precise delivery through microcatheters while achieving optimal radiopacity for visualization during procedures[4]. Their manufacturing process has evolved from small-batch production to large-scale GMP manufacturing with sophisticated quality control measures to ensure consistent performance across production lots. MicroVention has implemented automated filling and packaging systems specifically designed for their injectable hydrogels to maintain sterility and precision dosing[7]. The company has successfully navigated regulatory pathways in multiple global markets, establishing scalable production capabilities that maintain product integrity while meeting increasing clinical demand.
Strengths: Established commercial presence with FDA-approved injectable hydrogel products; specialized delivery systems optimized for neurovascular applications; proven scale-up capabilities with consistent product quality. Weaknesses: Technology primarily focused on embolic applications rather than broader regenerative medicine uses; DMSO carrier may limit certain applications due to potential cytotoxicity concerns; relatively specialized market focus compared to broader hydrogel platforms.
President & Fellows of Harvard College
Technical Solution: Harvard has pioneered significant advancements in injectable hydrogel technology through their patented shear-thinning biomaterial (STB) platform. Their approach utilizes nanofiber-based hydrogels with unique rheological properties that allow the material to flow under applied stress and rapidly self-heal when the stress is removed[1]. This technology enables minimally invasive delivery through small-gauge needles while maintaining structural integrity post-injection. Harvard researchers have developed dual-network hydrogels combining physical and chemical crosslinking mechanisms to enhance mechanical properties and tissue integration[3]. Their platform incorporates bioactive molecules and cells within the hydrogel matrix to promote tissue regeneration and controlled drug delivery. Harvard's manufacturing protocols have evolved to address scalability challenges through standardized production methods that maintain batch-to-batch consistency while preserving the hydrogel's functional properties[5].
Strengths: Superior shear-thinning properties allowing excellent injectability through fine needles; highly customizable platform for various biomedical applications; strong intellectual property portfolio. Weaknesses: Complex manufacturing processes may increase production costs; potential challenges in maintaining consistent mechanical properties during scale-up; longer regulatory pathway due to novel material combinations.
Critical Patents and Innovations in Hydrogel Technology
An injectable hydrogel with ph sensitivity and a process for the preparation thereof
PatentPendingIN202331064343A
Innovation
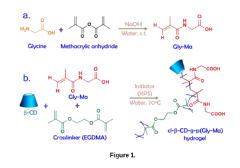
- Development of a pH-sensitive, biodegradable, and biocompatible hydrogel made from glycine and β-Cyclodextrin, which exhibits shear-thinning behavior and self-recovery properties, synthesized through a two-step process involving the formation of a crosslinked hydrogel with amino acid and methacrylic anhydride-based monomers.
Hydrogels and uses thereof
PatentInactiveUS7884185B2
Innovation
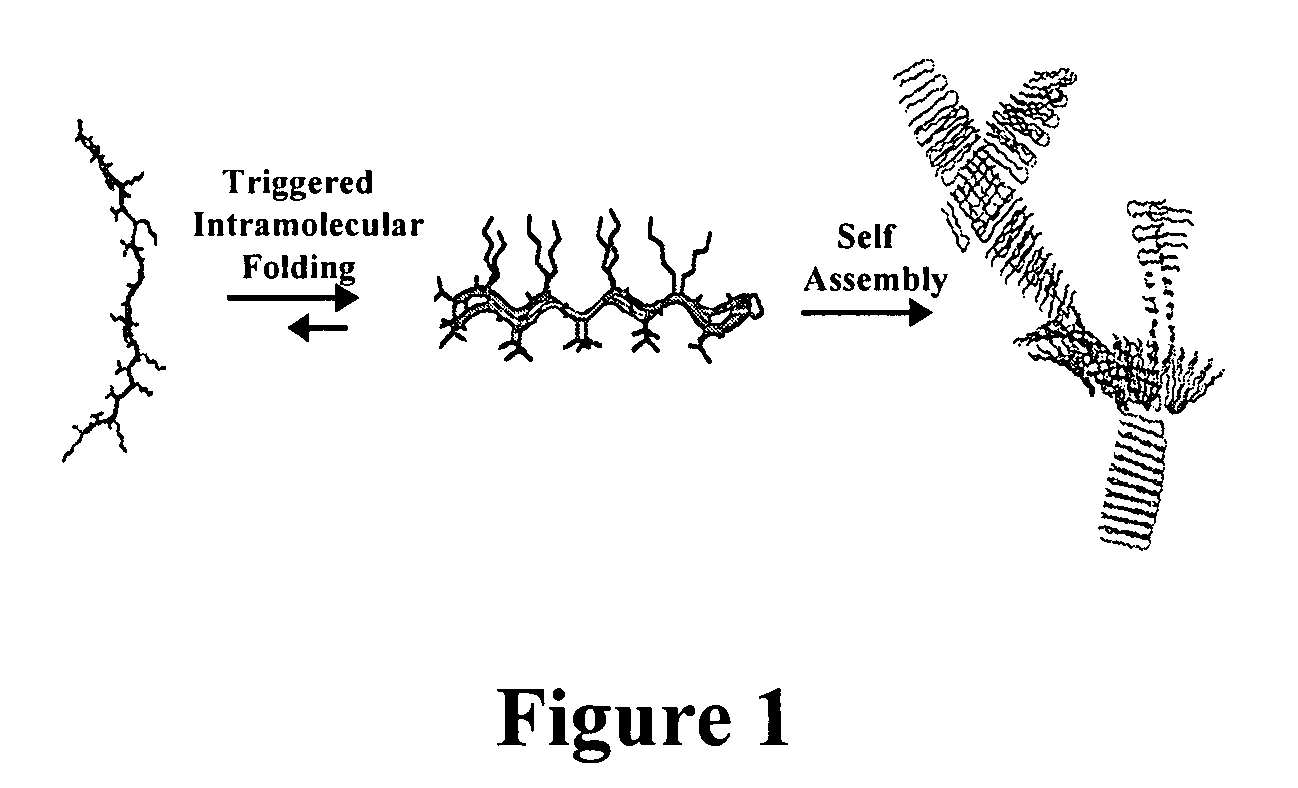
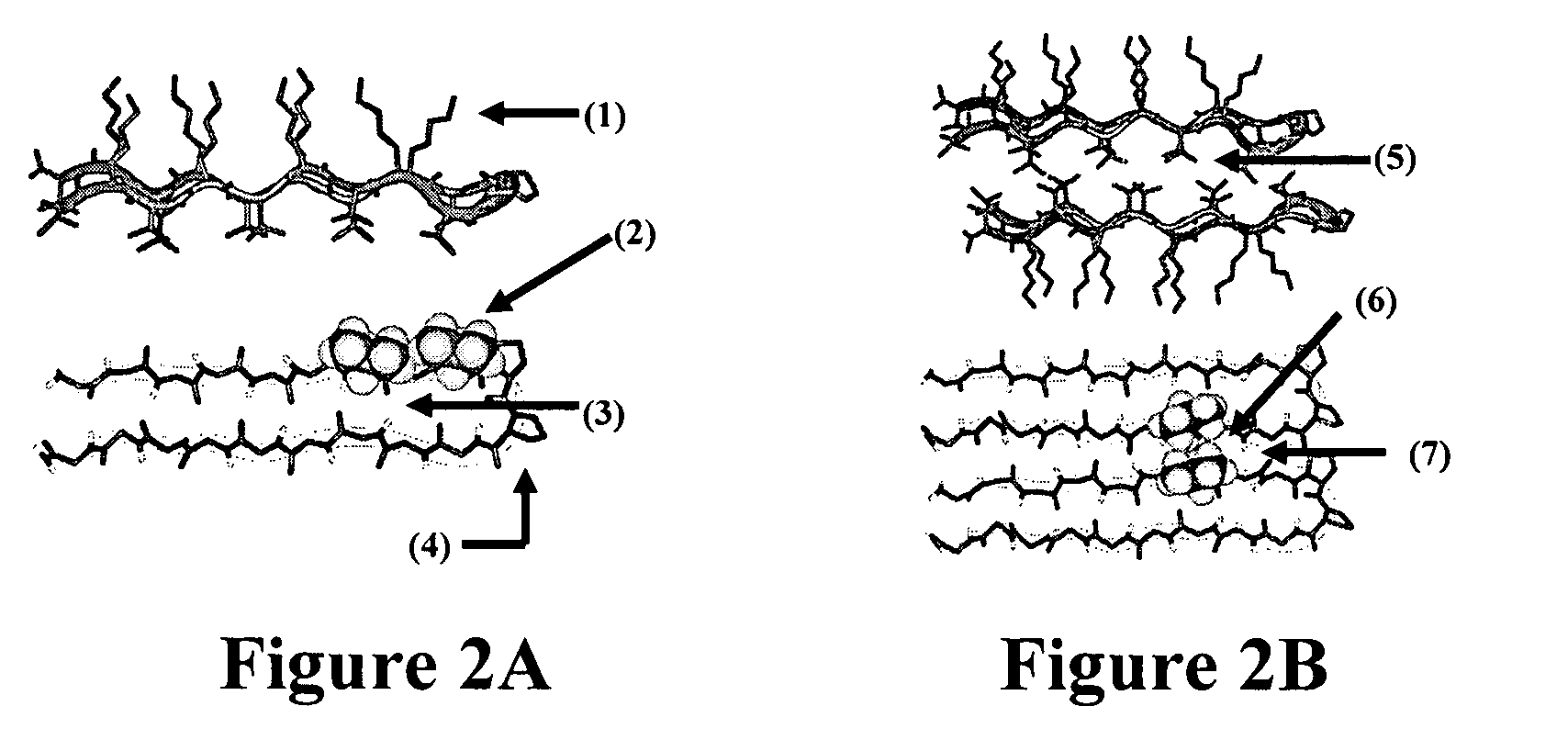
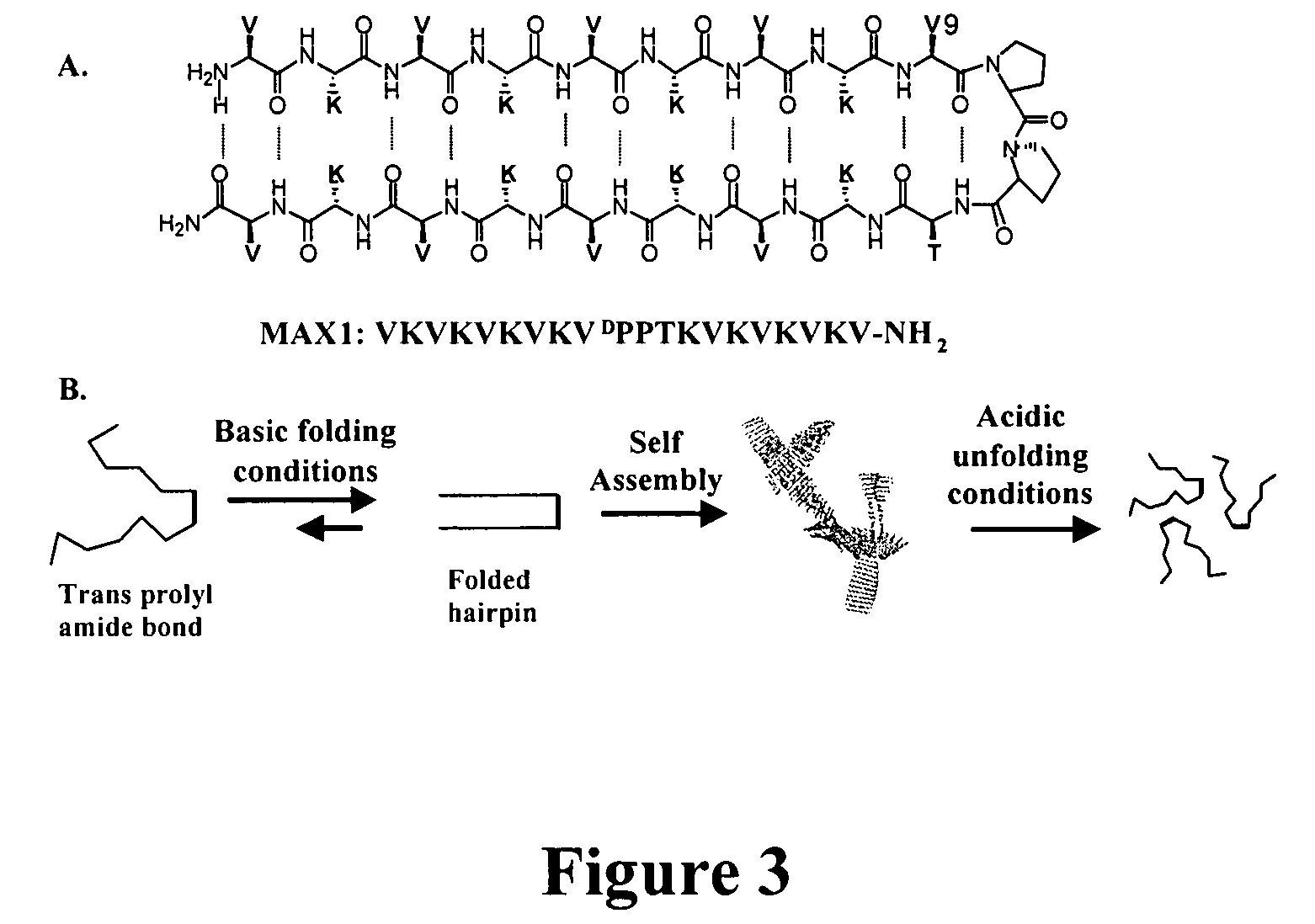
- The development of novel peptide-based hydrogels that undergo self-assembly in response to environmental stimuli, such as pH, ionic strength, and temperature, forming a rigid and porous structure through intramolecular folding and intermolecular association, eliminating the need for exogenous crosslinking agents and allowing for controlled hydrogelation and reversibility.
Regulatory Framework for Injectable Biomaterials
The regulatory landscape for injectable hydrogels represents a complex framework that varies significantly across global jurisdictions. In the United States, the FDA categorizes injectable hydrogels primarily as medical devices, drugs, or combination products, depending on their primary mode of action. The 510(k) clearance pathway is commonly utilized for hydrogels similar to previously approved products, while novel formulations typically require the more rigorous Premarket Approval (PMA) process, demanding extensive clinical trials and safety documentation.
The European Union, under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), implements a classification system based on risk levels, with most injectable hydrogels falling into Class III due to their invasive nature and long-term tissue interaction. This classification necessitates thorough clinical evaluation reports and post-market surveillance plans, creating significant regulatory hurdles for manufacturers.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the Sakigake designation system, which accelerates the approval process for innovative medical technologies, including certain injectable hydrogels. This system has facilitated faster market entry for breakthrough biomaterials while maintaining rigorous safety standards.
Quality management systems represent another critical regulatory component, with ISO 13485 certification being the international standard for medical device manufacturing. For injectable hydrogels, this encompasses stringent requirements for sterility assurance, biocompatibility testing according to ISO 10993 standards, and comprehensive stability studies to ensure shelf-life claims are substantiated.
Biocompatibility testing presents particular challenges for injectable hydrogels due to their dynamic nature post-injection. Regulatory bodies increasingly require in vivo degradation studies and long-term tissue response evaluations beyond standard cytotoxicity, sensitization, and irritation tests. The FDA's guidance on the use of International Standard ISO 10993 specifically addresses these considerations for implantable materials.
Recent regulatory trends indicate a shift toward harmonization efforts through the International Medical Device Regulators Forum (IMDRF), aiming to standardize requirements across major markets. Additionally, there is growing emphasis on real-world evidence collection post-approval, with conditional approval pathways emerging that allow market access while gathering additional safety data.
For manufacturers scaling up production of injectable hydrogels, compliance with Good Manufacturing Practices (GMP) presents significant challenges, particularly in maintaining batch-to-batch consistency and sterility assurance during large-scale production. The transition from laboratory to industrial scale often necessitates process validation studies specifically designed to address the unique rheological properties of hydrogel systems.
The European Union, under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), implements a classification system based on risk levels, with most injectable hydrogels falling into Class III due to their invasive nature and long-term tissue interaction. This classification necessitates thorough clinical evaluation reports and post-market surveillance plans, creating significant regulatory hurdles for manufacturers.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the Sakigake designation system, which accelerates the approval process for innovative medical technologies, including certain injectable hydrogels. This system has facilitated faster market entry for breakthrough biomaterials while maintaining rigorous safety standards.
Quality management systems represent another critical regulatory component, with ISO 13485 certification being the international standard for medical device manufacturing. For injectable hydrogels, this encompasses stringent requirements for sterility assurance, biocompatibility testing according to ISO 10993 standards, and comprehensive stability studies to ensure shelf-life claims are substantiated.
Biocompatibility testing presents particular challenges for injectable hydrogels due to their dynamic nature post-injection. Regulatory bodies increasingly require in vivo degradation studies and long-term tissue response evaluations beyond standard cytotoxicity, sensitization, and irritation tests. The FDA's guidance on the use of International Standard ISO 10993 specifically addresses these considerations for implantable materials.
Recent regulatory trends indicate a shift toward harmonization efforts through the International Medical Device Regulators Forum (IMDRF), aiming to standardize requirements across major markets. Additionally, there is growing emphasis on real-world evidence collection post-approval, with conditional approval pathways emerging that allow market access while gathering additional safety data.
For manufacturers scaling up production of injectable hydrogels, compliance with Good Manufacturing Practices (GMP) presents significant challenges, particularly in maintaining batch-to-batch consistency and sterility assurance during large-scale production. The transition from laboratory to industrial scale often necessitates process validation studies specifically designed to address the unique rheological properties of hydrogel systems.
Scalability Challenges and Solutions for Mass Production
The transition from laboratory-scale production to industrial manufacturing of injectable hydrogels presents significant challenges that must be addressed to ensure commercial viability. Current production methods often rely on batch processes with limited throughput, creating bottlenecks when scaling to meet market demands. These limitations stem from the complex physicochemical properties of hydrogels and the precision required during synthesis.
A primary challenge in mass production is maintaining consistent crosslinking density and mechanical properties across large batches. Small variations in reaction conditions can lead to significant differences in the final product's performance characteristics. This necessitates sophisticated monitoring systems and precise control over temperature, pH, and mixing parameters throughout the manufacturing process.
Raw material sourcing represents another critical hurdle, as industrial-scale production requires consistent, high-quality precursors. The variability in natural polymers like alginate or hyaluronic acid can impact product uniformity, while synthetic alternatives must meet stringent purity requirements. Establishing robust supply chains with appropriate quality control measures is essential for sustainable manufacturing.
Sterilization processes present unique challenges for hydrogel production. Traditional methods like autoclaving may compromise the structural integrity of the polymer network, while radiation-based approaches can induce unwanted crosslinking or degradation. Aseptic processing techniques offer an alternative but introduce complexity and cost to the manufacturing workflow.
Several innovative solutions have emerged to address these scalability challenges. Continuous flow reactors represent a significant advancement, allowing for consistent reaction conditions and reduced batch-to-batch variability. These systems enable precise control over residence time and mixing efficiency, facilitating the production of hydrogels with reproducible properties at industrial scales.
Microfluidic technologies have demonstrated promise for producing uniform hydrogel particles with controlled size distributions. By leveraging laminar flow conditions and precise fluid handling, these platforms can generate consistent products while minimizing waste. Integration with automated quality control systems further enhances manufacturing reliability.
Modular manufacturing approaches offer flexibility in production capacity, allowing companies to adapt to changing market demands without significant capital investment. These systems typically employ standardized process units that can be reconfigured or expanded as needed, reducing the risks associated with scaling production.
Advanced process analytical technologies (PAT) provide real-time monitoring of critical quality attributes during manufacturing. By implementing spectroscopic methods and machine learning algorithms, manufacturers can detect deviations from target specifications and make adjustments before product quality is compromised, ensuring consistent performance across production scales.
A primary challenge in mass production is maintaining consistent crosslinking density and mechanical properties across large batches. Small variations in reaction conditions can lead to significant differences in the final product's performance characteristics. This necessitates sophisticated monitoring systems and precise control over temperature, pH, and mixing parameters throughout the manufacturing process.
Raw material sourcing represents another critical hurdle, as industrial-scale production requires consistent, high-quality precursors. The variability in natural polymers like alginate or hyaluronic acid can impact product uniformity, while synthetic alternatives must meet stringent purity requirements. Establishing robust supply chains with appropriate quality control measures is essential for sustainable manufacturing.
Sterilization processes present unique challenges for hydrogel production. Traditional methods like autoclaving may compromise the structural integrity of the polymer network, while radiation-based approaches can induce unwanted crosslinking or degradation. Aseptic processing techniques offer an alternative but introduce complexity and cost to the manufacturing workflow.
Several innovative solutions have emerged to address these scalability challenges. Continuous flow reactors represent a significant advancement, allowing for consistent reaction conditions and reduced batch-to-batch variability. These systems enable precise control over residence time and mixing efficiency, facilitating the production of hydrogels with reproducible properties at industrial scales.
Microfluidic technologies have demonstrated promise for producing uniform hydrogel particles with controlled size distributions. By leveraging laminar flow conditions and precise fluid handling, these platforms can generate consistent products while minimizing waste. Integration with automated quality control systems further enhances manufacturing reliability.
Modular manufacturing approaches offer flexibility in production capacity, allowing companies to adapt to changing market demands without significant capital investment. These systems typically employ standardized process units that can be reconfigured or expanded as needed, reducing the risks associated with scaling production.
Advanced process analytical technologies (PAT) provide real-time monitoring of critical quality attributes during manufacturing. By implementing spectroscopic methods and machine learning algorithms, manufacturers can detect deviations from target specifications and make adjustments before product quality is compromised, ensuring consistent performance across production scales.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!