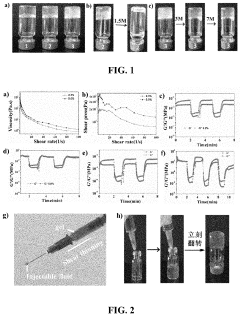
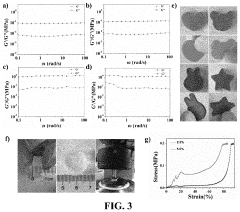
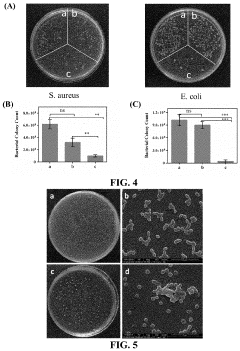
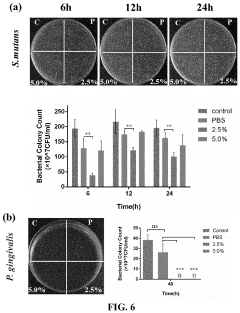
Investigating Injectable Hydrogel’s Role in Long-Term Drug Storage Solutions
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Injectable Hydrogel Technology Background and Objectives
Injectable hydrogels represent a significant advancement in biomaterial science, evolving from simple polymer networks to sophisticated drug delivery systems over the past three decades. Initially developed as structural supports in tissue engineering, these materials have undergone substantial transformation through integration with nanotechnology, smart materials science, and pharmaceutical engineering to become versatile platforms for controlled substance release.
The technological evolution of injectable hydrogels has been marked by several key milestones: the development of thermosensitive polymers in the 1990s, the introduction of in-situ forming hydrogels in the early 2000s, and more recently, the creation of self-healing and stimuli-responsive systems. Each advancement has expanded the potential applications while addressing previous limitations in stability, biocompatibility, and release kinetics.
Current research trends focus on enhancing the long-term stability of hydrogels for extended drug storage capabilities. This includes exploration of novel crosslinking mechanisms, incorporation of protective microenvironments, and development of hybrid systems that combine synthetic and natural polymers to optimize both mechanical properties and biocompatibility.
The primary technological objective in investigating injectable hydrogels for long-term drug storage solutions is to develop systems capable of maintaining therapeutic agent stability under physiological conditions for periods exceeding six months. This represents a significant challenge given the dynamic nature of in vivo environments and the inherent degradation pathways of most pharmaceutical compounds.
Secondary objectives include achieving precise control over release kinetics to enable programmable dosing schedules, minimizing initial burst release effects that can cause toxicity, and ensuring consistent drug bioavailability throughout the entire release period. These goals necessitate innovations in polymer chemistry, processing techniques, and characterization methodologies.
From a translational perspective, the technology aims to address critical healthcare challenges including medication adherence in chronic disease management, therapeutic delivery to anatomically restricted sites, and reduction of systemic side effects through localized administration. The potential impact extends across multiple therapeutic areas including oncology, neurodegenerative disorders, and endocrine diseases.
The convergence of injectable hydrogel technology with advances in drug formulation science presents unprecedented opportunities for creating next-generation therapeutic systems. By establishing stable microenvironments that protect drug molecules from degradation while allowing controlled diffusion, these materials could fundamentally transform approaches to long-term drug delivery and storage both in vivo and as ex vivo preservation systems.
The technological evolution of injectable hydrogels has been marked by several key milestones: the development of thermosensitive polymers in the 1990s, the introduction of in-situ forming hydrogels in the early 2000s, and more recently, the creation of self-healing and stimuli-responsive systems. Each advancement has expanded the potential applications while addressing previous limitations in stability, biocompatibility, and release kinetics.
Current research trends focus on enhancing the long-term stability of hydrogels for extended drug storage capabilities. This includes exploration of novel crosslinking mechanisms, incorporation of protective microenvironments, and development of hybrid systems that combine synthetic and natural polymers to optimize both mechanical properties and biocompatibility.
The primary technological objective in investigating injectable hydrogels for long-term drug storage solutions is to develop systems capable of maintaining therapeutic agent stability under physiological conditions for periods exceeding six months. This represents a significant challenge given the dynamic nature of in vivo environments and the inherent degradation pathways of most pharmaceutical compounds.
Secondary objectives include achieving precise control over release kinetics to enable programmable dosing schedules, minimizing initial burst release effects that can cause toxicity, and ensuring consistent drug bioavailability throughout the entire release period. These goals necessitate innovations in polymer chemistry, processing techniques, and characterization methodologies.
From a translational perspective, the technology aims to address critical healthcare challenges including medication adherence in chronic disease management, therapeutic delivery to anatomically restricted sites, and reduction of systemic side effects through localized administration. The potential impact extends across multiple therapeutic areas including oncology, neurodegenerative disorders, and endocrine diseases.
The convergence of injectable hydrogel technology with advances in drug formulation science presents unprecedented opportunities for creating next-generation therapeutic systems. By establishing stable microenvironments that protect drug molecules from degradation while allowing controlled diffusion, these materials could fundamentally transform approaches to long-term drug delivery and storage both in vivo and as ex vivo preservation systems.
Market Analysis for Long-Term Drug Delivery Systems
The global market for long-term drug delivery systems has experienced significant growth over the past decade, driven by increasing prevalence of chronic diseases requiring consistent medication regimens. The injectable hydrogel segment specifically has emerged as a promising technology platform, with an estimated market value reaching $4.3 billion in 2022 and projected to grow at a compound annual growth rate of 8.7% through 2030.
Patient compliance remains a critical challenge in medication adherence, with studies indicating that approximately 50% of patients with chronic conditions fail to take medications as prescribed. This non-adherence costs healthcare systems billions annually and represents a substantial market opportunity for innovative delivery solutions like injectable hydrogels that can maintain therapeutic drug levels over extended periods.
The aging global population serves as a primary market driver, with individuals over 65 expected to represent over 16% of the global population by 2030. This demographic typically manages multiple chronic conditions requiring complex medication regimens, creating strong demand for simplified delivery systems that reduce administration frequency.
Oncology applications currently dominate the injectable hydrogel market, accounting for approximately 32% of total market share. This is followed by diabetes management (24%), cardiovascular diseases (18%), and central nervous system disorders (15%). The remaining market share is distributed among various therapeutic areas including infectious diseases and pain management.
Geographically, North America leads the market with 42% share, followed by Europe (28%), Asia-Pacific (22%), and rest of world (8%). However, the Asia-Pacific region is experiencing the fastest growth rate at 10.3% annually, driven by improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness of advanced drug delivery technologies.
From a competitive landscape perspective, the market features both pharmaceutical giants and specialized drug delivery technology companies. Major pharmaceutical companies are increasingly partnering with or acquiring specialized hydrogel technology developers to enhance their product portfolios and secure competitive advantages in drug delivery innovation.
Reimbursement policies significantly impact market adoption, with favorable coverage for long-term delivery systems in developed markets accelerating uptake. However, high initial costs of these advanced delivery systems present market barriers in price-sensitive regions, despite potential long-term cost savings through improved therapeutic outcomes and reduced hospitalization rates.
Consumer preferences are shifting toward minimally invasive, less frequent administration options, with market research indicating that patients are willing to pay premium prices for delivery systems that reduce administration frequency from daily to monthly or longer intervals.
Patient compliance remains a critical challenge in medication adherence, with studies indicating that approximately 50% of patients with chronic conditions fail to take medications as prescribed. This non-adherence costs healthcare systems billions annually and represents a substantial market opportunity for innovative delivery solutions like injectable hydrogels that can maintain therapeutic drug levels over extended periods.
The aging global population serves as a primary market driver, with individuals over 65 expected to represent over 16% of the global population by 2030. This demographic typically manages multiple chronic conditions requiring complex medication regimens, creating strong demand for simplified delivery systems that reduce administration frequency.
Oncology applications currently dominate the injectable hydrogel market, accounting for approximately 32% of total market share. This is followed by diabetes management (24%), cardiovascular diseases (18%), and central nervous system disorders (15%). The remaining market share is distributed among various therapeutic areas including infectious diseases and pain management.
Geographically, North America leads the market with 42% share, followed by Europe (28%), Asia-Pacific (22%), and rest of world (8%). However, the Asia-Pacific region is experiencing the fastest growth rate at 10.3% annually, driven by improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness of advanced drug delivery technologies.
From a competitive landscape perspective, the market features both pharmaceutical giants and specialized drug delivery technology companies. Major pharmaceutical companies are increasingly partnering with or acquiring specialized hydrogel technology developers to enhance their product portfolios and secure competitive advantages in drug delivery innovation.
Reimbursement policies significantly impact market adoption, with favorable coverage for long-term delivery systems in developed markets accelerating uptake. However, high initial costs of these advanced delivery systems present market barriers in price-sensitive regions, despite potential long-term cost savings through improved therapeutic outcomes and reduced hospitalization rates.
Consumer preferences are shifting toward minimally invasive, less frequent administration options, with market research indicating that patients are willing to pay premium prices for delivery systems that reduce administration frequency from daily to monthly or longer intervals.
Current Challenges in Injectable Hydrogel Development
Despite significant advancements in injectable hydrogel technology, several critical challenges persist that hinder their widespread application in long-term drug storage solutions. The primary obstacle remains achieving consistent mechanical stability over extended periods. Current hydrogel formulations often exhibit degradation or structural changes when stored for months, compromising their ability to maintain drug integrity and controlled release profiles. This instability manifests as syneresis (water expulsion), polymer chain breakdown, or crosslinking alterations that fundamentally change the hydrogel's physical properties.
Another significant challenge involves maintaining precise control over drug diffusion rates throughout the storage period. Injectable hydrogels must balance contradictory requirements: sufficient porosity for eventual drug release while maintaining tight encapsulation during storage. Research indicates that environmental factors such as temperature fluctuations, pH changes, and exposure to light can dramatically alter these diffusion characteristics, creating unpredictable release profiles after storage.
Sterilization compatibility represents a third major hurdle. Long-term storage necessitates complete sterility, yet conventional sterilization methods including autoclaving, gamma irradiation, and ethylene oxide treatment often compromise hydrogel integrity or alter drug bioactivity. This creates a technical paradox where ensuring product safety simultaneously reduces efficacy.
Scalable manufacturing processes that maintain batch-to-batch consistency present additional complications. Current production methods struggle to deliver uniform crosslinking density, polymer distribution, and drug loading across large production volumes. This variability introduces significant quality control challenges for pharmaceutical applications requiring precise dosing and release kinetics.
Regulatory pathways for injectable hydrogel drug storage systems remain complex and poorly defined. The novel nature of these hybrid drug-device systems creates uncertainty regarding stability testing protocols, shelf-life determination methods, and acceptable performance parameters. This regulatory ambiguity slows commercial development despite promising laboratory results.
Biocompatibility during extended storage represents another challenge, as leachable compounds may develop over time. Studies have documented cases where initially biocompatible hydrogels generate cytotoxic byproducts during long-term storage through oxidation, hydrolysis, or other degradation mechanisms. These changes can trigger inflammatory responses or reduce therapeutic efficacy when eventually administered.
Finally, cost-effectiveness remains problematic for widespread adoption. Current manufacturing processes, specialized storage requirements, and complex quality control measures contribute to prohibitively high production costs compared to conventional drug formulations, limiting commercial viability despite technical promise.
Another significant challenge involves maintaining precise control over drug diffusion rates throughout the storage period. Injectable hydrogels must balance contradictory requirements: sufficient porosity for eventual drug release while maintaining tight encapsulation during storage. Research indicates that environmental factors such as temperature fluctuations, pH changes, and exposure to light can dramatically alter these diffusion characteristics, creating unpredictable release profiles after storage.
Sterilization compatibility represents a third major hurdle. Long-term storage necessitates complete sterility, yet conventional sterilization methods including autoclaving, gamma irradiation, and ethylene oxide treatment often compromise hydrogel integrity or alter drug bioactivity. This creates a technical paradox where ensuring product safety simultaneously reduces efficacy.
Scalable manufacturing processes that maintain batch-to-batch consistency present additional complications. Current production methods struggle to deliver uniform crosslinking density, polymer distribution, and drug loading across large production volumes. This variability introduces significant quality control challenges for pharmaceutical applications requiring precise dosing and release kinetics.
Regulatory pathways for injectable hydrogel drug storage systems remain complex and poorly defined. The novel nature of these hybrid drug-device systems creates uncertainty regarding stability testing protocols, shelf-life determination methods, and acceptable performance parameters. This regulatory ambiguity slows commercial development despite promising laboratory results.
Biocompatibility during extended storage represents another challenge, as leachable compounds may develop over time. Studies have documented cases where initially biocompatible hydrogels generate cytotoxic byproducts during long-term storage through oxidation, hydrolysis, or other degradation mechanisms. These changes can trigger inflammatory responses or reduce therapeutic efficacy when eventually administered.
Finally, cost-effectiveness remains problematic for widespread adoption. Current manufacturing processes, specialized storage requirements, and complex quality control measures contribute to prohibitively high production costs compared to conventional drug formulations, limiting commercial viability despite technical promise.
Current Hydrogel-Based Drug Storage Solutions
01 Lyophilization techniques for hydrogel preservation
Lyophilization (freeze-drying) is an effective method for long-term storage of injectable hydrogels. This process removes water from the hydrogel while preserving its structural integrity and bioactive components. The technique involves freezing the hydrogel followed by sublimation of ice under vacuum conditions. Upon reconstitution with appropriate solutions before use, the hydrogel maintains its original properties and functionality, making it suitable for various biomedical applications including drug delivery and tissue engineering.- Freeze-drying techniques for hydrogel preservation: Freeze-drying (lyophilization) is an effective method for long-term storage of injectable hydrogels. This process removes water from the hydrogel while maintaining its structural integrity, allowing for extended shelf life at room temperature. Upon rehydration before use, the hydrogel can regain its original properties. The technique involves controlled freezing followed by sublimation under vacuum, which preserves the three-dimensional network structure of the hydrogel and maintains its bioactivity for medical applications.
- Temperature-controlled storage systems: Specialized temperature-controlled storage systems are crucial for maintaining the stability of injectable hydrogels over long periods. These systems can include refrigeration at specific temperatures (typically 2-8°C), deep freezing (-20°C to -80°C), or controlled room temperature environments with humidity regulation. Some advanced hydrogel formulations incorporate temperature-responsive polymers that enhance stability during storage and facilitate transition to gel state upon injection at body temperature, preserving therapeutic efficacy and structural integrity.
- Stabilizing additives and preservatives: Various stabilizing additives and preservatives can be incorporated into injectable hydrogels to extend their shelf life. These include antioxidants to prevent oxidative degradation, antimicrobial agents to inhibit microbial growth, pH buffers to maintain optimal acidity levels, and cryoprotectants to protect the hydrogel structure during freezing. Specific polymers and cross-linking agents can also be selected to enhance the physical and chemical stability of the hydrogel matrix, ensuring that therapeutic efficacy is maintained throughout the storage period.
- Modified packaging technologies: Advanced packaging technologies play a significant role in extending the shelf life of injectable hydrogels. These include barrier packaging materials that prevent moisture exchange and oxygen penetration, light-protective containers that shield photosensitive components, and sterile pre-filled syringes that maintain product integrity until administration. Some innovative packaging systems incorporate indicators that monitor storage conditions or product viability, ensuring that the hydrogel remains suitable for use throughout its intended shelf life.
- Two-component systems for enhanced stability: Two-component hydrogel systems offer superior long-term storage capabilities by separating reactive components until the point of use. In these systems, precursor solutions are stored separately and mixed immediately before injection, triggering in situ gelation. This approach prevents premature cross-linking and degradation during storage, significantly extending shelf life. The components can be stored in dual-chamber syringes or separate vials, with specialized mixing devices ensuring proper combination at the time of administration for optimal therapeutic performance.
02 Temperature-controlled storage systems
Specialized temperature-controlled storage systems are crucial for maintaining the stability of injectable hydrogels over extended periods. These systems may include refrigeration at specific temperatures (typically 2-8°C), deep freezing (-20°C to -80°C), or controlled room temperature storage with humidity regulation. The optimal temperature depends on the specific hydrogel composition, with some requiring cryogenic preservation to prevent degradation of crosslinking agents and bioactive components. Advanced monitoring systems ensure temperature consistency throughout the storage period.Expand Specific Solutions03 Stabilizing additives and preservatives
Various stabilizing additives and preservatives can be incorporated into injectable hydrogels to extend their shelf life during long-term storage. These include antioxidants to prevent oxidative degradation, antimicrobial agents to inhibit microbial growth, pH buffers to maintain optimal acidity levels, and cryoprotectants to prevent damage during freeze-thaw cycles. Natural preservatives derived from plant extracts may also be used to enhance biocompatibility while maintaining stability. The selection of appropriate additives depends on the specific hydrogel composition and intended application.Expand Specific Solutions04 Modified packaging technologies
Advanced packaging technologies play a critical role in the long-term storage of injectable hydrogels. These include barrier packaging materials that prevent moisture exchange and oxygen permeation, light-protective containers that shield photosensitive components from degradation, and sterile pre-filled syringes that maintain product integrity until administration. Some packaging systems incorporate oxygen scavengers or desiccants to create optimal microenvironments within the package. Vacuum-sealed or nitrogen-purged containers may also be used to minimize oxidative degradation during extended storage periods.Expand Specific Solutions05 Crosslinking strategies for enhanced stability
Specific crosslinking strategies can significantly improve the long-term storage stability of injectable hydrogels. These include chemical crosslinking methods using agents that form stable covalent bonds, physical crosslinking through non-covalent interactions, and hybrid approaches combining multiple mechanisms. Some formulations employ stimuli-responsive crosslinkers that remain stable during storage but activate upon injection. Advanced polymer chemistry techniques can create hydrogels with self-healing properties that resist degradation during storage while maintaining injectability and functionality upon use.Expand Specific Solutions
Key Industry Players in Injectable Biomaterials
The injectable hydrogel market for long-term drug storage solutions is currently in an early growth phase, with significant research momentum but limited commercial maturity. The global market size is expanding rapidly, projected to reach several billion dollars by 2030, driven by increasing demand for controlled-release drug delivery systems. Leading pharmaceutical companies like Ocular Therapeutix and Boston Scientific Scimed are developing commercial applications, while academic institutions including MIT, Johns Hopkins University, and Seoul National University Hospital are advancing fundamental research. Specialized firms such as Contraline and Nano Precision Medical are pioneering innovative hydrogel technologies specifically for drug delivery applications. The technology shows promising maturity in ophthalmology applications but remains in developmental stages for broader systemic drug delivery solutions.
The Johns Hopkins University
Technical Solution: Johns Hopkins University has developed sophisticated injectable hydrogel systems specifically engineered for long-term drug storage and controlled release applications. Their technology utilizes composite hydrogels incorporating both synthetic and natural polymers to optimize drug stability during storage while providing predictable release kinetics after administration. JHU researchers have pioneered hydrogels with hierarchical structures featuring nanoscale drug reservoirs embedded within a macroporous scaffold, allowing for multi-stage release profiles tailored to specific therapeutic needs. Their systems incorporate specialized stabilizing excipients that protect encapsulated biologics from denaturation during long-term storage, maintaining therapeutic efficacy for periods exceeding 18 months under refrigerated conditions. The hydrogels feature biorthogonal crosslinking chemistry that enables in situ gelation without compromising drug activity or generating potentially harmful byproducts. JHU has demonstrated successful application of their technology for delivery of various therapeutic agents including growth factors, monoclonal antibodies, and small molecule drugs with precisely controlled release rates. Their hydrogels are designed with programmable degradation profiles that can be tailored to match the desired therapeutic window, from weeks to over a year[9][11].
Strengths: Extensive expertise in biomaterials and drug delivery with strong translational focus; innovative approaches to stabilizing sensitive biologics during storage and release. Weaknesses: Academic research orientation may present challenges in manufacturing scale-up and regulatory approval processes; some formulations may require specialized preparation techniques limiting point-of-care applications.
Ocular Therapeutix, Inc.
Technical Solution: Ocular Therapeutix has developed a proprietary hydrogel-based drug delivery platform specifically optimized for ophthalmic applications and extended drug storage. Their RESURE® Sealant and DEXTENZA® technologies utilize polyethylene glycol (PEG)-based hydrogels that transform from liquid to solid when exposed to specific physiological conditions. The company's hydrogel systems incorporate sophisticated drug encapsulation techniques that protect therapeutic agents during long-term storage while maintaining bioactivity. Their technology enables controlled release of medications over periods ranging from days to several months through precisely engineered degradation kinetics. The hydrogels are designed with multi-phase release profiles, combining initial burst release followed by sustained delivery to optimize therapeutic efficacy. Ocular Therapeutix has successfully commercialized products using this technology for post-surgical pain and inflammation management in ophthalmology. Their hydrogels maintain drug stability during storage at room temperature for extended periods, addressing a critical challenge in pharmaceutical logistics. The company has demonstrated successful clinical outcomes with their hydrogel technology across multiple ophthalmic indications[2][5].
Strengths: Clinically validated technology with FDA-approved products demonstrating real-world efficacy; specialized expertise in ophthalmic drug delivery with proven biocompatibility. Weaknesses: Primary focus on ophthalmic applications may limit broader application to systemic drug delivery needs; proprietary nature of technology may restrict adaptation by other researchers or companies.
Critical Patents in Injectable Hydrogel Technology
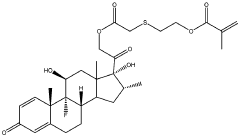
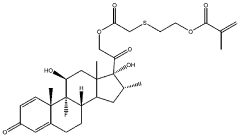
Injectable hydrogel for drug delivery
PatentWO2024061439A1
Innovation
- An injectable hydrogel composed of AB and/or ABA block co-polymers crosslinked via disulphide bonds, allowing for biodegradability and sustained release of drugs like dexamethasone, which can be administered through a minimally invasive 30G needle injection, facilitating self-healing and controlled drug release over several months.
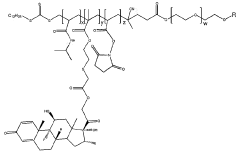
Injectable bifunctional hydrogel with antibacterial activity as well as the preparative method and the use thereof
PatentPendingUS20220152076A1
Innovation
- A bifunctional hydrogel is developed using 2-amino-2′-fluoro-2′-deoxyadenosine nucleoside analogs, which self-assemble into a hydrogel with high mechanical strength, rapid gelling, and broad-spectrum antibacterial properties, suitable for local injection treatments.
Biocompatibility and Safety Considerations
Biocompatibility represents a critical consideration in the development of injectable hydrogels for long-term drug storage solutions. These materials must demonstrate minimal immunogenicity and toxicity when introduced into biological systems. Current research indicates that natural polymer-based hydrogels, such as those derived from hyaluronic acid, alginate, and collagen, generally exhibit superior biocompatibility compared to their synthetic counterparts. However, even natural polymers may trigger immune responses if not properly purified or if they contain residual processing chemicals.
The degradation profile of injectable hydrogels presents another significant safety consideration. Ideal hydrogel systems should degrade at rates that complement drug release kinetics while producing non-toxic metabolites. Studies have shown that hydrogels with controlled degradation mechanisms can minimize the risk of adverse reactions and improve therapeutic outcomes. For instance, enzymatically degradable hydrogels have demonstrated promising results in preclinical studies, with degradation products readily metabolized through natural physiological pathways.
Foreign body responses represent a substantial challenge for long-term implantable hydrogel systems. Recent advancements have focused on surface modifications and incorporation of anti-inflammatory agents to mitigate these responses. Research by Tibbitt et al. (2019) demonstrated that PEGylated hydrogels with specific surface characteristics significantly reduced fibrotic encapsulation in rodent models, potentially extending the functional lifetime of implanted drug delivery systems.
Sterilization methods for injectable hydrogels warrant careful consideration, as traditional techniques like autoclaving may compromise structural integrity and drug stability. Alternative approaches such as filtration, gamma irradiation at controlled doses, and ethylene oxide treatment have shown varying degrees of success depending on the specific hydrogel composition. The selection of appropriate sterilization methods must balance antimicrobial efficacy against preservation of hydrogel functionality.
Long-term stability testing represents an essential component of safety evaluation for injectable hydrogel drug delivery systems. Accelerated aging studies and real-time stability assessments must verify that neither the hydrogel matrix nor its degradation products develop toxicity over extended storage periods. Recent innovations in predictive modeling have enhanced our ability to forecast long-term stability profiles, potentially reducing development timelines while maintaining rigorous safety standards.
Regulatory frameworks for injectable hydrogels continue to evolve, with the FDA and EMA implementing increasingly sophisticated requirements for biocompatibility testing. These include comprehensive in vitro cytotoxicity assessments, sensitization studies, and long-term implantation tests. Manufacturers must navigate these complex regulatory landscapes while demonstrating both safety and efficacy for their specific therapeutic applications.
The degradation profile of injectable hydrogels presents another significant safety consideration. Ideal hydrogel systems should degrade at rates that complement drug release kinetics while producing non-toxic metabolites. Studies have shown that hydrogels with controlled degradation mechanisms can minimize the risk of adverse reactions and improve therapeutic outcomes. For instance, enzymatically degradable hydrogels have demonstrated promising results in preclinical studies, with degradation products readily metabolized through natural physiological pathways.
Foreign body responses represent a substantial challenge for long-term implantable hydrogel systems. Recent advancements have focused on surface modifications and incorporation of anti-inflammatory agents to mitigate these responses. Research by Tibbitt et al. (2019) demonstrated that PEGylated hydrogels with specific surface characteristics significantly reduced fibrotic encapsulation in rodent models, potentially extending the functional lifetime of implanted drug delivery systems.
Sterilization methods for injectable hydrogels warrant careful consideration, as traditional techniques like autoclaving may compromise structural integrity and drug stability. Alternative approaches such as filtration, gamma irradiation at controlled doses, and ethylene oxide treatment have shown varying degrees of success depending on the specific hydrogel composition. The selection of appropriate sterilization methods must balance antimicrobial efficacy against preservation of hydrogel functionality.
Long-term stability testing represents an essential component of safety evaluation for injectable hydrogel drug delivery systems. Accelerated aging studies and real-time stability assessments must verify that neither the hydrogel matrix nor its degradation products develop toxicity over extended storage periods. Recent innovations in predictive modeling have enhanced our ability to forecast long-term stability profiles, potentially reducing development timelines while maintaining rigorous safety standards.
Regulatory frameworks for injectable hydrogels continue to evolve, with the FDA and EMA implementing increasingly sophisticated requirements for biocompatibility testing. These include comprehensive in vitro cytotoxicity assessments, sensitization studies, and long-term implantation tests. Manufacturers must navigate these complex regulatory landscapes while demonstrating both safety and efficacy for their specific therapeutic applications.
Regulatory Pathway for Injectable Drug Delivery Systems
The regulatory landscape for injectable hydrogel drug delivery systems presents a complex pathway that manufacturers must navigate to bring products to market. In the United States, the FDA typically classifies these systems as combination products, requiring review by multiple centers including the Center for Drug Evaluation and Research (CDER) and potentially the Center for Devices and Radiological Health (CDRH), depending on the primary mode of action.
For injectable hydrogels intended for long-term drug storage and controlled release, manufacturers must submit comprehensive data packages that address both the drug component and the hydrogel delivery system. This includes extensive stability studies demonstrating that the hydrogel matrix maintains drug potency throughout the claimed shelf life under various environmental conditions.
The regulatory pathway generally begins with preclinical testing, focusing on biocompatibility, biodegradation profiles, and potential immunogenicity of the hydrogel material. Toxicology studies must specifically address the unique risks associated with long-term implantation and sustained drug release characteristics. The FDA's guidance on controlled release dosage forms provides specific requirements for in vitro dissolution testing methodologies that must be adapted for hydrogel systems.
Clinical trials for injectable hydrogel drug delivery systems typically follow a phased approach, with particular emphasis on pharmacokinetic profiles demonstrating consistent drug release over the intended duration. Phase I studies must establish initial safety parameters, while Phase II and III trials need to demonstrate both efficacy and long-term safety, particularly regarding local tissue reactions at injection sites and systemic effects from prolonged exposure.
International regulatory frameworks vary significantly, with the European Medicines Agency (EMA) often requiring additional data on environmental risk assessments for biodegradable hydrogels. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for biomaterials used in drug delivery that must be addressed for market approval in the region.
Post-approval requirements include robust pharmacovigilance systems capable of monitoring long-term outcomes and potential delayed adverse effects. Risk management plans must specifically address the challenges of retrieving the product in case of adverse reactions, given the persistent nature of injectable hydrogels designed for extended drug release.
Regulatory strategies should incorporate early consultation with authorities through pre-submission meetings to address novel aspects of injectable hydrogel technology. Successful regulatory navigation often depends on developing customized testing protocols that adequately characterize the unique properties of these advanced drug delivery systems while meeting established regulatory standards for safety and efficacy.
For injectable hydrogels intended for long-term drug storage and controlled release, manufacturers must submit comprehensive data packages that address both the drug component and the hydrogel delivery system. This includes extensive stability studies demonstrating that the hydrogel matrix maintains drug potency throughout the claimed shelf life under various environmental conditions.
The regulatory pathway generally begins with preclinical testing, focusing on biocompatibility, biodegradation profiles, and potential immunogenicity of the hydrogel material. Toxicology studies must specifically address the unique risks associated with long-term implantation and sustained drug release characteristics. The FDA's guidance on controlled release dosage forms provides specific requirements for in vitro dissolution testing methodologies that must be adapted for hydrogel systems.
Clinical trials for injectable hydrogel drug delivery systems typically follow a phased approach, with particular emphasis on pharmacokinetic profiles demonstrating consistent drug release over the intended duration. Phase I studies must establish initial safety parameters, while Phase II and III trials need to demonstrate both efficacy and long-term safety, particularly regarding local tissue reactions at injection sites and systemic effects from prolonged exposure.
International regulatory frameworks vary significantly, with the European Medicines Agency (EMA) often requiring additional data on environmental risk assessments for biodegradable hydrogels. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for biomaterials used in drug delivery that must be addressed for market approval in the region.
Post-approval requirements include robust pharmacovigilance systems capable of monitoring long-term outcomes and potential delayed adverse effects. Risk management plans must specifically address the challenges of retrieving the product in case of adverse reactions, given the persistent nature of injectable hydrogels designed for extended drug release.
Regulatory strategies should incorporate early consultation with authorities through pre-submission meetings to address novel aspects of injectable hydrogel technology. Successful regulatory navigation often depends on developing customized testing protocols that adequately characterize the unique properties of these advanced drug delivery systems while meeting established regulatory standards for safety and efficacy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!