Cell-free protein expression for vaccine development.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Protein Expression Background and Objectives
Cell-free protein expression (CFPE) represents a revolutionary approach in biotechnology that has evolved significantly since its inception in the 1960s. This technology enables protein synthesis outside living cells by utilizing cell extracts containing the necessary machinery for transcription and translation. The evolution of CFPE systems has progressed from crude extracts to highly refined and optimized platforms capable of producing complex proteins with high yield and functionality.
The field has witnessed remarkable advancements over the past decades, transitioning from primarily research-focused applications to commercially viable production systems. Early CFPE systems derived from E. coli extracts have been complemented by eukaryotic systems from wheat germ, rabbit reticulocytes, insect cells, and human cell lines, each offering distinct advantages for specific protein expression requirements.
In the context of vaccine development, CFPE technology presents a particularly promising avenue. Traditional vaccine production methods often involve time-consuming cell culture processes, extensive purification steps, and significant biosafety considerations. CFPE systems circumvent many of these challenges by offering a cell-free environment that eliminates concerns about viral contamination and cellular toxicity while enabling rapid protein production.
The primary technical objectives for CFPE in vaccine development include enhancing expression yields to economically viable levels, improving post-translational modification capabilities to ensure proper protein folding and functionality, extending reaction durations to maximize productivity, and developing scalable production platforms suitable for commercial manufacturing.
Recent global health crises, particularly the COVID-19 pandemic, have underscored the critical need for rapid vaccine development technologies. CFPE systems offer the potential to dramatically accelerate the timeline from antigen identification to vaccine prototype production, potentially reducing development cycles from months to weeks or even days.
The convergence of CFPE technology with advances in synthetic biology, computational protein design, and high-throughput screening methodologies creates unprecedented opportunities for next-generation vaccine platforms. These systems aim to address emerging infectious diseases, cancer immunotherapies, and personalized medicine applications.
The ultimate goal of CFPE technology in vaccine development is to establish a versatile, rapid-response platform capable of producing safe, effective, and affordable vaccines against a wide spectrum of pathogens. This platform would ideally combine the speed and flexibility of cell-free systems with the scalability and regulatory compliance required for global vaccine distribution.
The field has witnessed remarkable advancements over the past decades, transitioning from primarily research-focused applications to commercially viable production systems. Early CFPE systems derived from E. coli extracts have been complemented by eukaryotic systems from wheat germ, rabbit reticulocytes, insect cells, and human cell lines, each offering distinct advantages for specific protein expression requirements.
In the context of vaccine development, CFPE technology presents a particularly promising avenue. Traditional vaccine production methods often involve time-consuming cell culture processes, extensive purification steps, and significant biosafety considerations. CFPE systems circumvent many of these challenges by offering a cell-free environment that eliminates concerns about viral contamination and cellular toxicity while enabling rapid protein production.
The primary technical objectives for CFPE in vaccine development include enhancing expression yields to economically viable levels, improving post-translational modification capabilities to ensure proper protein folding and functionality, extending reaction durations to maximize productivity, and developing scalable production platforms suitable for commercial manufacturing.
Recent global health crises, particularly the COVID-19 pandemic, have underscored the critical need for rapid vaccine development technologies. CFPE systems offer the potential to dramatically accelerate the timeline from antigen identification to vaccine prototype production, potentially reducing development cycles from months to weeks or even days.
The convergence of CFPE technology with advances in synthetic biology, computational protein design, and high-throughput screening methodologies creates unprecedented opportunities for next-generation vaccine platforms. These systems aim to address emerging infectious diseases, cancer immunotherapies, and personalized medicine applications.
The ultimate goal of CFPE technology in vaccine development is to establish a versatile, rapid-response platform capable of producing safe, effective, and affordable vaccines against a wide spectrum of pathogens. This platform would ideally combine the speed and flexibility of cell-free systems with the scalability and regulatory compliance required for global vaccine distribution.
Vaccine Development Market Analysis
The global vaccine market has experienced significant growth in recent years, reaching approximately $41 billion in 2022 and projected to expand at a compound annual growth rate (CAGR) of 7.2% through 2030. This growth is driven by increasing immunization programs worldwide, rising prevalence of infectious diseases, and technological advancements in vaccine development methodologies.
Cell-free protein expression systems are emerging as a disruptive technology within this expanding market, offering potential solutions to traditional vaccine development challenges. The market segment specifically for cell-free expression technologies in vaccine development was valued at around $580 million in 2022, with projections indicating growth to exceed $1.5 billion by 2028.
The COVID-19 pandemic has substantially accelerated market dynamics, creating unprecedented demand for rapid vaccine development platforms. This global health crisis highlighted the limitations of conventional vaccine manufacturing approaches and emphasized the need for flexible, scalable technologies like cell-free systems that can respond quickly to emerging threats.
Geographically, North America dominates the vaccine development market with approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 9.1% annually, driven by increasing healthcare expenditure, growing immunization awareness, and expanding manufacturing capabilities in countries like China and India.
The vaccine market segmentation reveals interesting trends relevant to cell-free expression systems. Recombinant vaccines represent the fastest-growing segment at 8.3% CAGR, while therapeutic vaccines for cancer and chronic diseases are expanding at 11.2% annually. These segments particularly benefit from the precision and flexibility offered by cell-free protein expression platforms.
Consumer demand patterns indicate growing preference for vaccines with improved safety profiles, reduced side effects, and faster production timelines - all potential advantages of cell-free systems. Additionally, there is increasing market pull for personalized vaccines and those targeting previously untreatable conditions, creating new opportunities for innovative development approaches.
Regulatory landscapes significantly impact market dynamics, with accelerated approval pathways being established for innovative vaccine technologies in major markets. The FDA's Emergency Use Authorization framework and similar mechanisms globally have created precedents for faster commercialization of novel vaccine platforms, potentially benefiting cell-free expression technologies.
Economic factors including production costs, scalability challenges, and supply chain considerations remain critical market determinants. Cell-free systems offer potential advantages in reducing capital expenditure requirements and enabling distributed manufacturing models, which could reshape traditional market structures in the vaccine industry.
Cell-free protein expression systems are emerging as a disruptive technology within this expanding market, offering potential solutions to traditional vaccine development challenges. The market segment specifically for cell-free expression technologies in vaccine development was valued at around $580 million in 2022, with projections indicating growth to exceed $1.5 billion by 2028.
The COVID-19 pandemic has substantially accelerated market dynamics, creating unprecedented demand for rapid vaccine development platforms. This global health crisis highlighted the limitations of conventional vaccine manufacturing approaches and emphasized the need for flexible, scalable technologies like cell-free systems that can respond quickly to emerging threats.
Geographically, North America dominates the vaccine development market with approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 9.1% annually, driven by increasing healthcare expenditure, growing immunization awareness, and expanding manufacturing capabilities in countries like China and India.
The vaccine market segmentation reveals interesting trends relevant to cell-free expression systems. Recombinant vaccines represent the fastest-growing segment at 8.3% CAGR, while therapeutic vaccines for cancer and chronic diseases are expanding at 11.2% annually. These segments particularly benefit from the precision and flexibility offered by cell-free protein expression platforms.
Consumer demand patterns indicate growing preference for vaccines with improved safety profiles, reduced side effects, and faster production timelines - all potential advantages of cell-free systems. Additionally, there is increasing market pull for personalized vaccines and those targeting previously untreatable conditions, creating new opportunities for innovative development approaches.
Regulatory landscapes significantly impact market dynamics, with accelerated approval pathways being established for innovative vaccine technologies in major markets. The FDA's Emergency Use Authorization framework and similar mechanisms globally have created precedents for faster commercialization of novel vaccine platforms, potentially benefiting cell-free expression technologies.
Economic factors including production costs, scalability challenges, and supply chain considerations remain critical market determinants. Cell-free systems offer potential advantages in reducing capital expenditure requirements and enabling distributed manufacturing models, which could reshape traditional market structures in the vaccine industry.
Technical Challenges in Cell-free Expression Systems
Despite the promising potential of cell-free protein expression systems for vaccine development, several significant technical challenges impede their widespread adoption and commercial application. One of the primary obstacles is the limited scalability of these systems. While laboratory-scale production is well-established, scaling up to industrial levels presents difficulties in maintaining consistent protein yield and quality across larger volumes. The economics of scale that benefit traditional cell-based systems are not as readily achievable in cell-free platforms.
Extract stability represents another critical challenge. Cell-free systems typically maintain optimal activity for only 2-4 hours before degradation of essential components occurs. This short operational window significantly constrains production capacity and necessitates either continuous supplementation of depleted components or development of stabilized extracts that can function for extended periods.
Energy supply limitations further complicate cell-free protein production. The absence of cellular metabolic networks means that ATP and other high-energy molecules must be externally supplied or generated through auxiliary enzymatic pathways. These energy systems often become rate-limiting factors in prolonged expression reactions, leading to premature termination of protein synthesis.
Post-translational modifications (PTMs) present a substantial hurdle for vaccine development applications. Many vaccine candidates require specific glycosylation patterns or other modifications for proper immunogenicity and efficacy. Current cell-free systems have limited capacity to perform complex PTMs compared to eukaryotic cell-based systems, potentially affecting the structural integrity and immunological properties of expressed antigens.
Batch-to-batch variability remains problematic for regulatory compliance. The preparation of cell extracts involves multiple steps that can introduce inconsistencies, resulting in variable protein yields and quality between production runs. This variability poses challenges for meeting the stringent quality control standards required for vaccine manufacturing.
Cost considerations also present significant barriers. The reagents required for cell-free systems, particularly nucleotides and energy regeneration components, remain expensive when compared to traditional fermentation approaches. Although recent advances have reduced costs considerably, the economic viability of cell-free systems for large-scale vaccine production remains questionable without further technological improvements.
Lastly, the preservation of functional integrity during storage and transport poses challenges. Cell-free extracts typically require ultra-low temperature storage to maintain activity, complicating distribution logistics and increasing costs, particularly for applications in resource-limited settings where cold chain infrastructure may be inadequate.
Extract stability represents another critical challenge. Cell-free systems typically maintain optimal activity for only 2-4 hours before degradation of essential components occurs. This short operational window significantly constrains production capacity and necessitates either continuous supplementation of depleted components or development of stabilized extracts that can function for extended periods.
Energy supply limitations further complicate cell-free protein production. The absence of cellular metabolic networks means that ATP and other high-energy molecules must be externally supplied or generated through auxiliary enzymatic pathways. These energy systems often become rate-limiting factors in prolonged expression reactions, leading to premature termination of protein synthesis.
Post-translational modifications (PTMs) present a substantial hurdle for vaccine development applications. Many vaccine candidates require specific glycosylation patterns or other modifications for proper immunogenicity and efficacy. Current cell-free systems have limited capacity to perform complex PTMs compared to eukaryotic cell-based systems, potentially affecting the structural integrity and immunological properties of expressed antigens.
Batch-to-batch variability remains problematic for regulatory compliance. The preparation of cell extracts involves multiple steps that can introduce inconsistencies, resulting in variable protein yields and quality between production runs. This variability poses challenges for meeting the stringent quality control standards required for vaccine manufacturing.
Cost considerations also present significant barriers. The reagents required for cell-free systems, particularly nucleotides and energy regeneration components, remain expensive when compared to traditional fermentation approaches. Although recent advances have reduced costs considerably, the economic viability of cell-free systems for large-scale vaccine production remains questionable without further technological improvements.
Lastly, the preservation of functional integrity during storage and transport poses challenges. Cell-free extracts typically require ultra-low temperature storage to maintain activity, complicating distribution logistics and increasing costs, particularly for applications in resource-limited settings where cold chain infrastructure may be inadequate.
Current Cell-free Expression Platforms for Vaccines
01 Cell-free protein synthesis systems
Cell-free protein synthesis systems enable protein production outside living cells by utilizing cellular extracts containing the necessary machinery for transcription and translation. These systems typically include ribosomes, enzymes, tRNAs, amino acids, and energy sources required for protein synthesis. They offer advantages such as rapid protein production, ability to express toxic proteins, and simplified purification processes compared to cell-based methods.- Cell-free protein synthesis systems: Cell-free protein synthesis systems allow for the production of proteins outside of living cells. These systems typically contain all the necessary components for transcription and translation, including ribosomes, enzymes, tRNAs, amino acids, and energy sources. They offer advantages such as rapid protein production, the ability to produce toxic proteins, and simplified purification processes. These systems can be derived from various organisms including bacteria, yeast, and mammalian cells, each with specific applications based on their properties.
- Enhanced cell-free expression methods: Various methods have been developed to enhance the efficiency and yield of cell-free protein expression. These include optimizing reaction conditions such as temperature, pH, and ion concentrations; supplementing with additional components like chaperones to assist protein folding; using continuous-flow systems to remove inhibitory byproducts; and incorporating specialized translation factors. These enhancements can significantly increase protein yield and maintain activity for extended periods compared to conventional batch reactions.
- Applications in therapeutic protein production: Cell-free protein expression systems are increasingly used for the production of therapeutic proteins and vaccines. These systems allow for rapid production of proteins with post-translational modifications, which is crucial for therapeutic efficacy. They are particularly valuable for producing proteins that are difficult to express in traditional cell-based systems due to toxicity or instability. The ability to quickly produce therapeutic candidates makes these systems especially useful in responding to emerging diseases and pandemics.
- Genetic and metabolic engineering for improved expression: Genetic and metabolic engineering approaches have been developed to optimize cell-free protein expression systems. These include modifying the genetic components to enhance transcription and translation efficiency, engineering energy regeneration pathways to sustain protein synthesis for longer periods, and optimizing codon usage for improved expression. Additionally, the incorporation of non-standard amino acids allows for the production of proteins with novel functionalities not found in nature.
- Microfluidic and high-throughput cell-free systems: Microfluidic and high-throughput technologies have been integrated with cell-free protein expression systems to enable rapid screening and optimization. These platforms allow for miniaturization of reactions, reducing reagent consumption and costs while increasing throughput. They facilitate the simultaneous testing of multiple conditions and variants, accelerating protein engineering and drug discovery processes. Additionally, these systems can be automated for continuous production and real-time monitoring of protein synthesis.
02 Enhanced expression yields in cell-free systems
Various approaches have been developed to improve protein expression yields in cell-free systems. These include optimizing reaction conditions, supplementing with additional components like chaperones, modifying energy regeneration systems, and engineering the translation machinery. Such enhancements can significantly increase protein production efficiency and enable the synthesis of complex proteins that are difficult to express in conventional systems.Expand Specific Solutions03 Cell-free expression for therapeutic applications
Cell-free protein expression systems are increasingly used for the production of therapeutic proteins and vaccines. These systems allow for rapid production of pharmaceuticals, which is particularly valuable in pandemic situations or personalized medicine. The absence of cellular contaminants can also simplify downstream purification processes and regulatory approval pathways for therapeutic products.Expand Specific Solutions04 Continuous cell-free protein expression
Continuous cell-free protein expression systems have been developed to overcome the limited reaction duration of batch processes. These systems employ various strategies such as continuous supply of substrates and removal of inhibitory byproducts through dialysis membranes or microfluidic devices. This approach enables prolonged protein synthesis and higher yields compared to traditional batch reactions.Expand Specific Solutions05 Cell-free expression for protein engineering and screening
Cell-free protein expression systems are valuable tools for protein engineering and high-throughput screening applications. They enable rapid testing of protein variants, directed evolution experiments, and functional characterization without the need for cell transformation and cultivation steps. This accelerates the discovery and optimization of proteins with desired properties for various applications in biotechnology and medicine.Expand Specific Solutions
Leading Companies and Research Institutions
Cell-free protein expression for vaccine development is currently in a growth phase, with increasing market size driven by demand for rapid vaccine production. The technology is maturing but still evolving, with key players demonstrating varying levels of expertise. Companies like Sutro Biopharma and Cellfree Sciences have established specialized platforms, while Bavarian Nordic and SK bioscience bring commercial vaccine development experience. Academic institutions including Northwestern University and RIKEN Institute contribute fundamental research advancements. Pharmaceutical companies such as MedImmune (AstraZeneca) are integrating cell-free systems into their development pipelines. The competitive landscape features both specialized biotechnology firms and larger established players, with innovation focused on improving expression yields, scalability, and regulatory compliance for clinical applications.
Bavarian Nordic A/S
Technical Solution: Bavarian Nordic has integrated cell-free protein expression into their MVA-BN® vaccine platform technology to accelerate antigen discovery and optimization. Their approach combines in vitro transcription and translation systems with their proprietary Modified Vaccinia Ankara (MVA) vector technology. The company utilizes cell-free systems to rapidly screen multiple antigen candidates before incorporating the most promising ones into their viral vector platform. Their technology employs rabbit reticulocyte lysate and wheat germ extract systems to evaluate protein expression, solubility, and antigenicity prior to vector construction. This hybrid approach allows for rapid iteration of antigen designs without the time-consuming process of generating multiple viral vectors. Bavarian Nordic has successfully applied this methodology to develop vaccines against smallpox, Ebola, RSV, and COVID-19. Their cell-free screening platform has demonstrated the ability to predict antigen expression in the final MVA vector with high accuracy, significantly reducing development timelines. The company has further enhanced their system by incorporating high-throughput immunological assays that can evaluate T-cell responses to cell-free expressed antigens, providing early insights into potential vaccine efficacy.
Strengths: Accelerated antigen screening process; seamless integration with established viral vector platform; reduced development timelines; ability to test multiple antigen variants simultaneously. Weaknesses: Limited scale-up potential for the cell-free component; reliance on viral vectors for final vaccine formulation; higher complexity compared to pure cell-free approaches; regulatory considerations for combined technology platforms.
SK bioscience Co., Ltd.
Technical Solution: SK bioscience has developed a proprietary cell-free protein expression platform called "GreenCF" that utilizes chloroplast extracts from green algae for vaccine antigen production. This novel approach combines the advantages of plant-based expression systems with the speed and flexibility of cell-free technology. Their system harnesses the natural protein folding machinery of chloroplasts, which is particularly effective for complex antigens containing multiple disulfide bonds. The GreenCF platform incorporates specialized chaperones and redox-controlling elements that facilitate proper folding of vaccine antigens. SK bioscience has demonstrated successful expression of various viral antigens, including those from SARS-CoV-2, influenza, and human papillomavirus. The company has optimized their system for high-yield production by engineering the algal strains to overexpress translation factors and eliminating components that inhibit protein synthesis. Their technology enables rapid production cycles (12-24 hours) and has been scaled to intermediate production volumes suitable for clinical trial material manufacturing. SK bioscience has further enhanced their platform by developing proprietary purification methods specifically designed for antigens produced in their chloroplast-derived cell-free system, resulting in highly pure vaccine candidates with minimal processing-related modifications.
Strengths: Environmentally sustainable production system; efficient disulfide bond formation; absence of endotoxins and human pathogens; cost-effective scaling potential. Weaknesses: Limited track record in late-stage clinical development; potential for plant-specific glycosylation patterns; regulatory pathway less established than traditional systems; challenges in producing certain mammalian proteins.
Key Patents and Breakthroughs in Cell-free Systems
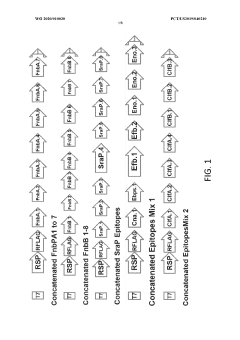
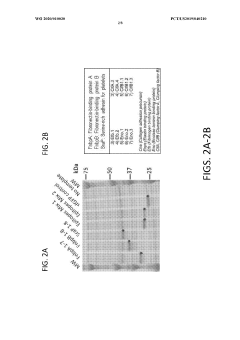
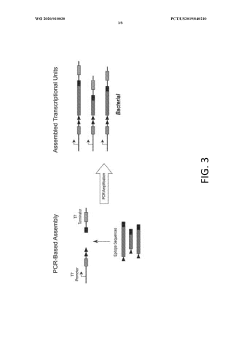
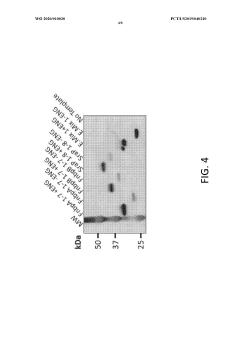
Materials and methods for cell-free expression of vaccine epitope concatemers
PatentWO2020010030A1
Innovation
- A cell-free protein synthesis platform is used for high-throughput, large-scale, and unbiased expression of concatenated epitopes, employing a protein expression cassette with a heterologous promoter, N-terminal peptide tag, and C-terminal peptide tag, allowing for flexible use of eukaryotic or prokaryotic lysates and integrated purification modules to optimize protein production.
Cell-free synthesis of proteins containing unnatural amino acids
PatentActiveAU2007325952A8
Innovation
- The method involves using a cell-free reaction mixture with orthogonal tRNA aminoacylated with unnatural amino acids, where the orthogonal tRNA base pairs with nonsense codons, and includes exogenously synthesized tRNA synthetase to minimize degradation, along with optimized conditions for oxidative phosphorylation and the addition of protein chaperones to ensure high yields and biological activity of the synthesized proteins.
Regulatory Pathway for Cell-free Derived Vaccines
The regulatory landscape for cell-free derived vaccines represents a complex and evolving framework that manufacturers must navigate carefully. Traditional vaccine approval pathways were designed primarily for conventional vaccine technologies, creating unique challenges for novel cell-free protein expression platforms. Regulatory agencies including the FDA, EMA, and WHO have begun developing specific guidance documents addressing these innovative manufacturing approaches, though comprehensive frameworks remain under development.
Cell-free derived vaccines typically follow a regulatory classification as biological products, requiring extensive characterization of both the expression system components and the final vaccine product. Critical regulatory considerations include the source and quality of cell extracts, potential contaminants from the cell-free system, and the consistency of protein expression across manufacturing batches.
Pre-clinical regulatory requirements focus heavily on demonstrating the absence of host cell proteins and nucleic acids that might trigger adverse immune responses. Manufacturers must implement robust purification processes with validated analytical methods to ensure product purity. The cell-free nature of these vaccines may offer advantages in regulatory review by eliminating concerns about viral contamination associated with cell-based production systems.
Clinical trial designs for cell-free derived vaccines must address specific regulatory expectations regarding safety monitoring, particularly for novel adjuvants or delivery systems that may accompany the vaccine antigen. Accelerated approval pathways may be accessible for vaccines targeting urgent public health needs, as demonstrated during the COVID-19 pandemic when several novel vaccine technologies received emergency use authorization.
Post-approval regulatory oversight includes enhanced pharmacovigilance requirements and potential post-marketing studies to monitor long-term safety profiles. Manufacturers must maintain detailed records of raw material sourcing, quality control procedures, and batch-to-batch consistency to satisfy ongoing regulatory compliance obligations.
International harmonization efforts are underway to standardize regulatory approaches to cell-free vaccine technologies across major markets. The International Council for Harmonisation (ICH) has established working groups focused on developing consistent guidelines for characterization and quality control of novel biological products, including cell-free expression systems.
Regulatory success ultimately depends on early and frequent engagement with relevant authorities through scientific advice meetings and formal consultation processes. Companies developing cell-free vaccine platforms should establish regulatory strategies that anticipate evolving requirements and incorporate flexibility to address emerging regulatory concerns throughout the development lifecycle.
Cell-free derived vaccines typically follow a regulatory classification as biological products, requiring extensive characterization of both the expression system components and the final vaccine product. Critical regulatory considerations include the source and quality of cell extracts, potential contaminants from the cell-free system, and the consistency of protein expression across manufacturing batches.
Pre-clinical regulatory requirements focus heavily on demonstrating the absence of host cell proteins and nucleic acids that might trigger adverse immune responses. Manufacturers must implement robust purification processes with validated analytical methods to ensure product purity. The cell-free nature of these vaccines may offer advantages in regulatory review by eliminating concerns about viral contamination associated with cell-based production systems.
Clinical trial designs for cell-free derived vaccines must address specific regulatory expectations regarding safety monitoring, particularly for novel adjuvants or delivery systems that may accompany the vaccine antigen. Accelerated approval pathways may be accessible for vaccines targeting urgent public health needs, as demonstrated during the COVID-19 pandemic when several novel vaccine technologies received emergency use authorization.
Post-approval regulatory oversight includes enhanced pharmacovigilance requirements and potential post-marketing studies to monitor long-term safety profiles. Manufacturers must maintain detailed records of raw material sourcing, quality control procedures, and batch-to-batch consistency to satisfy ongoing regulatory compliance obligations.
International harmonization efforts are underway to standardize regulatory approaches to cell-free vaccine technologies across major markets. The International Council for Harmonisation (ICH) has established working groups focused on developing consistent guidelines for characterization and quality control of novel biological products, including cell-free expression systems.
Regulatory success ultimately depends on early and frequent engagement with relevant authorities through scientific advice meetings and formal consultation processes. Companies developing cell-free vaccine platforms should establish regulatory strategies that anticipate evolving requirements and incorporate flexibility to address emerging regulatory concerns throughout the development lifecycle.
Scalability and Manufacturing Considerations
Scaling up cell-free protein expression systems for vaccine development presents unique challenges compared to traditional cell-based manufacturing. The transition from laboratory-scale production to industrial manufacturing requires significant optimization of reaction conditions, component sourcing, and process engineering. Currently, most cell-free protein expression systems operate efficiently at milliliter scales, but vaccine production demands liter to thousands of liter scales to meet global immunization needs.
The economics of large-scale cell-free systems must be carefully considered. The cost of reagents, particularly enzymes, nucleotides, and energy sources, represents a substantial portion of production expenses. Recent advancements have focused on developing cost-effective crude extract preparations and recycling key components to improve economic viability. Companies like Sutro Biopharma and Greenlight Biosciences have made notable progress in reducing production costs by optimizing extract preparation protocols and implementing continuous-flow bioreactor systems.
Quality control and consistency present another critical consideration for manufacturing cell-free vaccine components. Unlike cell-based systems where cellular homeostasis provides some buffering capacity, cell-free systems require precise control of reaction conditions. Temperature, pH, oxygen levels, and nutrient availability must be tightly regulated across scaled-up volumes to ensure consistent protein quality and yield. Advanced monitoring technologies using real-time sensors and automated feedback systems have emerged to address these challenges.
Regulatory pathways for cell-free produced vaccines remain less defined than traditional manufacturing routes. Regulatory agencies require extensive characterization of the production process, including the source of cellular extracts, potential contaminants, and batch-to-batch consistency metrics. Several companies are currently working with regulatory bodies to establish standardized frameworks for cell-free vaccine approval processes, which will be crucial for widespread implementation.
Infrastructure requirements differ significantly from traditional biomanufacturing. Cell-free systems eliminate the need for cell culture facilities but require specialized equipment for extract preparation, reaction mixing, and downstream processing. Modular and portable manufacturing platforms are being developed to enable distributed production, potentially allowing vaccine manufacturing closer to points of need during outbreaks or in resource-limited settings.
Supply chain resilience must be considered when scaling cell-free vaccine production. The dependency on specialized reagents and components necessitates robust supplier networks. Recent global disruptions have highlighted the importance of diversifying supply sources and developing stockpiling strategies for critical components to ensure manufacturing continuity during crises.
The economics of large-scale cell-free systems must be carefully considered. The cost of reagents, particularly enzymes, nucleotides, and energy sources, represents a substantial portion of production expenses. Recent advancements have focused on developing cost-effective crude extract preparations and recycling key components to improve economic viability. Companies like Sutro Biopharma and Greenlight Biosciences have made notable progress in reducing production costs by optimizing extract preparation protocols and implementing continuous-flow bioreactor systems.
Quality control and consistency present another critical consideration for manufacturing cell-free vaccine components. Unlike cell-based systems where cellular homeostasis provides some buffering capacity, cell-free systems require precise control of reaction conditions. Temperature, pH, oxygen levels, and nutrient availability must be tightly regulated across scaled-up volumes to ensure consistent protein quality and yield. Advanced monitoring technologies using real-time sensors and automated feedback systems have emerged to address these challenges.
Regulatory pathways for cell-free produced vaccines remain less defined than traditional manufacturing routes. Regulatory agencies require extensive characterization of the production process, including the source of cellular extracts, potential contaminants, and batch-to-batch consistency metrics. Several companies are currently working with regulatory bodies to establish standardized frameworks for cell-free vaccine approval processes, which will be crucial for widespread implementation.
Infrastructure requirements differ significantly from traditional biomanufacturing. Cell-free systems eliminate the need for cell culture facilities but require specialized equipment for extract preparation, reaction mixing, and downstream processing. Modular and portable manufacturing platforms are being developed to enable distributed production, potentially allowing vaccine manufacturing closer to points of need during outbreaks or in resource-limited settings.
Supply chain resilience must be considered when scaling cell-free vaccine production. The dependency on specialized reagents and components necessitates robust supplier networks. Recent global disruptions have highlighted the importance of diversifying supply sources and developing stockpiling strategies for critical components to ensure manufacturing continuity during crises.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!