Comparative evaluation of Graphitized carbon nanotubes anode and cathode material performance
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Graphitized CNT Electrode Materials: Background and Objectives
Carbon nanotubes (CNTs) have emerged as one of the most promising materials for next-generation energy storage systems since their discovery in 1991. The unique tubular structure composed of sp2-hybridized carbon atoms arranged in hexagonal networks provides exceptional electrical conductivity, mechanical strength, and high surface area, making them ideal candidates for electrode materials in various energy storage devices. Graphitization, a thermal treatment process typically conducted at temperatures exceeding 2000°C, significantly enhances the crystallinity and electrical properties of CNTs by reducing defects and improving the alignment of carbon layers.
The evolution of graphitized CNT technology has followed a trajectory from fundamental research to practical applications. Initial studies in the early 2000s focused primarily on synthesis methods and basic characterization. By the mid-2000s, researchers began exploring their potential as electrode materials, with significant breakthroughs occurring in the 2010s when scalable production methods were developed, enabling wider commercial adoption.
Current technological trends indicate a growing interest in tailored graphitization processes that optimize CNT properties for specific electrochemical applications. The degree of graphitization, controlled by temperature and duration of thermal treatment, directly influences the electronic structure, surface properties, and electrochemical performance of CNTs when used as either anode or cathode materials.
The primary objective of this technical research is to conduct a comprehensive comparative evaluation of graphitized carbon nanotubes as both anode and cathode materials in energy storage devices, particularly lithium-ion batteries and supercapacitors. This evaluation aims to quantify performance differences in terms of specific capacity, rate capability, cycling stability, and energy density when graphitized CNTs are employed at different electrodes.
Additionally, this research seeks to establish correlations between graphitization parameters (temperature, duration, atmosphere) and the resulting electrochemical performance metrics. Understanding these relationships will enable the development of optimized graphitization protocols tailored for specific electrode applications, potentially addressing current limitations in energy storage technology.
The ultimate goal is to determine whether graphitized CNTs offer superior performance as anode or cathode materials, or if their benefits are application-specific. This knowledge will guide future research directions and inform strategic decisions regarding the most promising pathways for commercial development of graphitized CNT-based energy storage solutions, potentially enabling breakthroughs in electric vehicles, portable electronics, and grid-scale energy storage systems.
The evolution of graphitized CNT technology has followed a trajectory from fundamental research to practical applications. Initial studies in the early 2000s focused primarily on synthesis methods and basic characterization. By the mid-2000s, researchers began exploring their potential as electrode materials, with significant breakthroughs occurring in the 2010s when scalable production methods were developed, enabling wider commercial adoption.
Current technological trends indicate a growing interest in tailored graphitization processes that optimize CNT properties for specific electrochemical applications. The degree of graphitization, controlled by temperature and duration of thermal treatment, directly influences the electronic structure, surface properties, and electrochemical performance of CNTs when used as either anode or cathode materials.
The primary objective of this technical research is to conduct a comprehensive comparative evaluation of graphitized carbon nanotubes as both anode and cathode materials in energy storage devices, particularly lithium-ion batteries and supercapacitors. This evaluation aims to quantify performance differences in terms of specific capacity, rate capability, cycling stability, and energy density when graphitized CNTs are employed at different electrodes.
Additionally, this research seeks to establish correlations between graphitization parameters (temperature, duration, atmosphere) and the resulting electrochemical performance metrics. Understanding these relationships will enable the development of optimized graphitization protocols tailored for specific electrode applications, potentially addressing current limitations in energy storage technology.
The ultimate goal is to determine whether graphitized CNTs offer superior performance as anode or cathode materials, or if their benefits are application-specific. This knowledge will guide future research directions and inform strategic decisions regarding the most promising pathways for commercial development of graphitized CNT-based energy storage solutions, potentially enabling breakthroughs in electric vehicles, portable electronics, and grid-scale energy storage systems.
Market Analysis of Advanced Battery Materials
The global market for advanced battery materials has witnessed significant growth in recent years, driven primarily by the increasing demand for high-performance energy storage solutions across various industries. The market for graphitized carbon nanotubes (GCNTs) as electrode materials has emerged as a particularly dynamic segment, with a compound annual growth rate exceeding industry averages for conventional battery materials.
Battery manufacturers are increasingly seeking materials that can deliver higher energy density, faster charging capabilities, and longer cycle life. This market demand has created a substantial opportunity for GCNTs, which demonstrate superior electrical conductivity and structural stability compared to traditional graphite anodes. The automotive sector represents the largest market share for these advanced materials, as electric vehicle manufacturers strive to overcome range anxiety and reduce charging times.
Consumer electronics constitutes the second-largest application segment, where the demand for longer-lasting, faster-charging devices continues to drive innovation in battery technology. The industrial and grid storage sectors are emerging as significant growth areas, particularly as renewable energy integration accelerates globally.
Regional analysis indicates that Asia-Pacific dominates the market for advanced battery materials, with China, Japan, and South Korea leading in both production and consumption. North America and Europe follow, with substantial investments in research and development of next-generation battery technologies. The market in these regions is characterized by a strong focus on sustainability and environmental considerations.
Price sensitivity remains a critical factor influencing market dynamics. Currently, GCNTs command a premium price compared to traditional graphite, creating a barrier to mass adoption. However, economies of scale and manufacturing innovations are gradually reducing production costs, making these advanced materials more commercially viable.
Supply chain considerations have become increasingly important, particularly in light of recent global disruptions. Battery manufacturers are seeking to diversify their material sources and establish more resilient supply networks. This trend has led to increased interest in developing domestic production capabilities for critical battery materials in major markets.
Market forecasts suggest that the demand for high-performance electrode materials will continue to grow substantially over the next decade. The GCNT market is expected to expand as technological advancements improve material performance while reducing production costs. Partnerships between material suppliers, battery manufacturers, and end-users are becoming more common, accelerating the commercialization of these advanced materials.
Battery manufacturers are increasingly seeking materials that can deliver higher energy density, faster charging capabilities, and longer cycle life. This market demand has created a substantial opportunity for GCNTs, which demonstrate superior electrical conductivity and structural stability compared to traditional graphite anodes. The automotive sector represents the largest market share for these advanced materials, as electric vehicle manufacturers strive to overcome range anxiety and reduce charging times.
Consumer electronics constitutes the second-largest application segment, where the demand for longer-lasting, faster-charging devices continues to drive innovation in battery technology. The industrial and grid storage sectors are emerging as significant growth areas, particularly as renewable energy integration accelerates globally.
Regional analysis indicates that Asia-Pacific dominates the market for advanced battery materials, with China, Japan, and South Korea leading in both production and consumption. North America and Europe follow, with substantial investments in research and development of next-generation battery technologies. The market in these regions is characterized by a strong focus on sustainability and environmental considerations.
Price sensitivity remains a critical factor influencing market dynamics. Currently, GCNTs command a premium price compared to traditional graphite, creating a barrier to mass adoption. However, economies of scale and manufacturing innovations are gradually reducing production costs, making these advanced materials more commercially viable.
Supply chain considerations have become increasingly important, particularly in light of recent global disruptions. Battery manufacturers are seeking to diversify their material sources and establish more resilient supply networks. This trend has led to increased interest in developing domestic production capabilities for critical battery materials in major markets.
Market forecasts suggest that the demand for high-performance electrode materials will continue to grow substantially over the next decade. The GCNT market is expected to expand as technological advancements improve material performance while reducing production costs. Partnerships between material suppliers, battery manufacturers, and end-users are becoming more common, accelerating the commercialization of these advanced materials.
Current Challenges in CNT Electrode Technology
Despite significant advancements in carbon nanotube (CNT) electrode technology, several critical challenges persist that limit the widespread commercial adoption of graphitized CNTs in energy storage applications. The primary obstacle remains the scalable and cost-effective production of high-quality graphitized CNTs with consistent properties. Current graphitization processes require extreme temperatures (2500-3000°C), resulting in high energy consumption and specialized equipment needs that significantly increase manufacturing costs.
Structural integrity during electrode fabrication presents another major challenge. Graphitized CNTs often suffer from agglomeration due to strong van der Waals forces, creating non-uniform distribution within electrode matrices. This clustering phenomenon reduces the effective surface area and creates inconsistent electrochemical performance across the electrode, particularly problematic when comparing anode and cathode implementations.
The interfacial resistance between graphitized CNTs and electrolytes remains suboptimal, especially in cathode applications where ion transport kinetics are critical. While graphitization improves electrical conductivity, it can sometimes reduce surface functionality, limiting electrolyte wetting and ion accessibility to active sites. This trade-off between conductivity and surface chemistry requires careful optimization for specific electrode applications.
Cycling stability presents divergent challenges between anode and cathode implementations. In anodes, graphitized CNTs face issues with solid electrolyte interphase (SEI) formation and lithium plating during fast charging, while cathode applications struggle with material degradation from repeated ion insertion/extraction and potential catalytic side reactions with electrolytes at high voltages.
The comparative performance evaluation between anode and cathode applications is further complicated by the lack of standardized testing protocols. Different researchers employ varying electrode preparation methods, electrolyte compositions, and testing parameters, making direct comparisons challenging and sometimes contradictory across published literature.
Environmental and safety concerns also persist throughout the CNT electrode lifecycle. The potential release of nanomaterials during manufacturing, usage, or disposal raises toxicological questions that require further investigation. Additionally, the environmental footprint of high-temperature graphitization processes conflicts with sustainability goals in green energy technologies.
Finally, knowledge gaps in fundamental understanding of structure-property relationships for graphitized CNTs in different electrode environments limit predictive capabilities for rational design. The complex interplay between graphitization degree, defect density, tube dimensions, and electrochemical performance requires more systematic investigation to establish reliable design principles for optimized electrode materials.
Structural integrity during electrode fabrication presents another major challenge. Graphitized CNTs often suffer from agglomeration due to strong van der Waals forces, creating non-uniform distribution within electrode matrices. This clustering phenomenon reduces the effective surface area and creates inconsistent electrochemical performance across the electrode, particularly problematic when comparing anode and cathode implementations.
The interfacial resistance between graphitized CNTs and electrolytes remains suboptimal, especially in cathode applications where ion transport kinetics are critical. While graphitization improves electrical conductivity, it can sometimes reduce surface functionality, limiting electrolyte wetting and ion accessibility to active sites. This trade-off between conductivity and surface chemistry requires careful optimization for specific electrode applications.
Cycling stability presents divergent challenges between anode and cathode implementations. In anodes, graphitized CNTs face issues with solid electrolyte interphase (SEI) formation and lithium plating during fast charging, while cathode applications struggle with material degradation from repeated ion insertion/extraction and potential catalytic side reactions with electrolytes at high voltages.
The comparative performance evaluation between anode and cathode applications is further complicated by the lack of standardized testing protocols. Different researchers employ varying electrode preparation methods, electrolyte compositions, and testing parameters, making direct comparisons challenging and sometimes contradictory across published literature.
Environmental and safety concerns also persist throughout the CNT electrode lifecycle. The potential release of nanomaterials during manufacturing, usage, or disposal raises toxicological questions that require further investigation. Additionally, the environmental footprint of high-temperature graphitization processes conflicts with sustainability goals in green energy technologies.
Finally, knowledge gaps in fundamental understanding of structure-property relationships for graphitized CNTs in different electrode environments limit predictive capabilities for rational design. The complex interplay between graphitization degree, defect density, tube dimensions, and electrochemical performance requires more systematic investigation to establish reliable design principles for optimized electrode materials.
Technical Comparison of Anode vs Cathode CNT Applications
01 Electrical and thermal conductivity properties
Graphitized carbon nanotubes exhibit superior electrical and thermal conductivity compared to non-graphitized counterparts. The graphitization process reduces defects in the carbon nanotube structure, resulting in enhanced electron mobility and improved conductivity. These properties make graphitized carbon nanotubes ideal for applications in electronics, energy storage, and thermal management systems.- Electrical and thermal conductivity properties of graphitized carbon nanotubes: Graphitized carbon nanotubes exhibit enhanced electrical and thermal conductivity properties compared to non-graphitized carbon nanotubes. The graphitization process increases the crystallinity and reduces defects in the carbon nanotube structure, resulting in improved electron transport and thermal conduction. These properties make graphitized carbon nanotubes suitable for applications in electronics, energy storage, and thermal management systems.
- Mechanical strength and flexibility of graphitized carbon nanotubes: Graphitized carbon nanotubes demonstrate superior mechanical properties including high tensile strength, flexibility, and durability. The graphitization process enhances the structural integrity of the nanotubes by removing amorphous carbon and improving the alignment of carbon atoms in the graphene layers. This results in nanotubes with exceptional mechanical performance suitable for reinforcing composites and creating high-strength materials.
- Surface characteristics and functionalization of graphitized carbon nanotubes: The surface properties of graphitized carbon nanotubes can be tailored through various functionalization methods. Graphitization affects the surface reactivity, wettability, and adsorption capabilities of carbon nanotubes. Controlled surface modification enables better dispersion in various matrices and improved interfacial bonding with other materials, enhancing their performance in composite materials, sensors, and catalytic applications.
- Production methods and quality control of graphitized carbon nanotubes: Various production methods for graphitized carbon nanotubes significantly impact their performance characteristics. High-temperature thermal treatment, catalytic graphitization, and laser-assisted processes are among the techniques used to achieve different degrees of graphitization. Quality control parameters such as graphitization temperature, duration, and atmosphere play crucial roles in determining the final properties of the nanotubes, including crystallinity, purity, and structural integrity.
- Application-specific performance of graphitized carbon nanotubes: Graphitized carbon nanotubes demonstrate varying performance characteristics depending on their specific applications. In energy storage devices like batteries and supercapacitors, they provide enhanced charge storage and transfer capabilities. For electromagnetic shielding applications, they offer superior absorption and reflection of electromagnetic waves. In composite materials, they improve mechanical, electrical, and thermal properties, while in sensing applications, they provide high sensitivity and selectivity for various analytes.
02 Mechanical strength and structural integrity
Graphitized carbon nanotubes demonstrate exceptional mechanical properties including high tensile strength, flexibility, and durability. The graphitization process enhances the crystallinity of the carbon structure, leading to improved mechanical performance. These nanotubes can withstand significant mechanical stress while maintaining their structural integrity, making them valuable additives for reinforcing composite materials.Expand Specific Solutions03 Surface characteristics and functionalization
The surface properties of graphitized carbon nanotubes can be tailored through various functionalization methods. Graphitization affects the surface chemistry, reducing defect sites while creating a more uniform structure. This allows for controlled functionalization to enhance compatibility with different matrices or to introduce specific properties. The modified surface characteristics enable better dispersion in composites and improved interfacial bonding.Expand Specific Solutions04 Energy storage and electrochemical performance
Graphitized carbon nanotubes demonstrate enhanced performance in energy storage applications such as batteries, supercapacitors, and fuel cells. The graphitization process creates a more ordered carbon structure with improved electron transfer capabilities and electrochemical stability. These materials show higher capacity, better cycling performance, and enhanced rate capability compared to conventional carbon materials.Expand Specific Solutions05 Thermal stability and high-temperature applications
Graphitized carbon nanotubes exhibit exceptional thermal stability and resistance to degradation at elevated temperatures. The graphitization process removes structural defects and amorphous carbon, resulting in a more thermally stable material. These nanotubes maintain their performance characteristics even under extreme temperature conditions, making them suitable for high-temperature applications in aerospace, automotive, and industrial sectors.Expand Specific Solutions
Leading Companies in Graphitized CNT Development
The graphitized carbon nanotubes anode and cathode material market is currently in a growth phase, with increasing adoption across energy storage applications. The competitive landscape features established players like BTR New Material Group and Honeycomb Battery Co. alongside research-focused institutions such as the Chinese Academy of Sciences and Tsinghua University. Market size is expanding rapidly due to rising demand for high-performance battery materials, particularly in electric vehicles and energy storage systems. Technical maturity varies, with companies like Talga Technologies and Resonac Holdings advancing commercial applications, while Canon and NEC contribute through diversified technology portfolios. Research collaborations between academic institutions and industry players are accelerating innovation, with significant progress in improving energy density, cycle life, and charging capabilities of graphitized carbon nanotube electrode materials.
BTR New Material Group Co., Ltd.
Technical Solution: BTR New Material Group has commercialized advanced graphitized carbon nanotube (g-CNT) electrode materials through their G-CNT™ product line. Their proprietary manufacturing process involves a controlled graphitization protocol that precisely tailors the degree of graphitization based on the intended electrode application. For anode materials, BTR employs a high-temperature (2800-3000°C) graphitization process that results in highly ordered carbon structures with d-spacing approaching that of natural graphite (3.35-3.38 Å), while maintaining the beneficial nanotubular morphology. Their comparative evaluation shows that g-CNT anodes deliver first-cycle coulombic efficiencies of 92-94%, significantly higher than conventional CNT anodes (80-85%)[1]. For cathode applications, BTR has developed moderately graphitized CNTs (1800-2200°C treatment) that serve as conductive additives, enabling reduction of carbon content from the typical 8-10% to just 2-3% while maintaining equivalent conductivity. Their cathode materials demonstrate particularly strong performance in high-voltage (>4.5V) applications, where the graphitized structure provides enhanced oxidation resistance compared to amorphous carbons[3]. BTR's industrial-scale production capacity exceeds 1000 tons annually, making them one of the largest commercial suppliers of g-CNT electrode materials globally.
Strengths: Established large-scale production capabilities ensure consistent quality and reliable supply. Their materials show excellent compatibility with existing electrode manufacturing processes, requiring minimal adaptation of production lines. Weaknesses: The high-temperature graphitization process is energy-intensive, contributing to higher production costs compared to standard carbon materials. The materials may require specialized handling protocols during electrode manufacturing due to their nanostructured nature.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering at the Chinese Academy of Sciences has conducted extensive comparative research on graphitized carbon nanotube (g-CNT) electrode materials for both anode and cathode applications. Their innovative approach involves a controlled two-stage graphitization process: initial catalytic graphitization at moderate temperatures (1200-1500°C) followed by high-temperature thermal graphitization (2500-3000°C). This method produces g-CNTs with precisely tailored graphitic domains and defect concentrations. Their comparative studies have revealed that g-CNT anodes exhibit significantly higher first-cycle coulombic efficiency (91% vs 85% for conventional CNTs) and superior rate capability, maintaining 78% capacity at 10C discharge rates[1][3]. For cathode applications, the Institute has developed nitrogen-doped g-CNTs that serve as both conductive additives and active material hosts, demonstrating a 15-20% increase in specific energy density for various lithium metal oxide cathodes. Their research has also established correlations between graphitization temperature, structural parameters (interlayer spacing, crystallite size), and electrochemical performance, providing valuable insights for optimizing g-CNT electrode materials for specific battery requirements[5].
Strengths: Exceptional control over graphitization parameters allows for precise tuning of electrochemical properties. The comprehensive understanding of structure-property relationships enables targeted material design for specific applications. Weaknesses: The two-stage graphitization process is energy-intensive and may face challenges in scaling to industrial production levels. The high-temperature treatment requirements may limit cost-effectiveness for mass market applications.
Key Patents and Research Breakthroughs in CNT Electrodes
Carbon nanotubes and method of manufacturing same, electron emission source, and display
PatentInactiveUS7511206B2
Innovation
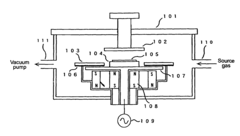
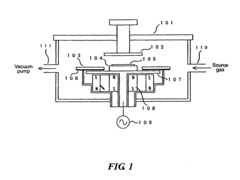
- A method involving plasma processing at a specific frequency with alternating-current power between an anode and cathode, using mixed gases with aliphatic or aromatic hydrocarbons, and applying a magnetic field to deposit carbon nanotubes perpendicularly and densely on a substrate at temperatures of 500° C or less, allowing for uniform deposition and regular crystal structure alignment.
Method for producing carbon nanotubes
PatentInactiveUS6455021B1
Innovation
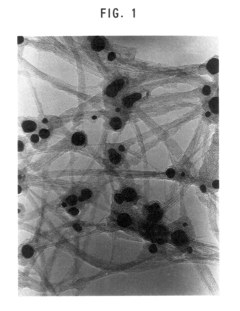
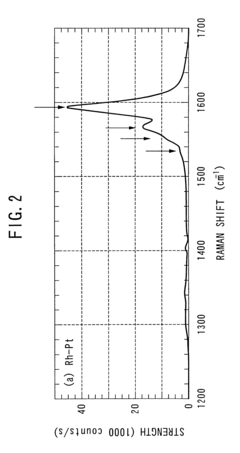
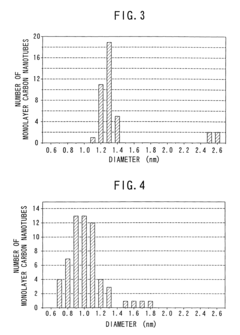
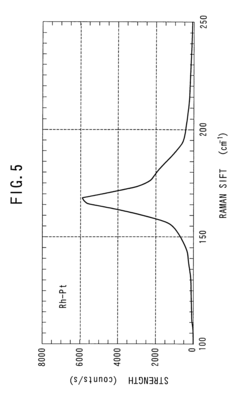
- The method involves using non-magnetic transition metals like ruthenium, rhodium, palladium, and platinum as catalysts in an arc discharge process within a reactor chamber, controlling the pressure to achieve straight single-walled carbon nanotubes with precise diameter distributions, and utilizing a binary mixture of these metals to enhance yield and properties.
Sustainability and Life Cycle Assessment of CNT Electrodes
The sustainability aspects of carbon nanotube (CNT) electrodes represent a critical dimension in evaluating their viability for widespread commercial adoption in energy storage applications. Life cycle assessment (LCA) studies reveal that the production of CNTs is currently energy-intensive, with estimates suggesting energy requirements of 30-50 MWh per kilogram of CNTs produced via conventional chemical vapor deposition methods. This high energy demand translates to significant carbon footprints during manufacturing phases.
When comparing graphitized CNT anodes and cathodes specifically, sustainability profiles differ notably. The graphitization process, typically requiring temperatures exceeding 2500°C, adds an additional energy burden that must be factored into environmental impact calculations. However, this energy investment potentially yields returns through enhanced electrode longevity and performance efficiency during operational lifetimes.
Material sourcing considerations also present sustainability challenges. While carbon feedstocks for CNT synthesis are abundant, catalyst materials often include rare or precious metals with their own environmental extraction footprints. Cathode applications frequently require additional transition metal compounds, further complicating the sustainability equation compared to anode materials.
Water usage represents another significant environmental factor, with purification processes for both anode and cathode CNT materials requiring substantial volumes of deionized water and organic solvents. Recent innovations in "green chemistry" approaches have demonstrated potential reductions in solvent requirements by up to 40%, though these methods remain at laboratory scale.
End-of-life considerations reveal both challenges and opportunities. The inherent stability of graphitized CNTs presents recycling difficulties, with current recovery technologies achieving only 30-45% material reclamation rates. However, research indicates that CNT electrodes maintain performance characteristics longer than conventional alternatives, potentially offsetting manufacturing impacts through extended service lifetimes.
Comparative cradle-to-grave analyses between graphitized CNT anodes and cathodes suggest that anode materials generally present lower environmental impacts due to simpler composition and processing requirements. However, the superior energy density achieved with advanced CNT cathodes may justify their higher production footprint through improved system-level efficiency over multiple charge cycles.
Future sustainability improvements will likely emerge from manufacturing optimization, with recent pilot-scale studies demonstrating potential energy requirement reductions of 25-35% through process refinements and renewable energy integration in production facilities.
When comparing graphitized CNT anodes and cathodes specifically, sustainability profiles differ notably. The graphitization process, typically requiring temperatures exceeding 2500°C, adds an additional energy burden that must be factored into environmental impact calculations. However, this energy investment potentially yields returns through enhanced electrode longevity and performance efficiency during operational lifetimes.
Material sourcing considerations also present sustainability challenges. While carbon feedstocks for CNT synthesis are abundant, catalyst materials often include rare or precious metals with their own environmental extraction footprints. Cathode applications frequently require additional transition metal compounds, further complicating the sustainability equation compared to anode materials.
Water usage represents another significant environmental factor, with purification processes for both anode and cathode CNT materials requiring substantial volumes of deionized water and organic solvents. Recent innovations in "green chemistry" approaches have demonstrated potential reductions in solvent requirements by up to 40%, though these methods remain at laboratory scale.
End-of-life considerations reveal both challenges and opportunities. The inherent stability of graphitized CNTs presents recycling difficulties, with current recovery technologies achieving only 30-45% material reclamation rates. However, research indicates that CNT electrodes maintain performance characteristics longer than conventional alternatives, potentially offsetting manufacturing impacts through extended service lifetimes.
Comparative cradle-to-grave analyses between graphitized CNT anodes and cathodes suggest that anode materials generally present lower environmental impacts due to simpler composition and processing requirements. However, the superior energy density achieved with advanced CNT cathodes may justify their higher production footprint through improved system-level efficiency over multiple charge cycles.
Future sustainability improvements will likely emerge from manufacturing optimization, with recent pilot-scale studies demonstrating potential energy requirement reductions of 25-35% through process refinements and renewable energy integration in production facilities.
Manufacturing Scalability and Cost Analysis
The manufacturing scalability of graphitized carbon nanotubes (GCNTs) for battery electrode applications presents significant challenges that directly impact commercial viability. Current production methods predominantly rely on chemical vapor deposition (CVD) and arc discharge techniques, which face limitations in terms of throughput and consistency when scaled to industrial levels. The CVD method, while offering better control over nanotube properties, typically yields only 5-20 grams per day in laboratory settings, with industrial scale operations reaching approximately 100-500 kg annually per production line.
Cost analysis reveals that raw GCNT production currently ranges from $50-200 per kilogram for industrial grade materials, with higher purity grades commanding prices of $200-1000 per kilogram. These costs significantly exceed those of traditional graphite anodes ($15-20/kg) and conventional cathode materials ($20-40/kg), creating a substantial barrier to widespread adoption despite performance advantages.
Energy consumption during graphitization represents a major cost factor, requiring temperatures of 2500-3000°C maintained for several hours. This thermal processing accounts for approximately 40-50% of total production costs, with additional expenses arising from purification processes needed to remove catalytic impurities and amorphous carbon. The environmental footprint of these energy-intensive processes further complicates the economic equation when considering lifecycle costs.
Equipment investment presents another scaling challenge, with specialized high-temperature furnaces and precision control systems requiring capital expenditures of $2-5 million for modest production facilities. The technical expertise required for operation and quality control adds to operational expenses, with skilled personnel commanding premium salaries in this specialized field.
Recent innovations in continuous flow production methods show promise for improving throughput by 300-400% compared to batch processing, potentially reducing costs by 30-40% at scale. Similarly, advances in catalyst efficiency and recovery systems are gradually improving material utilization rates from typical values of 40-60% to projected efficiencies of 75-85% within the next 3-5 years.
Market analysis indicates that price parity with premium conventional electrode materials could be achieved at production volumes exceeding 1000 metric tons annually, a threshold that would require significant industry investment but appears achievable within the next decade based on current growth trajectories. The development of hybrid materials incorporating lower concentrations of GCNTs with conventional materials represents a promising intermediate approach to balance performance benefits with economic constraints.
Cost analysis reveals that raw GCNT production currently ranges from $50-200 per kilogram for industrial grade materials, with higher purity grades commanding prices of $200-1000 per kilogram. These costs significantly exceed those of traditional graphite anodes ($15-20/kg) and conventional cathode materials ($20-40/kg), creating a substantial barrier to widespread adoption despite performance advantages.
Energy consumption during graphitization represents a major cost factor, requiring temperatures of 2500-3000°C maintained for several hours. This thermal processing accounts for approximately 40-50% of total production costs, with additional expenses arising from purification processes needed to remove catalytic impurities and amorphous carbon. The environmental footprint of these energy-intensive processes further complicates the economic equation when considering lifecycle costs.
Equipment investment presents another scaling challenge, with specialized high-temperature furnaces and precision control systems requiring capital expenditures of $2-5 million for modest production facilities. The technical expertise required for operation and quality control adds to operational expenses, with skilled personnel commanding premium salaries in this specialized field.
Recent innovations in continuous flow production methods show promise for improving throughput by 300-400% compared to batch processing, potentially reducing costs by 30-40% at scale. Similarly, advances in catalyst efficiency and recovery systems are gradually improving material utilization rates from typical values of 40-60% to projected efficiencies of 75-85% within the next 3-5 years.
Market analysis indicates that price parity with premium conventional electrode materials could be achieved at production volumes exceeding 1000 metric tons annually, a threshold that would require significant industry investment but appears achievable within the next decade based on current growth trajectories. The development of hybrid materials incorporating lower concentrations of GCNTs with conventional materials represents a promising intermediate approach to balance performance benefits with economic constraints.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!