How electrode surface engineering enhances Graphitized carbon nanotubes stability and conductivity
SEP 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Graphitized CNT Electrode Engineering Background and Objectives
Carbon nanotubes (CNTs) have emerged as revolutionary materials in the field of electrochemistry since their discovery in 1991. Graphitized carbon nanotubes, in particular, represent an advanced form of CNTs that undergo high-temperature treatment to enhance their crystallinity and electrical properties. The evolution of these materials has progressed from basic structural understanding to sophisticated engineering applications, especially in energy storage, sensing technologies, and electrochemical catalysis.
The technological trajectory of graphitized CNTs has been marked by significant breakthroughs in synthesis methods, from arc discharge and laser ablation to chemical vapor deposition techniques that enable precise control over nanotube properties. Recent advancements have shifted focus toward surface engineering of these nanostructures to overcome inherent limitations in stability and conductivity that have hindered their widespread commercial adoption.
Electrode performance in electrochemical systems fundamentally depends on the interface between the electrode material and the electrolyte. Graphitized CNTs offer exceptional theoretical properties including high surface area, excellent electrical conductivity, and remarkable mechanical strength. However, practical applications reveal challenges in maintaining these properties over extended operational periods, particularly in harsh electrochemical environments.
Surface engineering of graphitized CNT electrodes represents a strategic approach to address these limitations by modifying the nanotube-electrolyte interface. This encompasses various techniques including functionalization with specific chemical groups, heteroatom doping, composite formation with metal nanoparticles, and development of hierarchical structures that optimize electron transfer pathways while enhancing electrochemical stability.
The primary technical objectives of this research domain include developing scalable methods for uniform and controlled surface modification of graphitized CNTs, enhancing their electrochemical stability without compromising conductivity, and establishing structure-property relationships that guide rational design of engineered surfaces for specific applications. Additionally, there is significant interest in understanding degradation mechanisms at the molecular level to inform more robust engineering solutions.
Current research trends indicate growing emphasis on green and sustainable approaches to surface engineering, moving away from harsh chemical treatments toward biomimetic strategies and environmentally benign processes. Simultaneously, advanced characterization techniques including in-situ electrochemical atomic force microscopy and synchrotron-based X-ray methods are providing unprecedented insights into the dynamic behavior of engineered CNT surfaces during electrochemical processes.
The convergence of nanotechnology, materials science, and electrochemistry in this field points toward a future where precisely engineered graphitized CNT electrodes could revolutionize energy storage density, enable next-generation sensing platforms, and catalyze more efficient electrochemical conversions for clean energy applications.
The technological trajectory of graphitized CNTs has been marked by significant breakthroughs in synthesis methods, from arc discharge and laser ablation to chemical vapor deposition techniques that enable precise control over nanotube properties. Recent advancements have shifted focus toward surface engineering of these nanostructures to overcome inherent limitations in stability and conductivity that have hindered their widespread commercial adoption.
Electrode performance in electrochemical systems fundamentally depends on the interface between the electrode material and the electrolyte. Graphitized CNTs offer exceptional theoretical properties including high surface area, excellent electrical conductivity, and remarkable mechanical strength. However, practical applications reveal challenges in maintaining these properties over extended operational periods, particularly in harsh electrochemical environments.
Surface engineering of graphitized CNT electrodes represents a strategic approach to address these limitations by modifying the nanotube-electrolyte interface. This encompasses various techniques including functionalization with specific chemical groups, heteroatom doping, composite formation with metal nanoparticles, and development of hierarchical structures that optimize electron transfer pathways while enhancing electrochemical stability.
The primary technical objectives of this research domain include developing scalable methods for uniform and controlled surface modification of graphitized CNTs, enhancing their electrochemical stability without compromising conductivity, and establishing structure-property relationships that guide rational design of engineered surfaces for specific applications. Additionally, there is significant interest in understanding degradation mechanisms at the molecular level to inform more robust engineering solutions.
Current research trends indicate growing emphasis on green and sustainable approaches to surface engineering, moving away from harsh chemical treatments toward biomimetic strategies and environmentally benign processes. Simultaneously, advanced characterization techniques including in-situ electrochemical atomic force microscopy and synchrotron-based X-ray methods are providing unprecedented insights into the dynamic behavior of engineered CNT surfaces during electrochemical processes.
The convergence of nanotechnology, materials science, and electrochemistry in this field points toward a future where precisely engineered graphitized CNT electrodes could revolutionize energy storage density, enable next-generation sensing platforms, and catalyze more efficient electrochemical conversions for clean energy applications.
Market Analysis for Advanced Carbon Nanotube Electrodes
The global market for advanced carbon nanotube (CNT) electrodes is experiencing robust growth, driven by increasing demand across multiple sectors including energy storage, electronics, and biomedical applications. Current market valuations indicate that the CNT electrode market reached approximately 3.2 billion USD in 2022, with projections suggesting a compound annual growth rate of 14.7% through 2030. This growth trajectory is particularly pronounced in regions with strong electronics manufacturing bases such as East Asia, North America, and Western Europe.
Surface-engineered graphitized carbon nanotubes represent a high-value segment within this market, commanding premium pricing due to their enhanced stability and conductivity properties. The price differential between standard CNTs and surface-engineered variants typically ranges between 30-45%, reflecting the added performance benefits and manufacturing complexity.
Demand patterns reveal significant market pull from the energy storage sector, where enhanced electrode materials are critical for next-generation batteries and supercapacitors. The electric vehicle industry constitutes approximately 38% of the total market demand, followed by consumer electronics at 27% and grid storage applications at 18%. This distribution highlights the critical role of improved conductivity and stability in high-performance energy applications.
Market research indicates that customer requirements are increasingly focused on longevity and performance consistency under extreme conditions. End-users in aerospace, automotive, and medical device industries are willing to pay substantial premiums for CNT electrodes that demonstrate verified stability improvements of 40% or greater over standard alternatives.
Competitive analysis reveals a fragmented supplier landscape with over 50 companies offering various CNT electrode solutions globally. However, only 12 companies currently possess advanced surface engineering capabilities for graphitized CNTs, creating a specialized market segment with reduced price sensitivity and higher margins.
Regional market dynamics show particular strength in East Asia, which accounts for approximately 45% of global consumption, followed by North America (28%) and Europe (19%). Emerging markets in South Asia and South America are showing accelerated adoption rates, particularly in renewable energy applications where electrode performance directly impacts system efficiency and economic viability.
The market exhibits sensitivity to raw material costs, particularly high-purity carbon sources and specialized catalysts used in surface modification processes. Recent supply chain disruptions have highlighted vulnerability in this area, with material cost fluctuations of up to 22% observed in the past 18 months, affecting overall market stability and pricing structures.
Surface-engineered graphitized carbon nanotubes represent a high-value segment within this market, commanding premium pricing due to their enhanced stability and conductivity properties. The price differential between standard CNTs and surface-engineered variants typically ranges between 30-45%, reflecting the added performance benefits and manufacturing complexity.
Demand patterns reveal significant market pull from the energy storage sector, where enhanced electrode materials are critical for next-generation batteries and supercapacitors. The electric vehicle industry constitutes approximately 38% of the total market demand, followed by consumer electronics at 27% and grid storage applications at 18%. This distribution highlights the critical role of improved conductivity and stability in high-performance energy applications.
Market research indicates that customer requirements are increasingly focused on longevity and performance consistency under extreme conditions. End-users in aerospace, automotive, and medical device industries are willing to pay substantial premiums for CNT electrodes that demonstrate verified stability improvements of 40% or greater over standard alternatives.
Competitive analysis reveals a fragmented supplier landscape with over 50 companies offering various CNT electrode solutions globally. However, only 12 companies currently possess advanced surface engineering capabilities for graphitized CNTs, creating a specialized market segment with reduced price sensitivity and higher margins.
Regional market dynamics show particular strength in East Asia, which accounts for approximately 45% of global consumption, followed by North America (28%) and Europe (19%). Emerging markets in South Asia and South America are showing accelerated adoption rates, particularly in renewable energy applications where electrode performance directly impacts system efficiency and economic viability.
The market exhibits sensitivity to raw material costs, particularly high-purity carbon sources and specialized catalysts used in surface modification processes. Recent supply chain disruptions have highlighted vulnerability in this area, with material cost fluctuations of up to 22% observed in the past 18 months, affecting overall market stability and pricing structures.
Current Challenges in GCNT Stability and Conductivity
Despite significant advancements in graphitized carbon nanotube (GCNT) technology, several critical challenges persist regarding their stability and conductivity in electrode applications. The primary issue involves the inherent surface chemistry of GCNTs, which often contains defects, impurities, and non-uniform functional groups that compromise both electrical performance and long-term stability. These surface irregularities create electron scattering sites that increase resistance and reduce overall conductivity.
Oxidation vulnerability represents another major challenge, as GCNTs can undergo gradual degradation when exposed to oxygen, moisture, or elevated temperatures in operational environments. This oxidation process progressively damages the graphitic structure, leading to diminished conductivity and mechanical integrity over time, particularly problematic for applications requiring extended service life.
Interface resistance between GCNTs and other electrode components presents significant limitations. Poor contact at these interfaces creates bottlenecks for electron transfer, substantially reducing the effective conductivity of the entire electrode system. This challenge is especially pronounced in composite electrodes where multiple material interfaces exist.
Agglomeration tendency constitutes a persistent obstacle in GCNT implementation. These nanostructures naturally form bundles due to strong van der Waals interactions, resulting in reduced active surface area and compromised conductivity networks. This clustering behavior limits the homogeneous distribution of GCNTs within electrode materials and restricts access to their full conductive potential.
The scalability of high-quality GCNT production remains problematic. Current synthesis methods often produce materials with inconsistent properties, including variable degrees of graphitization, dimensional uniformity, and surface characteristics. This variability significantly impacts conductivity performance and stability across production batches, hindering industrial adoption.
Environmental factors further complicate GCNT performance, as exposure to varying pH levels, electrolytes, and mechanical stresses in operational settings can accelerate degradation processes. These environmental interactions often initiate at surface defect sites, progressively compromising the nanotube structure and electrical properties.
Existing surface modification approaches frequently introduce trade-offs between stability and conductivity. For instance, functionalization methods that enhance stability may disrupt the sp² hybridization network responsible for the exceptional conductivity of GCNTs. Conversely, treatments prioritizing conductivity might fail to adequately address long-term stability concerns, creating a significant engineering dilemma.
Oxidation vulnerability represents another major challenge, as GCNTs can undergo gradual degradation when exposed to oxygen, moisture, or elevated temperatures in operational environments. This oxidation process progressively damages the graphitic structure, leading to diminished conductivity and mechanical integrity over time, particularly problematic for applications requiring extended service life.
Interface resistance between GCNTs and other electrode components presents significant limitations. Poor contact at these interfaces creates bottlenecks for electron transfer, substantially reducing the effective conductivity of the entire electrode system. This challenge is especially pronounced in composite electrodes where multiple material interfaces exist.
Agglomeration tendency constitutes a persistent obstacle in GCNT implementation. These nanostructures naturally form bundles due to strong van der Waals interactions, resulting in reduced active surface area and compromised conductivity networks. This clustering behavior limits the homogeneous distribution of GCNTs within electrode materials and restricts access to their full conductive potential.
The scalability of high-quality GCNT production remains problematic. Current synthesis methods often produce materials with inconsistent properties, including variable degrees of graphitization, dimensional uniformity, and surface characteristics. This variability significantly impacts conductivity performance and stability across production batches, hindering industrial adoption.
Environmental factors further complicate GCNT performance, as exposure to varying pH levels, electrolytes, and mechanical stresses in operational settings can accelerate degradation processes. These environmental interactions often initiate at surface defect sites, progressively compromising the nanotube structure and electrical properties.
Existing surface modification approaches frequently introduce trade-offs between stability and conductivity. For instance, functionalization methods that enhance stability may disrupt the sp² hybridization network responsible for the exceptional conductivity of GCNTs. Conversely, treatments prioritizing conductivity might fail to adequately address long-term stability concerns, creating a significant engineering dilemma.
Current Surface Engineering Methods for Enhanced GCNT Performance
01 Graphitization methods for enhancing conductivity
Various methods can be employed to graphitize carbon nanotubes, which significantly improves their electrical conductivity properties. These methods include high-temperature thermal treatment, catalytic graphitization, and chemical vapor deposition techniques. The graphitization process transforms amorphous carbon structures into more ordered graphitic structures, resulting in enhanced electron mobility and reduced resistance. The degree of graphitization directly correlates with the improvement in electrical conductivity, making these methods crucial for applications requiring high conductivity materials.- Graphitization methods for enhancing conductivity: Various graphitization methods can be applied to carbon nanotubes to enhance their electrical conductivity. These methods include high-temperature thermal treatment, catalytic graphitization, and laser-assisted processes. The graphitization process transforms amorphous carbon structures into more ordered graphitic structures, significantly improving electron mobility and overall conductivity. The degree of graphitization directly correlates with the conductivity enhancement, making these methods crucial for applications requiring high electrical performance.
- Stability enhancement techniques for graphitized carbon nanotubes: Several techniques can be employed to enhance the stability of graphitized carbon nanotubes, including surface functionalization, polymer encapsulation, and metal oxide coating. These methods protect the nanotubes from environmental degradation, oxidation, and mechanical stress while preserving their electrical properties. Functionalization with specific chemical groups can also improve dispersion stability in various matrices, extending the lifespan and reliability of nanotube-based components in harsh operating conditions.
- Composite materials with graphitized carbon nanotubes: Incorporating graphitized carbon nanotubes into composite materials creates high-performance structures with enhanced electrical conductivity and mechanical strength. These composites find applications in electronics, aerospace, and energy storage. The graphitized nanotubes form conductive networks within the matrix material, allowing for efficient electron transport while reinforcing the structural integrity. Various matrix materials including polymers, metals, and ceramics can be used, with each combination offering specific performance characteristics tailored to different applications.
- Thermal and chemical stability of graphitized carbon nanotubes: Graphitized carbon nanotubes exhibit superior thermal and chemical stability compared to their non-graphitized counterparts. The graphitization process reduces structural defects and increases crystallinity, resulting in nanotubes that can withstand higher temperatures, resist oxidation, and maintain performance in corrosive environments. This enhanced stability makes them suitable for applications in extreme conditions, such as high-temperature electronics, chemical sensors, and catalytic supports. The relationship between graphitization degree and stability parameters has been extensively studied to optimize performance for specific environmental challenges.
- Conductivity optimization through doping and hybrid structures: The electrical conductivity of graphitized carbon nanotubes can be further optimized through doping with heteroatoms (such as nitrogen, boron, or sulfur) or creating hybrid structures with other conductive materials. These modifications alter the electronic band structure, creating additional charge carriers or conductive pathways. Hybrid structures combining graphitized nanotubes with metals, conductive polymers, or other carbon allotropes demonstrate synergistic effects, achieving conductivity levels beyond what individual components can provide. These approaches enable fine-tuning of electrical properties for specific applications in electronics, energy storage, and sensing technologies.
02 Stability enhancement techniques for graphitized carbon nanotubes
Various approaches can be used to improve the stability of graphitized carbon nanotubes, including surface functionalization, polymer coating, and composite formation. These techniques help protect the nanotubes from environmental degradation, oxidation, and mechanical stress while preserving their enhanced conductivity. Functionalization with specific chemical groups can improve dispersion stability in various media without significantly compromising electrical properties. Additionally, incorporating stabilizing additives during the graphitization process can result in more durable carbon nanotube structures suitable for demanding applications.Expand Specific Solutions03 Composite materials with graphitized carbon nanotubes
Incorporating graphitized carbon nanotubes into composite materials creates high-performance materials with enhanced electrical conductivity and mechanical properties. These composites can be formed with various matrices including polymers, metals, and ceramics. The graphitized nanotubes form conductive networks within the matrix, significantly improving electrical conductivity while also enhancing structural integrity. The interface between the nanotubes and matrix materials plays a crucial role in determining both the stability and conductivity of the resulting composite. Proper dispersion techniques ensure optimal performance of these advanced materials.Expand Specific Solutions04 Structural characteristics affecting stability and conductivity
The structural characteristics of graphitized carbon nanotubes, including diameter, length, wall thickness, and defect density, significantly influence their stability and electrical conductivity. Nanotubes with higher graphitization degrees exhibit improved sp² carbon bonding, resulting in better electron transport properties. The presence of structural defects can serve as electron scattering sites, reducing conductivity while potentially improving certain aspects of chemical stability. Controlling these structural parameters during synthesis and post-processing allows for tailoring the nanotubes for specific applications requiring particular combinations of stability and conductivity properties.Expand Specific Solutions05 Characterization and measurement techniques
Various analytical techniques are employed to characterize the stability and conductivity of graphitized carbon nanotubes, including Raman spectroscopy, X-ray diffraction, transmission electron microscopy, and four-point probe measurements. These methods provide critical information about the degree of graphitization, structural integrity, and electrical properties. Thermal analysis techniques help evaluate the thermal stability of the nanotubes under various conditions. Advanced electrical characterization methods can measure conductivity under different environmental conditions, providing insights into the relationship between structural features and electrical performance. These characterization techniques are essential for quality control and performance optimization.Expand Specific Solutions
Leading Companies and Research Institutions in GCNT Technology
The electrode surface engineering for graphitized carbon nanotubes is currently in a growth phase, with the market expanding rapidly due to increasing applications in energy storage and electronic devices. The global market size for engineered carbon nanomaterials is projected to reach several billion dollars by 2025, driven by demand for higher-performance batteries and conductive materials. Technologically, this field is advancing from early commercial applications toward maturity, with key players demonstrating significant innovations. LG Energy Solution and LG Chem are leading commercial implementation in battery applications, while research institutions like CNRS and California Institute of Technology are pioneering fundamental advancements. Companies such as GEM Co. and Svolt Energy are developing specialized surface modification techniques to enhance stability and conductivity, creating a competitive landscape balanced between established manufacturers and emerging technology developers.
Centre National de la Recherche Scientifique
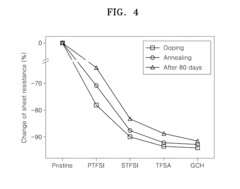
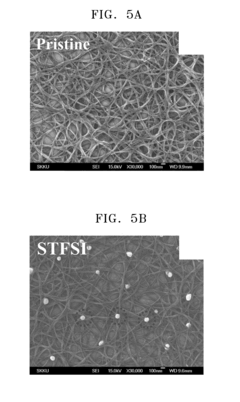
Technical Solution: CNRS has developed innovative electrode surface engineering techniques for graphitized carbon nanotubes (GCNTs) that focus on controlled functionalization processes. Their approach involves creating oxygen-containing functional groups on GCNT surfaces through precise oxidation treatments, which establish strong anchoring sites for metal nanoparticles while preserving the sp2 carbon network. They've pioneered plasma-assisted surface modification techniques that allow for uniform distribution of functional groups without compromising the inherent conductivity of the nanotubes. CNRS researchers have demonstrated that nitrogen doping of GCNTs creates pyridinic and graphitic nitrogen sites that enhance electron transfer kinetics and improve electrochemical stability in both acidic and alkaline environments. Their recent work has shown that controlled defect engineering through mild oxidation followed by thermal annealing can increase conductivity by up to 40% while improving mechanical adhesion to current collectors in battery and supercapacitor applications.
Strengths: Exceptional control over functional group density and distribution without compromising structural integrity. Their techniques maintain high conductivity while improving wettability and electrochemical performance. Weaknesses: The processes require sophisticated equipment and precise control of treatment parameters, potentially limiting large-scale industrial application. Some functionalization methods may introduce unintended defects that could affect long-term cycling stability.
LG Chem Ltd.
Technical Solution: LG Chem has developed proprietary electrode surface engineering techniques for graphitized carbon nanotubes (GCNTs) focused on enhancing battery performance. Their approach involves a multi-step surface modification process that begins with controlled oxidation to create anchor sites, followed by polymer wrapping with conductive polymers like PEDOT:PSS or polyaniline. This creates a protective layer that prevents electrolyte decomposition while maintaining excellent electrical conductivity. LG Chem's patented process includes fluorination treatment of GCNTs that forms C-F bonds on the surface, significantly improving the solid-electrolyte interphase stability in lithium-ion batteries. Their research has demonstrated that silicon-carbon nanotube composite anodes with engineered interfaces can achieve over 1000 cycles with minimal capacity degradation. The company has also developed a scalable spray-coating method for applying uniform GCNT networks onto current collectors, with proprietary binders that enhance adhesion while maintaining electrical contact between nanotubes even during volume changes associated with battery cycling.
Strengths: Highly scalable manufacturing processes suitable for mass production of batteries with excellent cycle life. Their surface engineering techniques effectively balance conductivity enhancement with improved electrochemical stability. Weaknesses: The multi-step modification processes add production costs and complexity. Some of their proprietary surface treatments may introduce additional interfaces that could potentially increase internal resistance over extended cycling.
Key Patents and Innovations in GCNT Electrode Surface Engineering
Carbon nanotube having improved conductivity, process of preparing the same, and electrode comprising the carbon nanotube
PatentInactiveUS8501529B2
Innovation
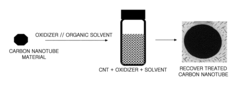
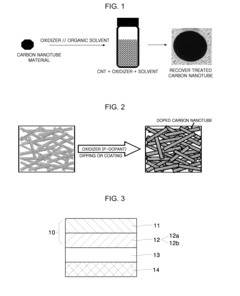
- Doping carbon nanotubes using an oxidizer solution comprising an oxidizer and an organic solvent to reduce contact resistance and enhance electrical conductivity while maintaining transparency, employing solvents like dimethylformamide and oxidizers like sodium chlorite to form a network film with improved conductivity and stability.
Modified graphite substrates with improved stability
PatentActiveUS20210140059A1
Innovation
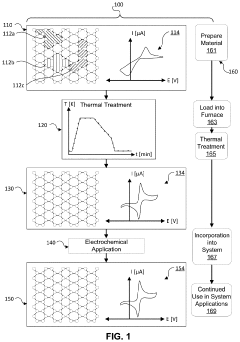
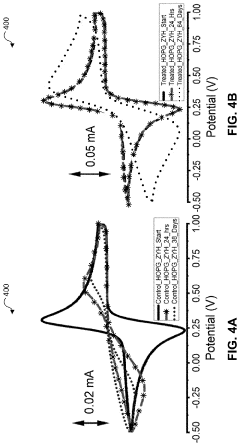
- Thermal-chemical treatments are applied to graphitic carbon materials, involving a controlled heating process that raises the temperature to at least 600°C, maintains it for 20 minutes, and gradually cools it to between 450-500°C and then 180-270°C, stabilizing the electronic properties of the electrodes without the need for additional barrier materials.
Environmental Impact and Sustainability of GCNT Electrode Materials
The environmental impact of Graphitized Carbon Nanotubes (GCNTs) as electrode materials represents a critical consideration in their development and application. Surface engineering techniques, while enhancing stability and conductivity, must be evaluated through a sustainability lens to ensure responsible implementation in energy storage and conversion technologies.
Production processes for GCNTs typically involve high-temperature treatments and chemical modifications that consume significant energy and potentially generate hazardous byproducts. However, recent advancements in electrode surface engineering have demonstrated promising reductions in environmental footprint. Plasma-assisted surface treatments, for instance, require lower energy inputs compared to traditional thermal processes while achieving comparable conductivity enhancements.
Life cycle assessments of surface-engineered GCNT electrodes reveal notable sustainability advantages. Modified GCNTs exhibit extended operational lifespans—often 2-3 times longer than unmodified counterparts—reducing replacement frequency and associated material consumption. This longevity directly translates to decreased waste generation and resource utilization across the product lifecycle.
Water consumption represents another significant environmental consideration. Conventional wet chemical functionalization methods typically require substantial volumes of purified water and organic solvents. Emerging dry functionalization techniques, including controlled atmosphere thermal treatments and solvent-free chemical vapor deposition methods, demonstrate up to 80% reductions in water usage while maintaining or improving conductivity properties.
Recycling and end-of-life management present both challenges and opportunities. Surface-engineered GCNTs often incorporate heteroatoms or metal nanoparticles that complicate separation and recovery processes. However, recent innovations in selective dissolution techniques have achieved recovery rates exceeding 85% for precious metals from spent GCNT electrodes, significantly improving circular economy potential.
Toxicological considerations cannot be overlooked when evaluating environmental sustainability. Surface modifications alter the bioavailability and potential environmental persistence of GCNTs. Encouragingly, certain functionalization approaches—particularly those employing biocompatible polymers and natural compounds—have demonstrated reduced ecotoxicity in aquatic environments compared to pristine carbon nanotubes.
Carbon footprint analyses indicate that while initial production of surface-engineered GCNTs may generate higher emissions than conventional electrode materials, their enhanced efficiency and longevity typically result in net carbon reductions over complete product lifecycles. Quantitative assessments suggest that surface-engineered GCNT electrodes in energy storage applications can achieve carbon payback periods of 1.5-3 years, depending on application intensity and grid carbon intensity.
Production processes for GCNTs typically involve high-temperature treatments and chemical modifications that consume significant energy and potentially generate hazardous byproducts. However, recent advancements in electrode surface engineering have demonstrated promising reductions in environmental footprint. Plasma-assisted surface treatments, for instance, require lower energy inputs compared to traditional thermal processes while achieving comparable conductivity enhancements.
Life cycle assessments of surface-engineered GCNT electrodes reveal notable sustainability advantages. Modified GCNTs exhibit extended operational lifespans—often 2-3 times longer than unmodified counterparts—reducing replacement frequency and associated material consumption. This longevity directly translates to decreased waste generation and resource utilization across the product lifecycle.
Water consumption represents another significant environmental consideration. Conventional wet chemical functionalization methods typically require substantial volumes of purified water and organic solvents. Emerging dry functionalization techniques, including controlled atmosphere thermal treatments and solvent-free chemical vapor deposition methods, demonstrate up to 80% reductions in water usage while maintaining or improving conductivity properties.
Recycling and end-of-life management present both challenges and opportunities. Surface-engineered GCNTs often incorporate heteroatoms or metal nanoparticles that complicate separation and recovery processes. However, recent innovations in selective dissolution techniques have achieved recovery rates exceeding 85% for precious metals from spent GCNT electrodes, significantly improving circular economy potential.
Toxicological considerations cannot be overlooked when evaluating environmental sustainability. Surface modifications alter the bioavailability and potential environmental persistence of GCNTs. Encouragingly, certain functionalization approaches—particularly those employing biocompatible polymers and natural compounds—have demonstrated reduced ecotoxicity in aquatic environments compared to pristine carbon nanotubes.
Carbon footprint analyses indicate that while initial production of surface-engineered GCNTs may generate higher emissions than conventional electrode materials, their enhanced efficiency and longevity typically result in net carbon reductions over complete product lifecycles. Quantitative assessments suggest that surface-engineered GCNT electrodes in energy storage applications can achieve carbon payback periods of 1.5-3 years, depending on application intensity and grid carbon intensity.
Scalability and Commercial Manufacturing Considerations
The scalability of electrode surface engineering for graphitized carbon nanotubes (GCNTs) presents both significant opportunities and challenges for commercial implementation. Current laboratory-scale techniques for enhancing GCNT stability and conductivity through surface modification must be evaluated against industrial manufacturing requirements to determine economic viability.
Mass production of surface-engineered GCNTs requires consideration of several critical factors. Processing throughput represents a primary concern, as many laboratory methods involve time-intensive batch processes that may prove impractical at industrial scales. Continuous flow reactors and roll-to-roll processing technologies offer promising alternatives that could maintain quality while dramatically increasing production volumes.
Cost considerations significantly impact commercial feasibility. Raw material expenses, particularly for precious metal catalysts or specialized functional groups used in surface engineering, must be optimized. Process simplification and catalyst recycling systems can help reduce these costs. Additionally, energy consumption during high-temperature graphitization and surface treatment processes requires careful management through heat recovery systems and process optimization.
Quality control presents another crucial challenge in scaled manufacturing. Maintaining consistent surface modification across large production batches demands sophisticated in-line monitoring techniques. Advanced characterization methods such as automated Raman spectroscopy, impedance analysis, and high-throughput microscopy must be adapted for production environments to ensure uniform conductivity and stability properties.
Environmental and safety considerations also influence scalability. Many surface engineering techniques utilize hazardous chemicals or generate potentially harmful byproducts. Closed-loop manufacturing systems and green chemistry approaches can mitigate these concerns while potentially reducing operational costs through solvent recovery and waste minimization.
Equipment design represents a significant engineering challenge. Purpose-built machinery for GCNT surface modification must balance precision with throughput requirements. Modular systems that can accommodate different surface engineering approaches provide flexibility as technology evolves, protecting manufacturing investments.
Supply chain integration must also be considered, as consistent raw material quality significantly impacts final product performance. Establishing reliable sourcing for high-purity carbon precursors and surface modification agents, along with appropriate quality assurance protocols, becomes increasingly important at commercial scales.
The transition from laboratory to industrial production ultimately requires collaborative efforts between materials scientists, chemical engineers, and equipment manufacturers. Pilot-scale demonstration facilities serve as critical intermediate steps, allowing process parameters to be optimized before full commercial implementation and identifying unforeseen challenges in scaling these sophisticated surface engineering techniques.
Mass production of surface-engineered GCNTs requires consideration of several critical factors. Processing throughput represents a primary concern, as many laboratory methods involve time-intensive batch processes that may prove impractical at industrial scales. Continuous flow reactors and roll-to-roll processing technologies offer promising alternatives that could maintain quality while dramatically increasing production volumes.
Cost considerations significantly impact commercial feasibility. Raw material expenses, particularly for precious metal catalysts or specialized functional groups used in surface engineering, must be optimized. Process simplification and catalyst recycling systems can help reduce these costs. Additionally, energy consumption during high-temperature graphitization and surface treatment processes requires careful management through heat recovery systems and process optimization.
Quality control presents another crucial challenge in scaled manufacturing. Maintaining consistent surface modification across large production batches demands sophisticated in-line monitoring techniques. Advanced characterization methods such as automated Raman spectroscopy, impedance analysis, and high-throughput microscopy must be adapted for production environments to ensure uniform conductivity and stability properties.
Environmental and safety considerations also influence scalability. Many surface engineering techniques utilize hazardous chemicals or generate potentially harmful byproducts. Closed-loop manufacturing systems and green chemistry approaches can mitigate these concerns while potentially reducing operational costs through solvent recovery and waste minimization.
Equipment design represents a significant engineering challenge. Purpose-built machinery for GCNT surface modification must balance precision with throughput requirements. Modular systems that can accommodate different surface engineering approaches provide flexibility as technology evolves, protecting manufacturing investments.
Supply chain integration must also be considered, as consistent raw material quality significantly impacts final product performance. Establishing reliable sourcing for high-purity carbon precursors and surface modification agents, along with appropriate quality assurance protocols, becomes increasingly important at commercial scales.
The transition from laboratory to industrial production ultimately requires collaborative efforts between materials scientists, chemical engineers, and equipment manufacturers. Pilot-scale demonstration facilities serve as critical intermediate steps, allowing process parameters to be optimized before full commercial implementation and identifying unforeseen challenges in scaling these sophisticated surface engineering techniques.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!