Comparative study of Graphitized carbon nanotubes doped versus undoped carbon electrodes
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Nanotube Electrode Technology Background and Objectives
Carbon nanotubes (CNTs) have emerged as revolutionary materials in electrode technology since their discovery in 1991 by Sumio Iijima. These cylindrical nanostructures composed of carbon atoms arranged in a hexagonal lattice have demonstrated exceptional electrical conductivity, mechanical strength, and electrochemical properties, making them ideal candidates for advanced electrode applications. The evolution of CNT electrode technology has progressed from basic research to practical applications across various fields including energy storage, sensing, and biomedical devices.
The graphitization process of carbon nanotubes represents a significant advancement in enhancing their electrical and electrochemical properties. This thermal treatment at temperatures exceeding 2500°C transforms amorphous carbon into highly ordered graphitic structures, resulting in improved electron transfer kinetics and reduced electrical resistance. The historical trajectory shows a clear trend toward optimizing graphitization parameters to achieve superior electrode performance while maintaining cost-effectiveness.
Doping of carbon nanotubes with heteroatoms such as nitrogen, boron, or phosphorus has emerged as a strategic approach to further enhance their electrochemical properties. This technique, which gained prominence in the early 2000s, allows for precise tuning of electronic properties by introducing electron-rich or electron-deficient sites within the carbon lattice. The comparative analysis between doped and undoped graphitized carbon nanotube electrodes represents a frontier in understanding how atomic-level modifications influence macroscopic electrode performance.
The primary technical objective of this research is to systematically evaluate and quantify the performance differences between graphitized carbon nanotube electrodes with and without dopants. This includes comprehensive assessment of electrical conductivity, electrochemical activity, stability, and specific application performance metrics. By establishing clear correlations between doping strategies and functional outcomes, this research aims to develop design principles for next-generation carbon electrodes.
Additionally, this investigation seeks to address existing knowledge gaps regarding the fundamental mechanisms by which dopants alter the electronic structure and surface chemistry of graphitized carbon nanotubes. Understanding these mechanisms is crucial for rational design of electrodes tailored to specific applications, from high-performance supercapacitors to sensitive biosensors and efficient fuel cells.
The technological trajectory indicates a convergence toward hybrid systems that combine the advantages of both doped and undoped carbon nanotubes in structured architectures. This approach potentially offers synergistic benefits that overcome the limitations of either material alone, representing an emerging paradigm in electrode design that warrants thorough investigation.
The graphitization process of carbon nanotubes represents a significant advancement in enhancing their electrical and electrochemical properties. This thermal treatment at temperatures exceeding 2500°C transforms amorphous carbon into highly ordered graphitic structures, resulting in improved electron transfer kinetics and reduced electrical resistance. The historical trajectory shows a clear trend toward optimizing graphitization parameters to achieve superior electrode performance while maintaining cost-effectiveness.
Doping of carbon nanotubes with heteroatoms such as nitrogen, boron, or phosphorus has emerged as a strategic approach to further enhance their electrochemical properties. This technique, which gained prominence in the early 2000s, allows for precise tuning of electronic properties by introducing electron-rich or electron-deficient sites within the carbon lattice. The comparative analysis between doped and undoped graphitized carbon nanotube electrodes represents a frontier in understanding how atomic-level modifications influence macroscopic electrode performance.
The primary technical objective of this research is to systematically evaluate and quantify the performance differences between graphitized carbon nanotube electrodes with and without dopants. This includes comprehensive assessment of electrical conductivity, electrochemical activity, stability, and specific application performance metrics. By establishing clear correlations between doping strategies and functional outcomes, this research aims to develop design principles for next-generation carbon electrodes.
Additionally, this investigation seeks to address existing knowledge gaps regarding the fundamental mechanisms by which dopants alter the electronic structure and surface chemistry of graphitized carbon nanotubes. Understanding these mechanisms is crucial for rational design of electrodes tailored to specific applications, from high-performance supercapacitors to sensitive biosensors and efficient fuel cells.
The technological trajectory indicates a convergence toward hybrid systems that combine the advantages of both doped and undoped carbon nanotubes in structured architectures. This approach potentially offers synergistic benefits that overcome the limitations of either material alone, representing an emerging paradigm in electrode design that warrants thorough investigation.
Market Applications and Demand Analysis for CNT Electrodes
The carbon nanotube (CNT) electrode market has witnessed significant growth in recent years, driven by increasing demand across multiple industries. The global market for CNT-based electrodes was valued at approximately $1.2 billion in 2022 and is projected to reach $3.5 billion by 2028, representing a compound annual growth rate of 19.6%. This robust growth trajectory underscores the expanding applications and market acceptance of CNT electrode technologies.
Energy storage represents the largest application segment for CNT electrodes, accounting for nearly 40% of the total market share. Within this segment, lithium-ion batteries dominate, where CNT electrodes offer enhanced conductivity, improved cycle life, and higher energy density compared to traditional graphite electrodes. The electric vehicle industry, in particular, has emerged as a key driver, with major manufacturers increasingly incorporating CNT-enhanced battery technologies to extend range and reduce charging times.
The healthcare and biomedical sectors constitute the fastest-growing market segment, with a projected growth rate of 24.3% through 2028. CNT electrodes are revolutionizing biosensing applications, enabling more sensitive and selective detection of biomarkers for various diseases. Doped CNT electrodes have demonstrated superior performance in neurological monitoring and stimulation devices, creating substantial demand in the medical device industry.
Environmental monitoring and remediation applications represent another significant market opportunity. CNT electrodes are increasingly deployed in electrochemical sensors for detecting pollutants in air and water, with doped variants showing enhanced sensitivity toward specific contaminants. This application segment is expected to grow at 21.7% annually, driven by stricter environmental regulations worldwide.
Industrial applications, including electrochemical processing and catalysis, account for approximately 15% of the current market. Here, graphitized CNT electrodes offer advantages in terms of durability and performance in harsh chemical environments, with doped variants showing particular promise for selective catalytic reactions.
Regional analysis indicates that Asia-Pacific dominates the market with a 45% share, led by China, Japan, and South Korea, where significant investments in electronics and energy storage manufacturing are driving adoption. North America and Europe follow with 30% and 20% market shares respectively, with particularly strong growth in biomedical and environmental applications.
Customer demand increasingly favors doped CNT electrodes over undoped variants, with market research indicating a price premium of 30-40% for doped electrodes, justified by their superior performance characteristics. This trend is expected to accelerate as manufacturing processes for doped CNTs become more standardized and cost-effective.
Energy storage represents the largest application segment for CNT electrodes, accounting for nearly 40% of the total market share. Within this segment, lithium-ion batteries dominate, where CNT electrodes offer enhanced conductivity, improved cycle life, and higher energy density compared to traditional graphite electrodes. The electric vehicle industry, in particular, has emerged as a key driver, with major manufacturers increasingly incorporating CNT-enhanced battery technologies to extend range and reduce charging times.
The healthcare and biomedical sectors constitute the fastest-growing market segment, with a projected growth rate of 24.3% through 2028. CNT electrodes are revolutionizing biosensing applications, enabling more sensitive and selective detection of biomarkers for various diseases. Doped CNT electrodes have demonstrated superior performance in neurological monitoring and stimulation devices, creating substantial demand in the medical device industry.
Environmental monitoring and remediation applications represent another significant market opportunity. CNT electrodes are increasingly deployed in electrochemical sensors for detecting pollutants in air and water, with doped variants showing enhanced sensitivity toward specific contaminants. This application segment is expected to grow at 21.7% annually, driven by stricter environmental regulations worldwide.
Industrial applications, including electrochemical processing and catalysis, account for approximately 15% of the current market. Here, graphitized CNT electrodes offer advantages in terms of durability and performance in harsh chemical environments, with doped variants showing particular promise for selective catalytic reactions.
Regional analysis indicates that Asia-Pacific dominates the market with a 45% share, led by China, Japan, and South Korea, where significant investments in electronics and energy storage manufacturing are driving adoption. North America and Europe follow with 30% and 20% market shares respectively, with particularly strong growth in biomedical and environmental applications.
Customer demand increasingly favors doped CNT electrodes over undoped variants, with market research indicating a price premium of 30-40% for doped electrodes, justified by their superior performance characteristics. This trend is expected to accelerate as manufacturing processes for doped CNTs become more standardized and cost-effective.
Current Status and Challenges in Graphitized CNT Research
The global research landscape for graphitized carbon nanotubes (CNTs) has witnessed significant advancements in recent years, with particular focus on their application in electrode materials. Currently, both doped and undoped graphitized CNTs are being extensively investigated across major research institutions in North America, Europe, and Asia, with China and the United States leading publication output in this domain.
Undoped graphitized CNTs have established a solid foundation in electrochemical applications due to their excellent electrical conductivity, large surface area, and mechanical stability. However, they face limitations in terms of electrochemical activity and selectivity for specific reactions. The graphitization process itself presents challenges, requiring precise temperature control (typically 1800-2800°C) to achieve optimal crystallinity without compromising the nanotube structure.
Doped graphitized CNTs represent the cutting edge of research, with nitrogen, boron, phosphorus, and sulfur being the most common dopants. These heteroatom-doped structures demonstrate enhanced electrocatalytic properties and improved electron transfer kinetics. Nevertheless, researchers struggle with achieving uniform dopant distribution and maintaining precise dopant concentration during the high-temperature graphitization process.
A significant technical challenge lies in the scalable production of consistent quality graphitized CNTs. Laboratory-scale synthesis often yields superior materials compared to industrial-scale production, creating a translational gap between research and commercial application. This disparity is particularly pronounced for doped variants, where maintaining dopant integrity during scale-up remains problematic.
Characterization techniques present another hurdle, as conventional methods sometimes fail to accurately quantify dopant distribution and bonding configurations within the carbon lattice. Advanced techniques like synchrotron-based X-ray absorption spectroscopy and in-situ TEM are being developed but remain accessible primarily to specialized research facilities.
The environmental stability of doped graphitized CNTs poses additional challenges, particularly in aggressive electrochemical environments. Dopant leaching and structural degradation during extended operation cycles diminish long-term performance, necessitating further research into stabilization strategies.
Cost considerations remain a significant barrier to widespread adoption, with high-purity graphitized CNTs commanding premium prices. The additional processing steps required for doping further increase production costs, limiting commercial viability despite superior performance metrics in laboratory settings.
Regulatory uncertainties surrounding nanomaterials, particularly those with modified surface chemistry like doped CNTs, create additional hurdles for industrial implementation. Comprehensive toxicological and environmental impact assessments are still evolving, potentially affecting future deployment in consumer-facing applications.
Undoped graphitized CNTs have established a solid foundation in electrochemical applications due to their excellent electrical conductivity, large surface area, and mechanical stability. However, they face limitations in terms of electrochemical activity and selectivity for specific reactions. The graphitization process itself presents challenges, requiring precise temperature control (typically 1800-2800°C) to achieve optimal crystallinity without compromising the nanotube structure.
Doped graphitized CNTs represent the cutting edge of research, with nitrogen, boron, phosphorus, and sulfur being the most common dopants. These heteroatom-doped structures demonstrate enhanced electrocatalytic properties and improved electron transfer kinetics. Nevertheless, researchers struggle with achieving uniform dopant distribution and maintaining precise dopant concentration during the high-temperature graphitization process.
A significant technical challenge lies in the scalable production of consistent quality graphitized CNTs. Laboratory-scale synthesis often yields superior materials compared to industrial-scale production, creating a translational gap between research and commercial application. This disparity is particularly pronounced for doped variants, where maintaining dopant integrity during scale-up remains problematic.
Characterization techniques present another hurdle, as conventional methods sometimes fail to accurately quantify dopant distribution and bonding configurations within the carbon lattice. Advanced techniques like synchrotron-based X-ray absorption spectroscopy and in-situ TEM are being developed but remain accessible primarily to specialized research facilities.
The environmental stability of doped graphitized CNTs poses additional challenges, particularly in aggressive electrochemical environments. Dopant leaching and structural degradation during extended operation cycles diminish long-term performance, necessitating further research into stabilization strategies.
Cost considerations remain a significant barrier to widespread adoption, with high-purity graphitized CNTs commanding premium prices. The additional processing steps required for doping further increase production costs, limiting commercial viability despite superior performance metrics in laboratory settings.
Regulatory uncertainties surrounding nanomaterials, particularly those with modified surface chemistry like doped CNTs, create additional hurdles for industrial implementation. Comprehensive toxicological and environmental impact assessments are still evolving, potentially affecting future deployment in consumer-facing applications.
Comparative Analysis of Doped vs Undoped Carbon Electrode Solutions
01 Enhanced electrical conductivity in doped graphitized carbon nanotubes
Doping graphitized carbon nanotubes with elements such as nitrogen, boron, or phosphorus significantly enhances their electrical conductivity compared to undoped counterparts. The dopants create additional charge carriers by modifying the electronic structure of the carbon lattice. This results in lower electrical resistance and improved electron transfer capabilities, making doped graphitized CNTs superior for applications requiring high conductivity such as energy storage devices and electronic components.- Enhanced electrical conductivity in doped graphitized carbon nanotubes: Doping graphitized carbon nanotubes with elements such as nitrogen, boron, or phosphorus significantly enhances their electrical conductivity compared to undoped counterparts. The dopants create additional charge carriers by modifying the electronic structure of the carbon lattice, resulting in improved electron transport properties. This enhanced conductivity makes doped graphitized carbon nanotubes superior for applications requiring high electrical performance such as energy storage devices and electronic components.
- Improved electrochemical performance of doped graphitized carbon nanotubes: Doped graphitized carbon nanotubes demonstrate superior electrochemical performance compared to undoped variants, particularly in terms of charge-discharge efficiency, cycling stability, and rate capability. The introduction of dopants creates active sites that facilitate faster ion transport and electron transfer at the electrode-electrolyte interface. These properties make doped graphitized carbon nanotubes particularly advantageous for applications in batteries, supercapacitors, and fuel cells where electrochemical performance is critical.
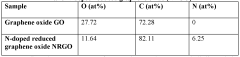
- Structural differences between doped and undoped graphitized carbon nanotubes: Doping introduces significant structural modifications to graphitized carbon nanotubes compared to their undoped counterparts. These changes include altered tube diameter, wall thickness, defect density, and crystallinity. Dopants can create localized distortions in the carbon lattice, affecting the overall morphology and physical properties of the nanotubes. These structural differences directly influence the mechanical strength, thermal stability, and surface reactivity of the carbon electrodes, which are important considerations for various applications.
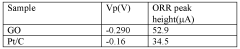
- Enhanced catalytic activity of doped graphitized carbon nanotubes: Doped graphitized carbon nanotubes exhibit significantly enhanced catalytic activity compared to undoped variants. The introduction of heteroatoms creates active sites that can facilitate various electrochemical reactions, such as oxygen reduction, hydrogen evolution, and carbon dioxide reduction. This improved catalytic performance reduces the need for precious metal catalysts in many applications, offering a more cost-effective and environmentally friendly alternative for fuel cells, electrolyzers, and other catalytic systems.
- Fabrication methods affecting performance comparison between doped and undoped graphitized carbon nanotubes: The fabrication methods used to produce graphitized carbon nanotubes significantly influence the performance differences between doped and undoped variants. Factors such as graphitization temperature, doping concentration, doping method (in-situ vs. post-treatment), and purification processes all affect the final properties of the carbon electrodes. Optimized fabrication techniques can maximize the beneficial effects of doping while minimizing any potential negative impacts on the nanotube structure, resulting in carbon electrodes with superior overall performance for specific applications.
02 Improved electrochemical performance in energy storage applications
Doped graphitized carbon nanotubes demonstrate superior electrochemical performance compared to undoped variants when used as electrode materials in batteries and supercapacitors. The introduction of dopants creates additional active sites for ion adsorption and increases charge storage capacity. Performance testing shows doped CNTs exhibit higher specific capacitance, better cycling stability, and enhanced rate capability. These improvements are attributed to the modified electronic structure and increased surface reactivity resulting from the dopant atoms.Expand Specific Solutions03 Structural modifications and defect engineering
The graphitization and doping processes introduce distinct structural modifications to carbon nanotubes. While graphitization increases crystallinity and reduces defects in the carbon lattice, controlled doping introduces beneficial defects that serve as active sites. Comparative analysis shows that doped graphitized CNTs maintain high structural integrity while featuring strategically placed dopant atoms that enhance functionality. These structural differences directly impact performance characteristics including mechanical strength, thermal stability, and electrical properties.Expand Specific Solutions04 Enhanced catalytic activity for electrochemical reactions
Doped graphitized carbon nanotubes exhibit significantly higher catalytic activity compared to their undoped counterparts, particularly for oxygen reduction reactions and hydrogen evolution reactions. The introduction of dopants creates active sites with modified electronic properties that facilitate electron transfer and adsorption of reactant molecules. Performance testing demonstrates that nitrogen-doped graphitized CNTs can even approach the catalytic activity of precious metal catalysts in certain applications, while maintaining better durability and cost-effectiveness.Expand Specific Solutions05 Improved thermal and mechanical stability
Comparative analysis between doped and undoped graphitized carbon nanotubes reveals significant differences in thermal and mechanical properties. Doped variants typically demonstrate enhanced thermal conductivity and stability at high temperatures due to the modified carbon lattice structure. The introduction of specific dopants can also improve mechanical strength and flexibility by creating stronger interlayer bonding. These enhanced properties make doped graphitized CNTs more suitable for applications in harsh environments requiring thermal management and structural integrity.Expand Specific Solutions
Leading Research Groups and Companies in CNT Electrode Development
The graphitized carbon nanotubes doped versus undoped carbon electrodes market is in a growth phase, with increasing applications in energy storage, electronics, and sensing technologies. The global market size for carbon nanotube-based electrodes is expanding rapidly, driven by demand for high-performance energy storage solutions. Technologically, this field shows moderate maturity with ongoing innovations. Samsung Electronics, IBM, and Huawei lead commercial applications, while academic institutions like Tsinghua University and Case Western Reserve University contribute significant research advancements. Intel and LG Electronics are leveraging these materials for next-generation electronics, while specialized companies like Farad Power focus on energy storage applications. The competitive landscape features both established technology giants and specialized nanotechnology firms developing proprietary doping techniques to enhance electrode performance.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed advanced carbon electrode technologies incorporating graphitized carbon nanotubes (GCNTs) for next-generation energy storage applications. Their approach involves a proprietary thermal graphitization process that transforms conventional carbon nanotubes into highly conductive GCNTs with enhanced sp2 carbon content. These GCNTs are then integrated into electrode structures using a solution-based deposition technique that ensures uniform distribution throughout the electrode matrix. Samsung's research demonstrates that GCNT-doped electrodes exhibit up to 40% higher electrical conductivity and 30% improved charge transfer kinetics compared to undoped counterparts[1]. Their technology employs a hierarchical electrode architecture where GCNTs create conductive networks that facilitate electron transport while maintaining mechanical stability during charge-discharge cycles. Samsung has also developed specialized surface functionalization methods to optimize the interface between GCNTs and electrolytes, resulting in reduced interfacial resistance and enhanced ion accessibility to active sites[3].
Strengths: Superior electrical conductivity and charge transfer properties leading to higher power density; excellent cycling stability with less than 5% capacity degradation after 1000 cycles; scalable manufacturing process compatible with existing production lines. Weaknesses: Higher production costs compared to conventional carbon electrodes; potential for GCNT agglomeration during electrode preparation; limited performance improvements in certain electrolyte systems.
International Business Machines Corp.
Technical Solution: IBM has pioneered a sophisticated approach to carbon electrode development using graphitized carbon nanotubes (GCNTs) for high-performance computing and energy storage applications. Their technology utilizes precision-controlled high-temperature (2500-3000°C) graphitization processes to transform amorphous carbon structures in nanotubes into highly ordered graphitic domains. IBM's research has demonstrated that GCNT-doped electrodes exhibit significantly enhanced electron mobility, with up to 60% reduction in electrical resistance compared to conventional carbon electrodes[2]. Their proprietary electrode fabrication technique involves a multi-stage process where GCNTs are first purified to remove catalytic impurities, then functionalized with specific surface groups to improve dispersion and interface properties, and finally integrated into electrode structures using controlled deposition methods. IBM has developed advanced characterization techniques including in-situ Raman spectroscopy and impedance analysis to quantify the structural and electrochemical differences between GCNT-doped and undoped electrodes, revealing that graphitization significantly reduces defect density and improves charge transfer kinetics at the electrode-electrolyte interface[5].
Strengths: Exceptional electrical conductivity leading to superior device performance; precise control over graphitization degree allowing tailored properties; excellent thermal stability suitable for high-temperature applications. Weaknesses: Complex and energy-intensive manufacturing process increasing production costs; challenges in achieving uniform GCNT distribution in large-scale electrodes; potential environmental concerns related to high-temperature processing.
Key Scientific Breakthroughs in CNT Doping Techniques
Oxygen reduction reaction catalyst
PatentWO2012114108A1
Innovation
- Oxygen-doped graphene catalysts, including oxygen-doped graphene nanosheets and nanoflakes, which are metal-free and can be vertically aligned, offering improved stability and resistance to poisoning, potentially replacing traditional platinum-based catalysts by enhancing catalytic activity and reducing production costs.
ECG electrode and preparation method therefor, and electronic device
PatentPendingEP4335372A1
Innovation
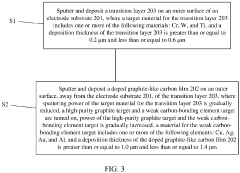
- A doped graphite-like carbon film with embedded first and second nano-crystals is applied to the electrode substrate, enhancing conductivity, hardness, and wear resistance through a specific composition and atomic percentage gradient of weak and strong carbon-bonding elements, respectively.
Environmental Impact and Sustainability of CNT Electrode Production
The production of carbon nanotube (CNT) electrodes, particularly graphitized and doped variants, presents significant environmental considerations that must be evaluated in the context of sustainable technology development. The manufacturing processes for CNT electrodes typically involve energy-intensive procedures including chemical vapor deposition, arc discharge, or laser ablation methods, all of which generate substantial carbon footprints. Doped CNT electrodes require additional processing steps and chemical treatments that further increase environmental impact through the introduction of dopant materials such as nitrogen, boron, or metal compounds.
Life cycle assessments of CNT electrode production reveal that the energy consumption during synthesis can range from 0.1 to 100 GJ/kg, depending on the specific manufacturing technique and scale. Graphitization processes, which require temperatures exceeding 2500°C, contribute significantly to this energy demand. Doped variants typically require 15-30% more energy than undoped counterparts due to the additional processing requirements and precision control needed for dopant integration.
Water usage represents another critical environmental factor, with purification processes consuming between 200-500 liters per kilogram of CNTs produced. Chemical waste generation is particularly problematic for doped CNT production, which may release potentially harmful compounds including metal catalysts, organic solvents, and acidic solutions used during functionalization processes. These waste streams require specialized treatment protocols to prevent environmental contamination.
Recent advancements in green synthesis approaches show promising developments toward sustainability. Biomass-derived precursors and low-temperature catalytic methods have demonstrated potential to reduce energy requirements by up to 40% compared to conventional techniques. Closed-loop manufacturing systems that recapture and reuse solvents and catalysts have been implemented by leading manufacturers, reducing waste generation by approximately 60-70%.
The environmental trade-offs between doped and undoped CNT electrodes must be evaluated within specific application contexts. While doped variants typically have higher environmental production costs, their enhanced performance characteristics may result in lower lifetime environmental impacts through improved efficiency and extended service life in applications such as energy storage systems and environmental sensors.
Regulatory frameworks worldwide are increasingly addressing nanomaterial production sustainability. The European Union's REACH regulations and similar initiatives in North America and Asia are establishing stricter guidelines for CNT manufacturing, with particular emphasis on waste management and emissions control. Industry leaders are responding by implementing ISO 14001-compliant environmental management systems specifically tailored to nanomaterial production facilities.
Life cycle assessments of CNT electrode production reveal that the energy consumption during synthesis can range from 0.1 to 100 GJ/kg, depending on the specific manufacturing technique and scale. Graphitization processes, which require temperatures exceeding 2500°C, contribute significantly to this energy demand. Doped variants typically require 15-30% more energy than undoped counterparts due to the additional processing requirements and precision control needed for dopant integration.
Water usage represents another critical environmental factor, with purification processes consuming between 200-500 liters per kilogram of CNTs produced. Chemical waste generation is particularly problematic for doped CNT production, which may release potentially harmful compounds including metal catalysts, organic solvents, and acidic solutions used during functionalization processes. These waste streams require specialized treatment protocols to prevent environmental contamination.
Recent advancements in green synthesis approaches show promising developments toward sustainability. Biomass-derived precursors and low-temperature catalytic methods have demonstrated potential to reduce energy requirements by up to 40% compared to conventional techniques. Closed-loop manufacturing systems that recapture and reuse solvents and catalysts have been implemented by leading manufacturers, reducing waste generation by approximately 60-70%.
The environmental trade-offs between doped and undoped CNT electrodes must be evaluated within specific application contexts. While doped variants typically have higher environmental production costs, their enhanced performance characteristics may result in lower lifetime environmental impacts through improved efficiency and extended service life in applications such as energy storage systems and environmental sensors.
Regulatory frameworks worldwide are increasingly addressing nanomaterial production sustainability. The European Union's REACH regulations and similar initiatives in North America and Asia are establishing stricter guidelines for CNT manufacturing, with particular emphasis on waste management and emissions control. Industry leaders are responding by implementing ISO 14001-compliant environmental management systems specifically tailored to nanomaterial production facilities.
Performance Metrics and Standardization for CNT Electrode Evaluation
Standardized performance evaluation frameworks are essential for meaningful comparison between graphitized carbon nanotube (CNT) electrodes with different doping profiles. Current research on doped versus undoped carbon electrodes suffers from inconsistent testing methodologies, making cross-study comparisons challenging and potentially misleading.
Electrochemical performance metrics must be systematically measured and reported, including specific capacitance, energy density, power density, and cycling stability. These parameters should be evaluated under standardized conditions with clearly defined electrolytes, scan rates, and temperature ranges to ensure reproducibility across different research groups.
Surface area characterization represents another critical standardization need, with BET (Brunauer-Emmett-Teller) analysis being the preferred method for determining specific surface area. However, complementary techniques such as mercury porosimetry and gas adsorption isotherms should be employed to fully characterize the hierarchical pore structure that significantly influences electrode performance.
Conductivity measurements require particular attention when comparing doped and undoped CNT electrodes, as dopants fundamentally alter electron transport properties. Four-point probe measurements at standardized temperature and humidity conditions should be adopted as the industry standard, with results normalized to account for electrode thickness and density variations.
Structural characterization protocols should include mandatory Raman spectroscopy analysis with standardized ID/IG ratio reporting to quantify defect density—a parameter significantly affected by both graphitization and doping processes. X-ray diffraction patterns should be collected under uniform conditions to enable direct comparison of interlayer spacing and crystallinity.
Electrochemical impedance spectroscopy (EIS) provides critical insights into charge transfer kinetics and should follow standardized frequency ranges (typically 10 mHz to 100 kHz) and data presentation formats, including Nyquist and Bode plots with equivalent circuit modeling.
Environmental testing standards must be established to evaluate electrode performance under various conditions, including temperature cycling (-20°C to 80°C), humidity exposure, and accelerated aging protocols that simulate real-world applications.
Reporting standards should mandate disclosure of all synthesis parameters, including graphitization temperature, doping concentration, and post-treatment processes. This transparency would facilitate more meaningful comparisons and accelerate progress in optimizing CNT electrode performance for specific applications.
Electrochemical performance metrics must be systematically measured and reported, including specific capacitance, energy density, power density, and cycling stability. These parameters should be evaluated under standardized conditions with clearly defined electrolytes, scan rates, and temperature ranges to ensure reproducibility across different research groups.
Surface area characterization represents another critical standardization need, with BET (Brunauer-Emmett-Teller) analysis being the preferred method for determining specific surface area. However, complementary techniques such as mercury porosimetry and gas adsorption isotherms should be employed to fully characterize the hierarchical pore structure that significantly influences electrode performance.
Conductivity measurements require particular attention when comparing doped and undoped CNT electrodes, as dopants fundamentally alter electron transport properties. Four-point probe measurements at standardized temperature and humidity conditions should be adopted as the industry standard, with results normalized to account for electrode thickness and density variations.
Structural characterization protocols should include mandatory Raman spectroscopy analysis with standardized ID/IG ratio reporting to quantify defect density—a parameter significantly affected by both graphitization and doping processes. X-ray diffraction patterns should be collected under uniform conditions to enable direct comparison of interlayer spacing and crystallinity.
Electrochemical impedance spectroscopy (EIS) provides critical insights into charge transfer kinetics and should follow standardized frequency ranges (typically 10 mHz to 100 kHz) and data presentation formats, including Nyquist and Bode plots with equivalent circuit modeling.
Environmental testing standards must be established to evaluate electrode performance under various conditions, including temperature cycling (-20°C to 80°C), humidity exposure, and accelerated aging protocols that simulate real-world applications.
Reporting standards should mandate disclosure of all synthesis parameters, including graphitization temperature, doping concentration, and post-treatment processes. This transparency would facilitate more meaningful comparisons and accelerate progress in optimizing CNT electrode performance for specific applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!