Comparison of Electrolytic vs. Catalytic Ammonia Synthesis Methods
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonia Synthesis Evolution and Objectives
Ammonia synthesis has undergone significant evolution since its industrial inception in the early 20th century. The Haber-Bosch process, developed in 1909, revolutionized ammonia production by enabling the direct combination of nitrogen and hydrogen under high pressure and temperature conditions using iron-based catalysts. This breakthrough technology has remained the dominant industrial method for over a century, supporting global food production through nitrogen fertilizers and serving as a foundation for numerous chemical industries.
The technological trajectory of ammonia synthesis reflects broader industrial challenges and innovations. While the Haber-Bosch process achieved remarkable efficiency improvements over decades, reducing energy consumption by approximately 30% since its introduction, it still accounts for 1-2% of global energy consumption and generates substantial carbon emissions—approximately 1.8 tons of CO2 per ton of ammonia produced.
Recent environmental imperatives have shifted research objectives toward more sustainable synthesis methods. The emergence of electrolytic ammonia synthesis represents a paradigm shift, offering potential pathways to decouple ammonia production from fossil fuels. This approach leverages renewable electricity to drive nitrogen reduction reactions at ambient conditions, promising significant carbon footprint reductions compared to conventional methods.
Catalytic innovations continue in parallel, with researchers exploring novel materials including ruthenium-based catalysts, metal nitrides, and lithium-mediated systems that can operate under milder conditions than traditional iron catalysts. These developments aim to reduce the energy intensity while maintaining or improving production efficiency.
The primary objectives driving current ammonia synthesis research include: reducing energy consumption below 30 GJ/ton (compared to approximately 35-45 GJ/ton in modern Haber-Bosch plants); enabling operation at near-ambient conditions to simplify infrastructure requirements; developing processes compatible with intermittent renewable energy sources; and achieving carbon-neutral or carbon-negative production pathways.
Technological convergence between electrolytic and catalytic approaches is emerging as a promising direction, with hybrid systems potentially offering advantages of both methodologies. These systems aim to overcome the inherent limitations of each approach—the energy efficiency challenges of electrolytic methods and the harsh operating conditions of traditional catalytic processes.
The ultimate goal remains developing economically viable, environmentally sustainable ammonia synthesis technologies that can operate at distributed scales, potentially transforming the centralized production model that has dominated the industry since its inception. Success in this endeavor would have profound implications for global food security, energy storage applications, and industrial decarbonization efforts.
The technological trajectory of ammonia synthesis reflects broader industrial challenges and innovations. While the Haber-Bosch process achieved remarkable efficiency improvements over decades, reducing energy consumption by approximately 30% since its introduction, it still accounts for 1-2% of global energy consumption and generates substantial carbon emissions—approximately 1.8 tons of CO2 per ton of ammonia produced.
Recent environmental imperatives have shifted research objectives toward more sustainable synthesis methods. The emergence of electrolytic ammonia synthesis represents a paradigm shift, offering potential pathways to decouple ammonia production from fossil fuels. This approach leverages renewable electricity to drive nitrogen reduction reactions at ambient conditions, promising significant carbon footprint reductions compared to conventional methods.
Catalytic innovations continue in parallel, with researchers exploring novel materials including ruthenium-based catalysts, metal nitrides, and lithium-mediated systems that can operate under milder conditions than traditional iron catalysts. These developments aim to reduce the energy intensity while maintaining or improving production efficiency.
The primary objectives driving current ammonia synthesis research include: reducing energy consumption below 30 GJ/ton (compared to approximately 35-45 GJ/ton in modern Haber-Bosch plants); enabling operation at near-ambient conditions to simplify infrastructure requirements; developing processes compatible with intermittent renewable energy sources; and achieving carbon-neutral or carbon-negative production pathways.
Technological convergence between electrolytic and catalytic approaches is emerging as a promising direction, with hybrid systems potentially offering advantages of both methodologies. These systems aim to overcome the inherent limitations of each approach—the energy efficiency challenges of electrolytic methods and the harsh operating conditions of traditional catalytic processes.
The ultimate goal remains developing economically viable, environmentally sustainable ammonia synthesis technologies that can operate at distributed scales, potentially transforming the centralized production model that has dominated the industry since its inception. Success in this endeavor would have profound implications for global food security, energy storage applications, and industrial decarbonization efforts.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability imperatives and technological innovation. Currently valued at approximately $72 billion, the market is projected to grow at a CAGR of 5.3% through 2030, with sustainable ammonia production methods gaining substantial traction. Traditional Haber-Bosch processes account for nearly 90% of current production but are increasingly challenged by emerging sustainable alternatives.
Demand for sustainable ammonia is being fueled by multiple sectors. The agricultural industry, which consumes roughly 80% of global ammonia production for fertilizers, is facing mounting pressure to reduce its carbon footprint. Industrial applications, including mining, textiles, and pharmaceuticals, represent about 15% of consumption and are similarly seeking greener alternatives. The emerging hydrogen carrier and fuel markets present particularly promising growth opportunities, with projections suggesting they could account for 25% of ammonia demand by 2040.
Regional market dynamics reveal significant variations. Europe leads in sustainable ammonia adoption, driven by stringent regulatory frameworks including the European Green Deal and carbon pricing mechanisms. North America follows with substantial investments in green ammonia projects, particularly in regions with abundant renewable energy resources. The Asia-Pacific region, while still heavily reliant on conventional production methods, is witnessing accelerated investment in sustainable technologies, especially in Japan, South Korea, and Australia.
Economic analysis indicates that while conventional ammonia production costs range from $250-450 per ton, electrolytic methods currently cost between $600-1,200 per ton, and catalytic innovations range from $500-900 per ton. However, cost projections suggest that technological advancements and economies of scale could reduce sustainable production costs by 40-60% by 2030, approaching cost parity with conventional methods.
Market barriers include high capital expenditure requirements for new production facilities, technological uncertainties, and infrastructure limitations. The sustainable ammonia market also faces regulatory challenges, with inconsistent policy frameworks across regions creating market fragmentation and investment uncertainty.
Customer willingness to pay premiums for sustainable ammonia varies significantly by sector. Agricultural end-users demonstrate price sensitivity with limited premium tolerance (5-10%), while industrial applications show moderate willingness (10-20%). The emerging energy sector exhibits the highest premium acceptance (20-30%), particularly in markets with strong decarbonization mandates.
Future market growth will likely be driven by technological breakthroughs reducing production costs, strengthened regulatory frameworks incentivizing low-carbon solutions, and expanding applications in the energy sector as ammonia gains traction as a hydrogen carrier and carbon-free fuel.
Demand for sustainable ammonia is being fueled by multiple sectors. The agricultural industry, which consumes roughly 80% of global ammonia production for fertilizers, is facing mounting pressure to reduce its carbon footprint. Industrial applications, including mining, textiles, and pharmaceuticals, represent about 15% of consumption and are similarly seeking greener alternatives. The emerging hydrogen carrier and fuel markets present particularly promising growth opportunities, with projections suggesting they could account for 25% of ammonia demand by 2040.
Regional market dynamics reveal significant variations. Europe leads in sustainable ammonia adoption, driven by stringent regulatory frameworks including the European Green Deal and carbon pricing mechanisms. North America follows with substantial investments in green ammonia projects, particularly in regions with abundant renewable energy resources. The Asia-Pacific region, while still heavily reliant on conventional production methods, is witnessing accelerated investment in sustainable technologies, especially in Japan, South Korea, and Australia.
Economic analysis indicates that while conventional ammonia production costs range from $250-450 per ton, electrolytic methods currently cost between $600-1,200 per ton, and catalytic innovations range from $500-900 per ton. However, cost projections suggest that technological advancements and economies of scale could reduce sustainable production costs by 40-60% by 2030, approaching cost parity with conventional methods.
Market barriers include high capital expenditure requirements for new production facilities, technological uncertainties, and infrastructure limitations. The sustainable ammonia market also faces regulatory challenges, with inconsistent policy frameworks across regions creating market fragmentation and investment uncertainty.
Customer willingness to pay premiums for sustainable ammonia varies significantly by sector. Agricultural end-users demonstrate price sensitivity with limited premium tolerance (5-10%), while industrial applications show moderate willingness (10-20%). The emerging energy sector exhibits the highest premium acceptance (20-30%), particularly in markets with strong decarbonization mandates.
Future market growth will likely be driven by technological breakthroughs reducing production costs, strengthened regulatory frameworks incentivizing low-carbon solutions, and expanding applications in the energy sector as ammonia gains traction as a hydrogen carrier and carbon-free fuel.
Current Technologies and Barriers in Ammonia Synthesis
Ammonia synthesis is dominated by two primary technological approaches: the traditional Haber-Bosch process and emerging electrolytic methods. The Haber-Bosch process, developed over a century ago, remains the industrial standard, accounting for approximately 80% of global ammonia production. This catalytic method combines nitrogen and hydrogen at high temperatures (400-500°C) and pressures (150-300 bar) over iron-based catalysts, consuming 1-2% of global energy and producing significant carbon emissions.
Recent advancements in catalytic technology have focused on developing more efficient catalysts that operate under milder conditions. Ruthenium-based catalysts have demonstrated activity at lower pressures, while research into metal nitrides and supported metal catalysts shows promise for reducing energy requirements. However, these improvements remain incremental rather than transformative.
Electrolytic ammonia synthesis has emerged as a potentially disruptive alternative. This approach uses electricity to drive nitrogen reduction reactions at ambient conditions, potentially eliminating the need for hydrogen feedstock and high-pressure equipment. Several variants exist, including solid-state electrolyte cells, molten salt electrolysis, and aqueous electrolyte systems, each with distinct advantages and limitations.
The primary barrier to widespread adoption of electrolytic methods is their low Faradaic efficiency, typically below 10% compared to the theoretical maximum. This inefficiency stems from competing hydrogen evolution reactions and the strong triple bond of nitrogen molecules. Current densities in electrolytic systems also remain too low for industrial viability, generally below 100 mA/cm², whereas commercial applications would require at least an order of magnitude improvement.
Catalyst selectivity presents another significant challenge. Most electrocatalysts struggle to preferentially reduce nitrogen over hydrogen, particularly in aqueous environments. Materials such as noble metals, transition metal nitrides, and metal-organic frameworks show promise but require further development to achieve practical selectivity levels.
Scalability remains problematic for both approaches. While Haber-Bosch plants operate efficiently at massive scales (1,000+ tons/day), they are economically unviable at smaller capacities needed for distributed production. Conversely, electrolytic methods show potential for modular, smaller-scale implementation but face significant engineering challenges in scaling up to even moderate production volumes.
Energy efficiency comparisons reveal that current electrolytic methods consume 60-90 MWh per ton of ammonia, substantially higher than the 10-12 MWh per ton for modern Haber-Bosch plants. This efficiency gap must narrow considerably before electrolytic approaches can compete economically, particularly in regions without abundant renewable electricity.
Recent advancements in catalytic technology have focused on developing more efficient catalysts that operate under milder conditions. Ruthenium-based catalysts have demonstrated activity at lower pressures, while research into metal nitrides and supported metal catalysts shows promise for reducing energy requirements. However, these improvements remain incremental rather than transformative.
Electrolytic ammonia synthesis has emerged as a potentially disruptive alternative. This approach uses electricity to drive nitrogen reduction reactions at ambient conditions, potentially eliminating the need for hydrogen feedstock and high-pressure equipment. Several variants exist, including solid-state electrolyte cells, molten salt electrolysis, and aqueous electrolyte systems, each with distinct advantages and limitations.
The primary barrier to widespread adoption of electrolytic methods is their low Faradaic efficiency, typically below 10% compared to the theoretical maximum. This inefficiency stems from competing hydrogen evolution reactions and the strong triple bond of nitrogen molecules. Current densities in electrolytic systems also remain too low for industrial viability, generally below 100 mA/cm², whereas commercial applications would require at least an order of magnitude improvement.
Catalyst selectivity presents another significant challenge. Most electrocatalysts struggle to preferentially reduce nitrogen over hydrogen, particularly in aqueous environments. Materials such as noble metals, transition metal nitrides, and metal-organic frameworks show promise but require further development to achieve practical selectivity levels.
Scalability remains problematic for both approaches. While Haber-Bosch plants operate efficiently at massive scales (1,000+ tons/day), they are economically unviable at smaller capacities needed for distributed production. Conversely, electrolytic methods show potential for modular, smaller-scale implementation but face significant engineering challenges in scaling up to even moderate production volumes.
Energy efficiency comparisons reveal that current electrolytic methods consume 60-90 MWh per ton of ammonia, substantially higher than the 10-12 MWh per ton for modern Haber-Bosch plants. This efficiency gap must narrow considerably before electrolytic approaches can compete economically, particularly in regions without abundant renewable electricity.
Comparative Analysis of Electrolytic and Catalytic Synthesis Approaches
01 Electrolytic ammonia synthesis methods
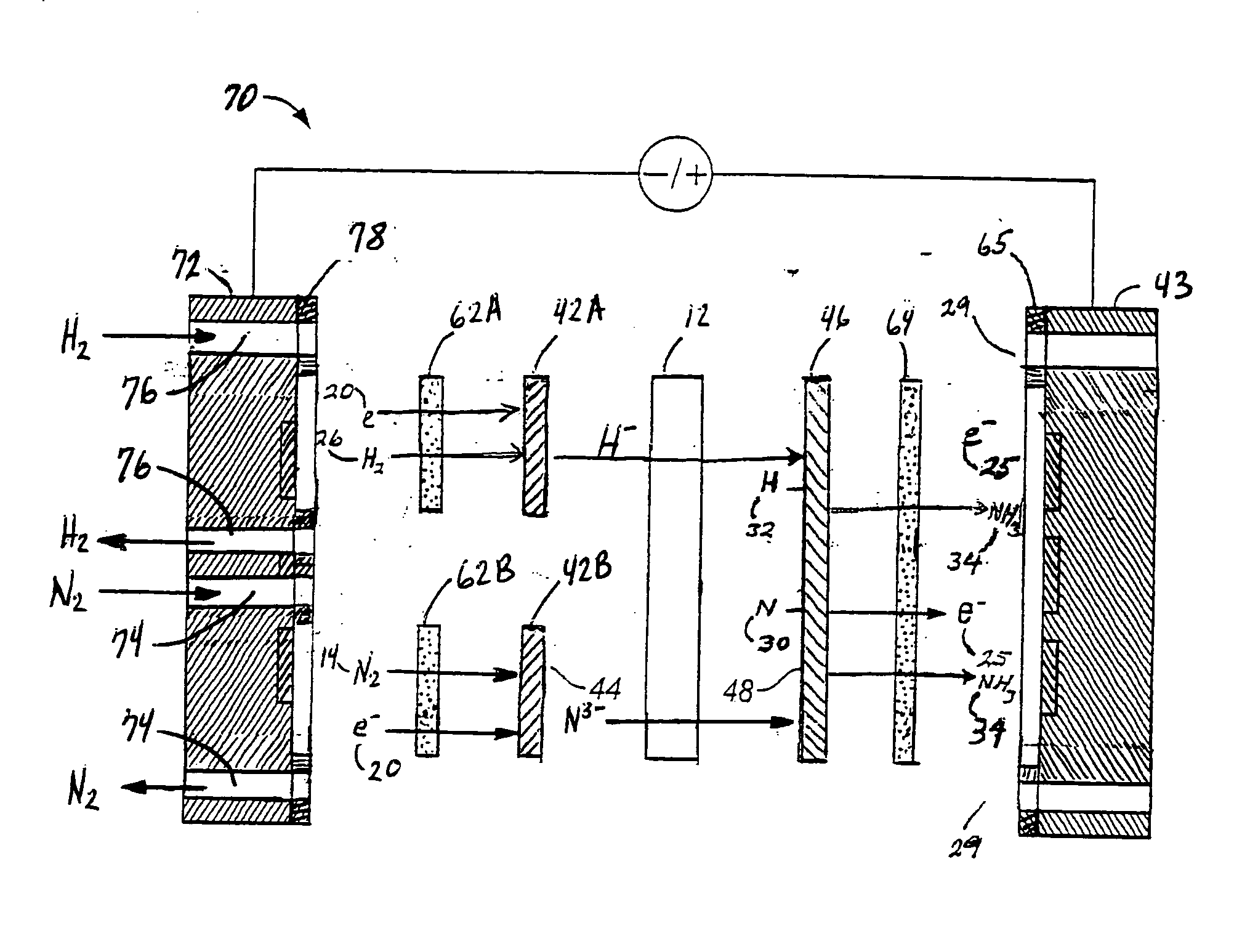
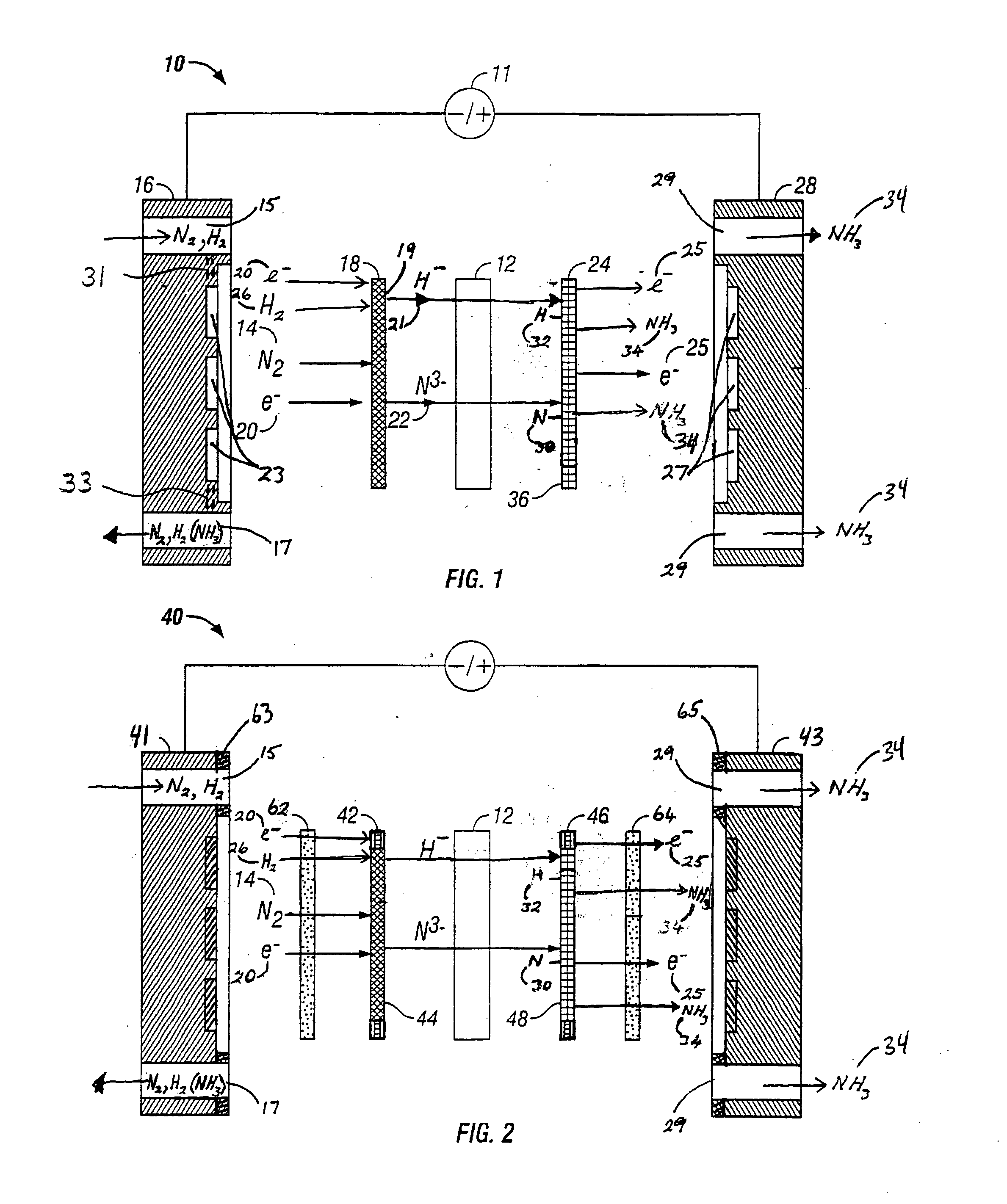
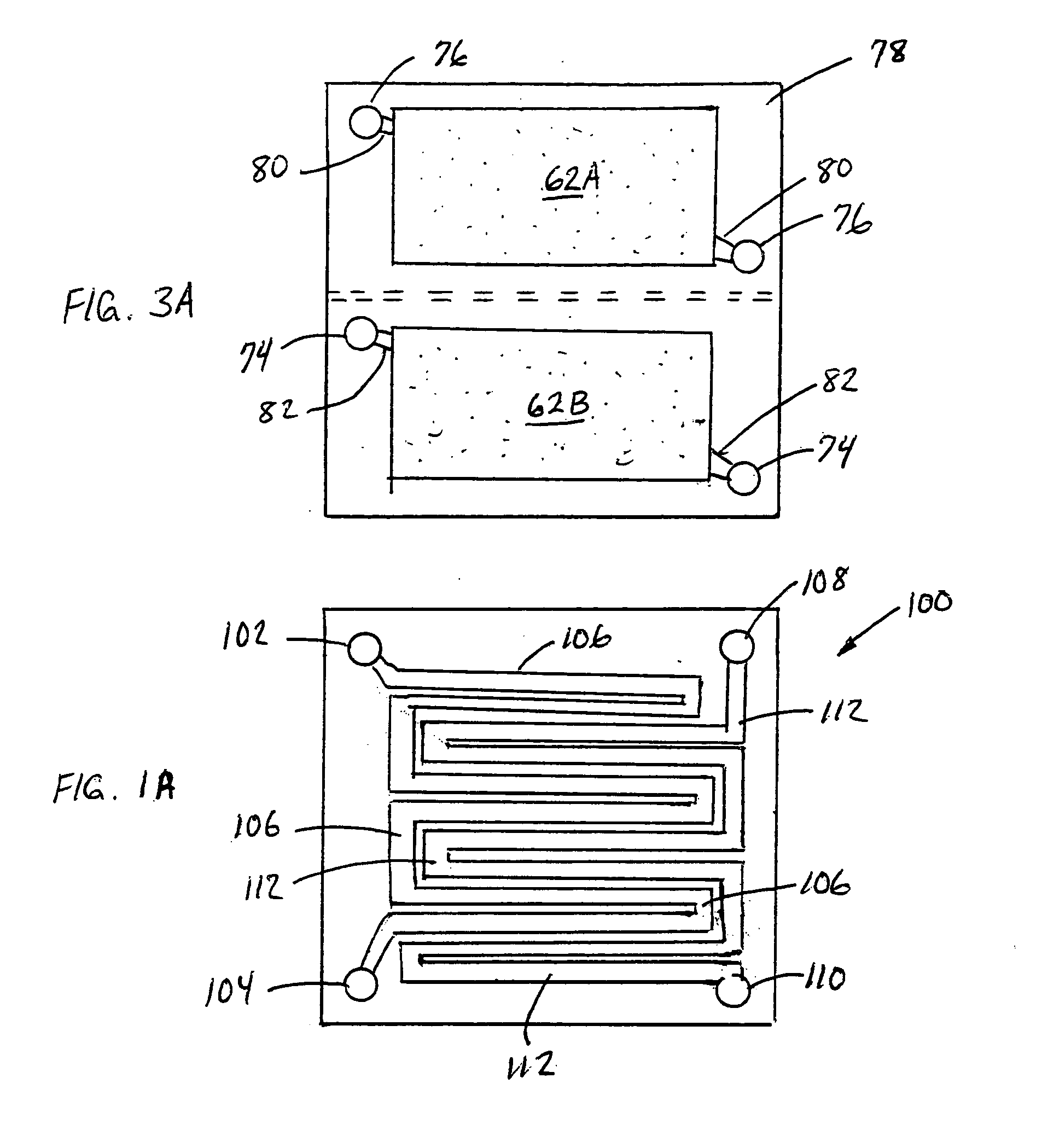
Electrolytic methods for ammonia synthesis involve the use of electricity to drive the reaction between nitrogen and hydrogen. These methods typically operate at milder conditions compared to traditional catalytic processes, often at ambient temperature and pressure. Electrolytic synthesis can utilize renewable electricity sources, making it a potentially more sustainable approach. The process typically involves electrochemical cells with specialized electrodes and electrolytes that facilitate the reduction of nitrogen to ammonia.- Electrolytic ammonia synthesis methods: Electrolytic methods for ammonia synthesis involve using electricity to drive the reaction between nitrogen and hydrogen. These processes typically operate at ambient temperatures and pressures, making them more energy-efficient than traditional high-pressure methods. Electrolytic cells with specialized catalysts and membranes facilitate the reaction by providing protons and electrons necessary for nitrogen reduction. These methods often use renewable electricity sources, making them environmentally friendly alternatives to conventional ammonia production.
- Catalytic ammonia synthesis using transition metals: Transition metal catalysts play a crucial role in ammonia synthesis by facilitating the breaking of the strong nitrogen triple bond. Iron-based catalysts, particularly those derived from the Haber-Bosch process, remain widely used in industrial applications. Other transition metals such as ruthenium, cobalt, and nickel, often supported on various substrates, have shown promising catalytic activity. These catalysts are typically designed to maximize surface area and active sites while minimizing energy requirements for the reaction.
- Low-pressure ammonia synthesis technologies: Low-pressure ammonia synthesis methods aim to overcome the energy-intensive nature of traditional high-pressure processes. These approaches utilize advanced catalyst designs, novel reactor configurations, and optimized process conditions to achieve ammonia formation at significantly reduced pressures. Some methods incorporate membrane reactors that continuously remove ammonia as it forms, shifting the reaction equilibrium toward product formation. These technologies can substantially reduce the capital and operating costs associated with ammonia production while improving energy efficiency.
- Plasma-assisted ammonia synthesis: Plasma-assisted methods utilize electrical discharges to create reactive nitrogen species that can more readily combine with hydrogen to form ammonia. By generating a plasma environment, these processes can activate nitrogen molecules at much milder conditions than conventional thermal processes. Various plasma technologies, including dielectric barrier discharge, microwave plasma, and glow discharge, have been employed for ammonia synthesis. These methods often operate at atmospheric pressure and relatively low temperatures, potentially offering energy savings compared to traditional approaches.
- Green ammonia production using renewable energy: Green ammonia production methods integrate renewable energy sources with ammonia synthesis to create a sustainable production pathway. These approaches typically couple electrolytic hydrogen generation from water using renewable electricity with nitrogen separation from air, followed by ammonia synthesis. Some systems directly utilize renewable electricity in electrochemical ammonia synthesis. By eliminating fossil fuel inputs, these methods can significantly reduce the carbon footprint of ammonia production, making it suitable for both fertilizer applications and as a potential carbon-free energy carrier.
02 Catalytic ammonia synthesis using traditional Haber-Bosch process
The traditional Haber-Bosch process uses iron-based catalysts to synthesize ammonia from nitrogen and hydrogen gases at high temperature and pressure. This well-established industrial method typically operates at 400-500°C and 150-300 bar pressure. The process requires significant energy input for maintaining reaction conditions and hydrogen production. Various improvements to the catalyst composition and reactor design have been developed to enhance efficiency and conversion rates.Expand Specific Solutions03 Novel catalyst systems for ammonia synthesis
Advanced catalyst systems have been developed to improve ammonia synthesis efficiency beyond traditional iron catalysts. These include ruthenium-based catalysts, bimetallic systems, and supported metal catalysts that can operate at lower temperatures or pressures. Some novel catalysts incorporate promoters or specific structures to enhance nitrogen activation and ammonia formation. These catalytic innovations aim to reduce the energy requirements while maintaining or improving conversion rates and selectivity.Expand Specific Solutions04 Plasma-assisted ammonia synthesis methods
Plasma-assisted methods utilize non-thermal plasma to activate nitrogen molecules for ammonia synthesis. These approaches can operate at atmospheric pressure and lower temperatures than conventional processes. The plasma creates reactive nitrogen species that can more readily combine with hydrogen to form ammonia. Various plasma generation techniques, including dielectric barrier discharge and microwave plasma, have been investigated. These methods often combine plasma activation with catalytic surfaces to enhance conversion efficiency.Expand Specific Solutions05 Integrated and hybrid ammonia production systems
Integrated systems combine different approaches to ammonia synthesis for improved efficiency. These include hybrid electrocatalytic-thermal systems, renewable energy integration with ammonia production, and processes that combine catalytic and electrolytic methods. Some systems incorporate hydrogen production from renewable sources directly with ammonia synthesis. These integrated approaches aim to reduce carbon footprint, improve energy efficiency, and enable distributed ammonia production at various scales.Expand Specific Solutions
Leading Companies and Research Institutions in Ammonia Technology
The ammonia synthesis technology landscape is evolving from traditional Haber-Bosch processes toward more sustainable electrolytic and catalytic methods, currently in early commercialization stages. The global market is expanding rapidly, driven by decarbonization initiatives and green hydrogen integration. Companies like Atmonia, GenCell, and Topsoe are pioneering electrolytic ammonia synthesis technologies, while established players such as Siemens, Saudi Aramco, and Toyota are investing in catalytic innovations. Academic institutions including University of Tokyo, Technical University of Denmark, and Carnegie Mellon are advancing fundamental research. The technology maturity varies significantly, with electrolytic methods at TRL 5-7 and advanced catalytic approaches at TRL 4-6, indicating promising but still developing alternatives to conventional energy-intensive production methods.
GenCell Ltd.
Technical Solution: GenCell has developed an innovative alkaline fuel cell technology that can be reversed for ammonia synthesis. Their approach centers on a unique alkaline membrane electrolytic cell operating at near-ambient temperatures (40-60°C) and pressures. The company's patented "Ammonia-to-Power" technology has been adapted to work bidirectionally, enabling both ammonia synthesis and power generation from ammonia. Their electrolytic system employs non-precious metal catalysts based on nickel and cobalt compounds, significantly reducing costs compared to platinum-group metal catalysts. The system achieves ammonia production rates of approximately 50-100 g NH₃/h per square meter of electrode area [4], with electrical efficiency of around 65-70%. GenCell's technology incorporates a proprietary ion exchange membrane that selectively allows nitrogen reduction while minimizing hydrogen evolution side reactions, improving faradaic efficiency to over 60% [5]. The modular design allows for scalable implementation and direct coupling with intermittent renewable energy sources without the need for constant operation.
Strengths: Ambient operating conditions reduce energy requirements; bidirectional capability allows flexible operation as either fuel cell or ammonia synthesizer; use of non-precious metal catalysts significantly reduces costs; modular design enables scalability. Weaknesses: Lower production rates compared to conventional Haber-Bosch process; still requires external hydrogen source for optimal operation; technology remains at demonstration scale rather than full commercial implementation; membrane durability issues under continuous operation conditions.
Siemens AG
Technical Solution: Siemens has developed an integrated green ammonia production system that combines their Silyzer PEM electrolysis technology with optimized Haber-Bosch synthesis. Their approach focuses on creating a flexible, modular system that can operate dynamically with variable renewable energy inputs. The Siemens system employs high-efficiency PEM electrolyzers achieving 75-80% electrical efficiency [9], producing high-purity hydrogen that feeds into a modified Haber-Bosch process. Their proprietary control system enables rapid response to fluctuating power inputs, with ramp-up times of less than 10 seconds to full load, allowing direct coupling with intermittent renewables. Siemens has optimized the catalytic synthesis stage with ruthenium-based catalysts that operate at lower pressures (80-100 bar) than conventional iron catalysts, reducing compression energy requirements by approximately 15-20%. Their demonstration projects have achieved production capacities of 50-300 kg NH₃/day with overall system efficiency (electricity-to-ammonia) of 60-65% [10]. A key innovation is their heat integration system that recovers waste heat from the exothermic synthesis reaction to support the endothermic electrolysis process, improving overall energy efficiency by 10-15% compared to non-integrated systems.
Strengths: High-efficiency PEM electrolysis technology; rapid response capability for integration with variable renewables; advanced system integration and heat recovery; modular design allows scalable implementation. Weaknesses: Still relies on modified Haber-Bosch process requiring moderate pressures; higher capital costs compared to conventional ammonia plants; PEM electrolyzer stack lifetime (60,000-80,000 hours) requires periodic replacement; technology primarily demonstrated at pilot scale rather than full industrial implementation.
Key Patents and Breakthroughs in Ammonia Synthesis
Electrochemical synthesis of ammonia
PatentInactiveUS20060049063A1
Innovation
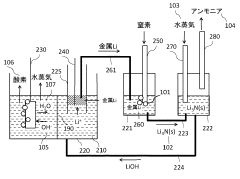
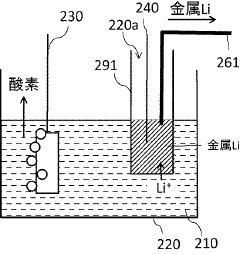
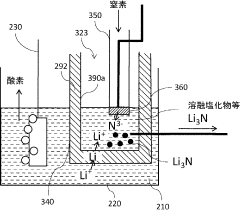
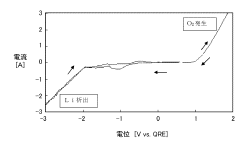
- An anodic electrochemical method using molten salts with dissolved nitride ions and a porous anode structure to oxidize nitride ions and react them with adsorbed hydrogen atoms to form ammonia, allowing for the production of ammonia at lower temperatures and pressures with improved conversion efficiencies.
Method and apparatus for electrolytically synthesizing ammonia
PatentActiveJP2012026036A
Innovation
- A method using a molten hydroxide-based electrolytic bath allows for the selection of various anode materials and structures by reducing nitrogen to form nitrides with alkali or alkaline earth metals, which are then reacted with steam to produce ammonia at lower temperatures.
Energy Efficiency and Carbon Footprint Assessment
Energy efficiency and carbon footprint considerations are critical factors in evaluating ammonia synthesis technologies, particularly when comparing electrolytic and catalytic methods. The Haber-Bosch process, the predominant catalytic method, currently consumes approximately 1-2% of global energy production and generates significant CO2 emissions—estimated at 1.6 tons of CO2 per ton of ammonia produced. This high carbon footprint stems from the process's reliance on natural gas for hydrogen production and the high-temperature, high-pressure operating conditions.
Electrolytic ammonia synthesis presents a potentially greener alternative when powered by renewable electricity sources. Recent studies indicate that renewable-powered electrolytic systems can reduce carbon emissions by 60-90% compared to conventional Haber-Bosch operations. However, current electrolytic methods face efficiency challenges, with energy conversion rates typically ranging from 40-60%, significantly lower than the 65-75% efficiency achieved in optimized catalytic systems.
The total energy requirement for electrolytic ammonia production currently stands at approximately 12-15 MWh per ton of ammonia, compared to 9-11 MWh for conventional methods. This efficiency gap represents a substantial barrier to widespread adoption, despite the carbon benefits. The higher energy demand translates to increased operational costs, estimated at 30-50% above conventional production methods at current electricity prices.
Life cycle assessments reveal that the environmental advantage of electrolytic systems is highly dependent on the electricity source. When powered by coal-generated electricity, electrolytic ammonia actually produces 20-30% more emissions than conventional methods. Conversely, wind or solar-powered systems can achieve carbon reductions of over 80%, highlighting the importance of renewable integration.
Recent technological developments show promising improvements in electrolytic efficiency. Advanced electrode materials and optimized cell designs have demonstrated laboratory-scale efficiency improvements of 15-25% over the past five years. Meanwhile, hybrid systems that combine renewable-powered electrolysis with more efficient catalytic conversion are emerging as a transitional technology, offering carbon reductions of 40-60% while maintaining competitive energy efficiency.
Economic modeling suggests that continued renewable energy cost reductions of 5-7% annually, combined with projected efficiency improvements in electrolytic systems, could achieve cost parity with conventional methods by 2030-2035. Carbon pricing mechanisms would accelerate this timeline, with a carbon price of $50-70 per ton potentially making renewable electrolytic ammonia economically competitive within this decade in regions with abundant renewable resources.
Electrolytic ammonia synthesis presents a potentially greener alternative when powered by renewable electricity sources. Recent studies indicate that renewable-powered electrolytic systems can reduce carbon emissions by 60-90% compared to conventional Haber-Bosch operations. However, current electrolytic methods face efficiency challenges, with energy conversion rates typically ranging from 40-60%, significantly lower than the 65-75% efficiency achieved in optimized catalytic systems.
The total energy requirement for electrolytic ammonia production currently stands at approximately 12-15 MWh per ton of ammonia, compared to 9-11 MWh for conventional methods. This efficiency gap represents a substantial barrier to widespread adoption, despite the carbon benefits. The higher energy demand translates to increased operational costs, estimated at 30-50% above conventional production methods at current electricity prices.
Life cycle assessments reveal that the environmental advantage of electrolytic systems is highly dependent on the electricity source. When powered by coal-generated electricity, electrolytic ammonia actually produces 20-30% more emissions than conventional methods. Conversely, wind or solar-powered systems can achieve carbon reductions of over 80%, highlighting the importance of renewable integration.
Recent technological developments show promising improvements in electrolytic efficiency. Advanced electrode materials and optimized cell designs have demonstrated laboratory-scale efficiency improvements of 15-25% over the past five years. Meanwhile, hybrid systems that combine renewable-powered electrolysis with more efficient catalytic conversion are emerging as a transitional technology, offering carbon reductions of 40-60% while maintaining competitive energy efficiency.
Economic modeling suggests that continued renewable energy cost reductions of 5-7% annually, combined with projected efficiency improvements in electrolytic systems, could achieve cost parity with conventional methods by 2030-2035. Carbon pricing mechanisms would accelerate this timeline, with a carbon price of $50-70 per ton potentially making renewable electrolytic ammonia economically competitive within this decade in regions with abundant renewable resources.
Regulatory Framework for Industrial Ammonia Production
The regulatory landscape governing industrial ammonia production has evolved significantly in response to environmental concerns, safety requirements, and energy efficiency standards. At the international level, the Paris Agreement has indirectly influenced ammonia production by encouraging the transition to lower-carbon manufacturing processes, which has accelerated interest in electrolytic methods over traditional catalytic approaches. The United Nations Framework Convention on Climate Change (UNFCCC) further provides guidelines that impact ammonia producers' carbon footprint reporting requirements.
In the United States, the Environmental Protection Agency (EPA) regulates ammonia production under the Clean Air Act and Clean Water Act, with specific provisions for nitrogen oxide emissions and wastewater discharge from production facilities. The Occupational Safety and Health Administration (OSHA) imposes strict safety protocols for handling ammonia, which affects facility design and operational procedures for both electrolytic and catalytic production methods. The Department of Energy (DOE) offers incentives for energy-efficient ammonia production technologies, currently favoring innovations in electrolytic synthesis.
The European Union implements more stringent regulations through its Industrial Emissions Directive (IED) and the EU Emissions Trading System (EU ETS), which places carbon pricing pressure on traditional Haber-Bosch facilities. The EU's REACH regulation (Registration, Evaluation, Authorization and Restriction of Chemicals) imposes additional compliance requirements for ammonia producers. The European Green Deal has established a regulatory framework that increasingly favors green ammonia production methods, providing competitive advantages to electrolytic technologies.
In Asia, China's 14th Five-Year Plan includes specific targets for reducing carbon intensity in chemical production, including ammonia synthesis. Japan's regulatory framework emphasizes energy efficiency through its Top Runner Program, which has implications for ammonia production technologies. India has implemented the Perform, Achieve and Trade (PAT) scheme that sets energy efficiency targets for ammonia producers.
Certification standards such as ISO 14001 for environmental management systems and ISO 50001 for energy management have become de facto regulatory requirements for global market access. Industry-specific standards from organizations like the International Fertilizer Association provide technical guidelines that influence regulatory compliance strategies.
Emerging regulatory trends indicate a shift toward lifecycle assessment approaches, where the entire environmental impact of ammonia production is considered from raw material extraction through end-use. This trend favors electrolytic methods when powered by renewable energy sources. Carbon border adjustment mechanisms being developed in several jurisdictions will likely impact the competitiveness of different ammonia production methods based on their carbon intensity, potentially creating market advantages for low-carbon electrolytic processes over traditional catalytic methods.
In the United States, the Environmental Protection Agency (EPA) regulates ammonia production under the Clean Air Act and Clean Water Act, with specific provisions for nitrogen oxide emissions and wastewater discharge from production facilities. The Occupational Safety and Health Administration (OSHA) imposes strict safety protocols for handling ammonia, which affects facility design and operational procedures for both electrolytic and catalytic production methods. The Department of Energy (DOE) offers incentives for energy-efficient ammonia production technologies, currently favoring innovations in electrolytic synthesis.
The European Union implements more stringent regulations through its Industrial Emissions Directive (IED) and the EU Emissions Trading System (EU ETS), which places carbon pricing pressure on traditional Haber-Bosch facilities. The EU's REACH regulation (Registration, Evaluation, Authorization and Restriction of Chemicals) imposes additional compliance requirements for ammonia producers. The European Green Deal has established a regulatory framework that increasingly favors green ammonia production methods, providing competitive advantages to electrolytic technologies.
In Asia, China's 14th Five-Year Plan includes specific targets for reducing carbon intensity in chemical production, including ammonia synthesis. Japan's regulatory framework emphasizes energy efficiency through its Top Runner Program, which has implications for ammonia production technologies. India has implemented the Perform, Achieve and Trade (PAT) scheme that sets energy efficiency targets for ammonia producers.
Certification standards such as ISO 14001 for environmental management systems and ISO 50001 for energy management have become de facto regulatory requirements for global market access. Industry-specific standards from organizations like the International Fertilizer Association provide technical guidelines that influence regulatory compliance strategies.
Emerging regulatory trends indicate a shift toward lifecycle assessment approaches, where the entire environmental impact of ammonia production is considered from raw material extraction through end-use. This trend favors electrolytic methods when powered by renewable energy sources. Carbon border adjustment mechanisms being developed in several jurisdictions will likely impact the competitiveness of different ammonia production methods based on their carbon intensity, potentially creating market advantages for low-carbon electrolytic processes over traditional catalytic methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!