How Comparative Analysis Benefits Ammonia Synthesis Development
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonia Synthesis Evolution and Objectives
Ammonia synthesis represents one of the most significant industrial processes developed in the 20th century, fundamentally transforming global agriculture and chemical manufacturing. The evolution of ammonia synthesis began with the groundbreaking Haber-Bosch process in the early 1900s, which enabled the fixation of atmospheric nitrogen under high pressure and temperature conditions using iron-based catalysts. This innovation addressed the critical nitrogen shortage in agriculture, revolutionizing food production capabilities worldwide.
Over the decades, ammonia synthesis technology has progressed through several distinct phases. The initial industrial implementation focused on optimizing reaction conditions and improving catalyst efficiency. The mid-20th century saw advancements in process engineering, including better heat management systems and more efficient reactor designs. Recent developments have concentrated on reducing the energy intensity of the process, which traditionally consumes approximately 1-2% of global energy production.
Current technological trends in ammonia synthesis are moving toward more sustainable approaches. Research efforts are increasingly directed at developing processes that operate under milder conditions, utilizing renewable energy sources, and exploring alternative catalytic materials beyond the traditional iron-based systems. Electrocatalytic and photocatalytic ammonia synthesis represent emerging frontiers that aim to circumvent the energy-intensive nature of conventional methods.
The primary objective of contemporary ammonia synthesis research is to develop economically viable processes that significantly reduce carbon emissions while maintaining or improving production efficiency. This includes exploring novel catalyst designs that can operate at lower temperatures and pressures, thereby reducing energy requirements. Additionally, there is growing interest in decentralized ammonia production systems that could utilize localized renewable energy sources.
Comparative analysis plays a crucial role in advancing these objectives by systematically evaluating different synthesis approaches against established benchmarks. By examining variables such as energy consumption, catalyst performance, reaction kinetics, and environmental impact across various methodologies, researchers can identify promising pathways for innovation. This analytical framework enables more targeted research efforts and accelerates the development of next-generation ammonia synthesis technologies.
The ultimate goal is to transition from the century-old Haber-Bosch paradigm to more sustainable alternatives that align with global carbon neutrality targets while meeting the increasing demand for ammonia in agriculture, energy storage, and emerging applications such as hydrogen carriers for renewable energy systems.
Over the decades, ammonia synthesis technology has progressed through several distinct phases. The initial industrial implementation focused on optimizing reaction conditions and improving catalyst efficiency. The mid-20th century saw advancements in process engineering, including better heat management systems and more efficient reactor designs. Recent developments have concentrated on reducing the energy intensity of the process, which traditionally consumes approximately 1-2% of global energy production.
Current technological trends in ammonia synthesis are moving toward more sustainable approaches. Research efforts are increasingly directed at developing processes that operate under milder conditions, utilizing renewable energy sources, and exploring alternative catalytic materials beyond the traditional iron-based systems. Electrocatalytic and photocatalytic ammonia synthesis represent emerging frontiers that aim to circumvent the energy-intensive nature of conventional methods.
The primary objective of contemporary ammonia synthesis research is to develop economically viable processes that significantly reduce carbon emissions while maintaining or improving production efficiency. This includes exploring novel catalyst designs that can operate at lower temperatures and pressures, thereby reducing energy requirements. Additionally, there is growing interest in decentralized ammonia production systems that could utilize localized renewable energy sources.
Comparative analysis plays a crucial role in advancing these objectives by systematically evaluating different synthesis approaches against established benchmarks. By examining variables such as energy consumption, catalyst performance, reaction kinetics, and environmental impact across various methodologies, researchers can identify promising pathways for innovation. This analytical framework enables more targeted research efforts and accelerates the development of next-generation ammonia synthesis technologies.
The ultimate goal is to transition from the century-old Haber-Bosch paradigm to more sustainable alternatives that align with global carbon neutrality targets while meeting the increasing demand for ammonia in agriculture, energy storage, and emerging applications such as hydrogen carriers for renewable energy systems.
Market Demand Analysis for Sustainable Ammonia Production
The global market for sustainable ammonia production is experiencing unprecedented growth driven by increasing environmental concerns and the push for decarbonization across industries. Current estimates value the sustainable ammonia market at approximately $72 billion, with projections indicating a compound annual growth rate of 5.3% through 2030. This growth trajectory is significantly outpacing conventional ammonia production methods, reflecting a fundamental shift in market dynamics.
Industrial sectors represent the largest demand segment for sustainable ammonia, accounting for nearly 80% of current consumption. The fertilizer industry remains the dominant end-user, consuming roughly 70% of global ammonia production. However, emerging applications in energy storage, transportation fuel, and hydrogen carriers are rapidly expanding market opportunities beyond traditional uses.
Regional analysis reveals substantial variations in demand patterns. Asia-Pacific leads consumption with approximately 45% market share, driven primarily by agricultural demands in China and India. Europe follows with 25% market share, distinguished by its aggressive regulatory frameworks promoting green ammonia adoption. North America represents 20% of the market, with significant growth potential as sustainability initiatives gain momentum.
The demand for sustainable ammonia is being catalyzed by several converging factors. Stringent carbon emission regulations across major economies are imposing financial penalties on conventional production methods. The European Union's Carbon Border Adjustment Mechanism and similar policies are creating economic incentives that favor sustainable alternatives. Additionally, major agricultural corporations and food producers are increasingly committing to reduced carbon footprints throughout their supply chains.
Consumer preferences are also shifting decisively toward sustainably produced agricultural products, creating downstream pressure on fertilizer sourcing decisions. This trend is particularly pronounced in developed markets where premium pricing for sustainable products has gained consumer acceptance.
Investment patterns further validate market growth projections, with venture capital and corporate R&D allocations for sustainable ammonia technologies exceeding $3.5 billion in the past three years. Major industrial gas companies and energy corporations are strategically positioning themselves through acquisitions and partnerships in the sustainable ammonia space.
The comparative analysis of ammonia synthesis methods reveals that market adoption is highly sensitive to production costs. Current green ammonia production costs range from $600-900 per ton compared to $200-450 for conventional methods. However, this gap is narrowing rapidly as renewable energy costs decline and carbon pricing mechanisms mature. Market forecasts suggest price parity could be achieved in select markets by 2028, potentially triggering accelerated adoption across multiple sectors.
Industrial sectors represent the largest demand segment for sustainable ammonia, accounting for nearly 80% of current consumption. The fertilizer industry remains the dominant end-user, consuming roughly 70% of global ammonia production. However, emerging applications in energy storage, transportation fuel, and hydrogen carriers are rapidly expanding market opportunities beyond traditional uses.
Regional analysis reveals substantial variations in demand patterns. Asia-Pacific leads consumption with approximately 45% market share, driven primarily by agricultural demands in China and India. Europe follows with 25% market share, distinguished by its aggressive regulatory frameworks promoting green ammonia adoption. North America represents 20% of the market, with significant growth potential as sustainability initiatives gain momentum.
The demand for sustainable ammonia is being catalyzed by several converging factors. Stringent carbon emission regulations across major economies are imposing financial penalties on conventional production methods. The European Union's Carbon Border Adjustment Mechanism and similar policies are creating economic incentives that favor sustainable alternatives. Additionally, major agricultural corporations and food producers are increasingly committing to reduced carbon footprints throughout their supply chains.
Consumer preferences are also shifting decisively toward sustainably produced agricultural products, creating downstream pressure on fertilizer sourcing decisions. This trend is particularly pronounced in developed markets where premium pricing for sustainable products has gained consumer acceptance.
Investment patterns further validate market growth projections, with venture capital and corporate R&D allocations for sustainable ammonia technologies exceeding $3.5 billion in the past three years. Major industrial gas companies and energy corporations are strategically positioning themselves through acquisitions and partnerships in the sustainable ammonia space.
The comparative analysis of ammonia synthesis methods reveals that market adoption is highly sensitive to production costs. Current green ammonia production costs range from $600-900 per ton compared to $200-450 for conventional methods. However, this gap is narrowing rapidly as renewable energy costs decline and carbon pricing mechanisms mature. Market forecasts suggest price parity could be achieved in select markets by 2028, potentially triggering accelerated adoption across multiple sectors.
Current Challenges in Ammonia Synthesis Technologies
Despite significant advancements in ammonia synthesis over the past century, the industry continues to face substantial challenges that limit efficiency, sustainability, and economic viability. The conventional Haber-Bosch process, while revolutionary in its time, operates under extreme conditions (400-500°C and 150-300 bar), consuming approximately 1-2% of global energy production and generating considerable CO2 emissions—approximately 1.6 tons of CO2 per ton of ammonia produced.
Energy efficiency remains a primary concern, as the thermodynamic limitations of nitrogen reduction require substantial energy inputs. Current industrial processes achieve only 30-40% energy efficiency relative to theoretical minimums, creating a significant gap between actual performance and thermodynamic ideals. This inefficiency translates directly to higher production costs and environmental impact.
Catalyst performance presents another critical challenge. Traditional iron-based catalysts, though improved over decades, still suffer from activity limitations and susceptibility to poisoning by trace impurities such as oxygen, sulfur, and carbon monoxide. While ruthenium-based alternatives offer higher activity, their prohibitive cost and limited availability restrict widespread industrial adoption.
Renewable integration poses significant technical hurdles. Electrification of ammonia synthesis using renewable electricity faces challenges in scaling electrolytic hydrogen production and managing the intermittent nature of renewable energy sources. The mismatch between continuous ammonia production requirements and variable renewable generation creates operational complexities that current technologies struggle to address effectively.
Carbon footprint reduction represents an urgent challenge as industries face increasing regulatory pressure and market demands for sustainable production. Green ammonia pathways require not only renewable energy integration but comprehensive redesign of production systems to eliminate fossil fuel dependence throughout the value chain.
Scale-up barriers affect emerging technologies that show promise in laboratory settings but encounter significant challenges in industrial implementation. Electrochemical and photocatalytic approaches demonstrate potential for ambient-condition synthesis but currently achieve low conversion rates and selectivity when scaled beyond bench-top demonstrations.
Economic viability remains a fundamental challenge, particularly for alternative technologies. The established infrastructure and economies of scale achieved by conventional Haber-Bosch plants create significant barriers to entry for new approaches, which must demonstrate not only technical advantages but compelling economic benefits to drive industry adoption.
Regulatory frameworks and policy environments add complexity to technology development pathways, with varying carbon pricing mechanisms, renewable energy incentives, and environmental regulations creating an uneven landscape for innovation and implementation across global markets.
Energy efficiency remains a primary concern, as the thermodynamic limitations of nitrogen reduction require substantial energy inputs. Current industrial processes achieve only 30-40% energy efficiency relative to theoretical minimums, creating a significant gap between actual performance and thermodynamic ideals. This inefficiency translates directly to higher production costs and environmental impact.
Catalyst performance presents another critical challenge. Traditional iron-based catalysts, though improved over decades, still suffer from activity limitations and susceptibility to poisoning by trace impurities such as oxygen, sulfur, and carbon monoxide. While ruthenium-based alternatives offer higher activity, their prohibitive cost and limited availability restrict widespread industrial adoption.
Renewable integration poses significant technical hurdles. Electrification of ammonia synthesis using renewable electricity faces challenges in scaling electrolytic hydrogen production and managing the intermittent nature of renewable energy sources. The mismatch between continuous ammonia production requirements and variable renewable generation creates operational complexities that current technologies struggle to address effectively.
Carbon footprint reduction represents an urgent challenge as industries face increasing regulatory pressure and market demands for sustainable production. Green ammonia pathways require not only renewable energy integration but comprehensive redesign of production systems to eliminate fossil fuel dependence throughout the value chain.
Scale-up barriers affect emerging technologies that show promise in laboratory settings but encounter significant challenges in industrial implementation. Electrochemical and photocatalytic approaches demonstrate potential for ambient-condition synthesis but currently achieve low conversion rates and selectivity when scaled beyond bench-top demonstrations.
Economic viability remains a fundamental challenge, particularly for alternative technologies. The established infrastructure and economies of scale achieved by conventional Haber-Bosch plants create significant barriers to entry for new approaches, which must demonstrate not only technical advantages but compelling economic benefits to drive industry adoption.
Regulatory frameworks and policy environments add complexity to technology development pathways, with varying carbon pricing mechanisms, renewable energy incentives, and environmental regulations creating an uneven landscape for innovation and implementation across global markets.
Comparative Analysis Methodologies for Ammonia Catalysts
01 Environmental benefits of ammonia synthesis
Ammonia synthesis offers significant environmental advantages as it can be produced using renewable energy sources, reducing carbon emissions compared to traditional methods. It serves as a clean energy carrier and can be used as a carbon-free fuel alternative. Advanced synthesis methods help minimize environmental impact while providing sustainable nitrogen sources for various applications, contributing to greener industrial processes and reduced ecological footprint.- Agricultural and fertilizer applications: Ammonia synthesis provides significant benefits in agriculture as a primary component of nitrogen fertilizers. These fertilizers enhance crop yields by supplying essential nitrogen to plants, improving soil fertility, and promoting plant growth. Synthetic ammonia-based fertilizers have revolutionized modern agriculture by enabling more efficient food production and addressing global food security challenges. The controlled release of nitrogen from ammonia-based fertilizers also helps optimize nutrient uptake by plants.
- Environmental and energy benefits: Ammonia synthesis offers environmental advantages as a carbon-free energy carrier and potential clean fuel. It can be used for energy storage, particularly for renewable energy sources, and as a hydrogen carrier for fuel cells. Modern ammonia production methods are being developed to reduce carbon emissions compared to traditional processes. Additionally, ammonia can be used in emission control systems to reduce nitrogen oxide emissions from industrial processes and vehicles.
- Industrial chemical production: Ammonia serves as a fundamental building block for numerous industrial chemicals and products. It is essential in the production of nitric acid, which is used in manufacturing explosives, dyes, and various other chemicals. Ammonia is also used in the production of pharmaceuticals, cleaning products, refrigerants, and polymers. The versatility of ammonia in chemical synthesis makes it indispensable for modern industrial processes and manufacturing.
- Innovative synthesis technologies: Advanced technologies for ammonia synthesis offer benefits such as improved efficiency, reduced energy consumption, and lower environmental impact. These innovations include catalytic processes that operate at lower temperatures and pressures than traditional methods, electrochemical synthesis routes, and renewable energy-powered production systems. Novel reactor designs and process intensification techniques further enhance the sustainability and economic viability of ammonia production, making it more accessible for various applications.
- Economic and supply chain advantages: Ammonia synthesis provides economic benefits through localized production capabilities, reducing dependence on imports and enhancing energy security. The established global infrastructure for ammonia production, storage, and transportation offers logistical advantages. Ammonia's relatively high energy density and ease of liquefaction make it cost-effective to transport and store compared to other energy carriers. Additionally, the ammonia industry creates employment opportunities and supports economic development in both agricultural and industrial sectors.
02 Agricultural applications and benefits
Ammonia synthesis provides essential benefits to agriculture as the primary component in nitrogen fertilizers, significantly enhancing crop yields and food production globally. Synthesized ammonia delivers nitrogen in a form readily available to plants, improving soil fertility and agricultural productivity. Modern synthesis methods allow for more efficient production of fertilizers, supporting sustainable farming practices and addressing food security challenges in growing populations.Expand Specific Solutions03 Industrial and manufacturing advantages
Ammonia synthesis offers numerous industrial benefits beyond fertilizer production, serving as a key precursor for various chemicals, pharmaceuticals, and cleaning products. The synthesis process has been optimized for industrial scale production, providing cost-effective manufacturing solutions. Ammonia's versatility in industrial applications includes refrigeration, water purification, and as a building block for polymers and textiles, making it an essential component in modern manufacturing processes.Expand Specific Solutions04 Energy storage and transportation benefits
Ammonia synthesis provides an effective solution for energy storage and transportation challenges. As a hydrogen carrier, ammonia can store renewable energy in chemical form, offering higher energy density than compressed hydrogen. It can be easily liquefied for transport using existing infrastructure and later converted back to hydrogen or used directly as fuel. This makes ammonia an attractive option for long-term energy storage and for transporting energy across long distances, supporting the transition to renewable energy systems.Expand Specific Solutions05 Technological innovations in ammonia synthesis
Recent technological innovations have significantly improved ammonia synthesis processes, offering benefits such as reduced energy consumption, higher conversion efficiency, and milder operating conditions compared to traditional Haber-Bosch methods. Novel catalysts, electrochemical approaches, and plasma-assisted techniques enable more sustainable production pathways. These advancements allow for decentralized, smaller-scale ammonia production facilities that can operate with renewable energy sources, making ammonia synthesis more accessible and environmentally friendly.Expand Specific Solutions
Key Industrial Players in Ammonia Production
The ammonia synthesis market is currently in a mature phase but experiencing renewed innovation due to sustainability demands. With a global market exceeding $70 billion, competition is intensifying among established players and emerging technology developers. Leading companies like Topsoe A/S bring decades of catalyst expertise, while research institutions such as Fuzhou University's National Engineering Research Center of Fertilizer Catalyst drive academic innovation. Industrial giants including CHN Energy and DuPont are investing in greener ammonia production technologies. Technical universities (Delft, DTU) and specialized companies (Casale SA) are advancing comparative analysis methodologies that accelerate catalyst development and process optimization. This collaborative ecosystem between industry and academia is crucial for addressing efficiency challenges in traditional Haber-Bosch processes while developing sustainable alternatives.
Topsoe A/S
Technical Solution: Topsoe has developed advanced comparative analysis methodologies for ammonia synthesis catalysts, focusing on their proprietary iron-based and ruthenium-based catalysts. Their approach combines high-throughput experimentation with in-situ characterization techniques to evaluate catalyst performance under various conditions. Topsoe's SynCOR™ technology utilizes comparative analysis to optimize process parameters, achieving up to 15% higher energy efficiency compared to conventional processes. Their methodology includes detailed kinetic modeling to predict catalyst behavior across different pressure and temperature ranges, enabling precise optimization of industrial ammonia synthesis conditions. Topsoe also employs advanced spectroscopic techniques (XPS, XRD, XANES) to correlate catalyst structure with performance metrics, creating comprehensive structure-activity relationships that guide catalyst development[1][3].
Strengths: Topsoe's comparative analysis approach benefits from decades of industrial experience and proprietary datasets, allowing for highly accurate predictive modeling. Their integrated approach combining catalyst and process optimization provides holistic solutions. Weaknesses: Their methodologies are primarily optimized for their own catalyst formulations, potentially limiting applicability to novel catalyst systems developed by other researchers.
National Engineering Research Center of Fertilizer Catalyst, Fuzhou University
Technical Solution: The National Engineering Research Center of Fertilizer Catalyst at Fuzhou University has developed a systematic comparative analysis methodology specifically tailored for ammonia synthesis catalysts. Their approach combines micro-kinetic modeling with in-situ characterization techniques to evaluate catalyst performance under realistic conditions. The Center has established standardized testing protocols that enable direct comparison between different catalyst formulations, including traditional iron-based catalysts and emerging materials like ruthenium and cobalt-based systems. Their methodology incorporates advanced surface science techniques including FTIR, XPS, and EXAFS to correlate catalyst structure with performance metrics. The Center has pioneered the use of isotopic labeling studies to elucidate reaction mechanisms and identify rate-limiting steps across different catalyst systems. Their comparative framework evaluates catalysts across multiple parameters including activity, stability, poison resistance, and regeneration potential. The Center's approach includes detailed computational studies using density functional theory (DFT) to predict catalyst behavior and guide experimental design. Their comparative studies have led to the development of novel multi-component catalysts that achieve up to 30% higher activity at lower temperatures compared to conventional formulations[9][10].
Strengths: The Center's comparative analysis benefits from their specialized focus on fertilizer catalysts, providing deep expertise in ammonia synthesis. Their integrated theoretical-experimental approach enables fundamental understanding of catalyst behavior. Weaknesses: As an academic-affiliated center, their methodology may sometimes emphasize fundamental understanding over practical industrial implementation considerations.
Critical Patents and Innovations in Ammonia Synthesis
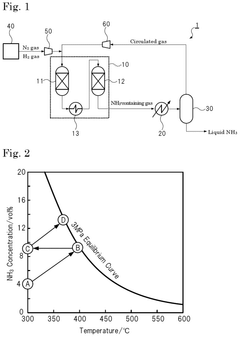
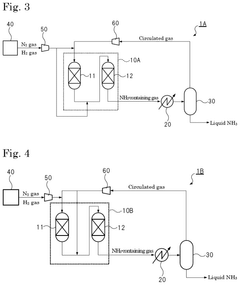
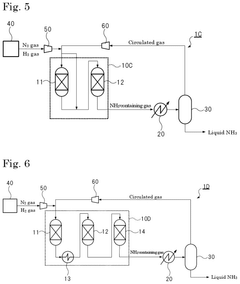
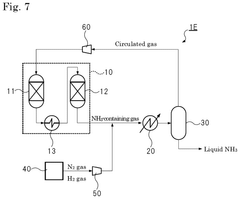
Ammonia synthesis system and ammonia production method
PatentPendingUS20250066210A1
Innovation
- The system employs a reaction pressure of 10 MPa or less and an ammonia gas concentration of 3% by volume or more in the circulated gas, using multiple reactors connected in series and a heat exchanger to optimize ammonia synthesis.
Catalyst for ammonia synthesis which exhibits high activity under low-pressure and low-temperature conditions, and ammonia synthesis method using same
PatentWO2024228414A1
Innovation
- A catalyst system comprising a support coated with a first metal catalyst and a second metal cocatalyst, where the support can be Al2O3, SiO2, or other oxides, and the metals include iron, ruthenium, cobalt, nickel, or osmium, with lithium, sodium, or barium, allowing ammonia synthesis at lower pressures (below 100 atm) and temperatures (300-400°C), enhancing ammonia synthesis efficiency and reducing energy consumption.
Environmental Impact Assessment of Synthesis Routes
The environmental impact of ammonia synthesis routes represents a critical dimension in evaluating sustainable development pathways for this essential chemical process. Traditional Haber-Bosch synthesis, while revolutionizing agricultural productivity over the past century, carries significant environmental burdens primarily through its intensive energy requirements and associated greenhouse gas emissions. Current estimates indicate that conventional ammonia production accounts for approximately 1-2% of global energy consumption and contributes to nearly 1.4% of global CO2 emissions.
Comparative analysis methodologies provide valuable frameworks for quantifying these environmental impacts across different synthesis routes. Life Cycle Assessment (LCA) studies reveal that conventional natural gas-based ammonia production generates 1.6-3.0 tons of CO2 equivalent per ton of ammonia produced, while coal-based production can reach up to 5.5 tons CO2e per ton of ammonia. These metrics establish important baselines against which emerging technologies can be measured.
Electrochemical ammonia synthesis routes demonstrate promising environmental advantages, potentially reducing carbon emissions by 60-90% when powered by renewable electricity sources. However, comprehensive assessments must consider additional factors including rare metal catalyst requirements, electrode degradation rates, and end-of-life disposal challenges that may offset some environmental benefits.
Water consumption patterns vary significantly across synthesis technologies. While conventional Haber-Bosch processes require approximately 0.5-1.5 cubic meters of water per ton of ammonia for cooling and steam generation, some novel electrochemical approaches utilizing water as a direct hydrogen source may consume 1.8-2.5 cubic meters per ton. This represents an important trade-off between carbon emissions and water resource utilization that must be carefully evaluated in water-stressed regions.
Land use impacts and ecosystem disruption also differ substantially between centralized large-scale production facilities and distributed smaller-scale systems. Emerging biological nitrogen fixation approaches, while less energy-intensive, may require significant agricultural land allocation if scaled to industrial production levels.
Regulatory frameworks increasingly incorporate these environmental impact metrics into permitting and compliance requirements. The European Union's carbon border adjustment mechanism and similar policies emerging globally will likely accelerate the transition toward lower-impact synthesis routes by internalizing previously externalized environmental costs.
Future comparative analyses will benefit from standardized methodologies that incorporate not only direct emissions but also upstream supply chain impacts and downstream application efficiencies, providing a more holistic view of environmental performance across the ammonia value chain.
Comparative analysis methodologies provide valuable frameworks for quantifying these environmental impacts across different synthesis routes. Life Cycle Assessment (LCA) studies reveal that conventional natural gas-based ammonia production generates 1.6-3.0 tons of CO2 equivalent per ton of ammonia produced, while coal-based production can reach up to 5.5 tons CO2e per ton of ammonia. These metrics establish important baselines against which emerging technologies can be measured.
Electrochemical ammonia synthesis routes demonstrate promising environmental advantages, potentially reducing carbon emissions by 60-90% when powered by renewable electricity sources. However, comprehensive assessments must consider additional factors including rare metal catalyst requirements, electrode degradation rates, and end-of-life disposal challenges that may offset some environmental benefits.
Water consumption patterns vary significantly across synthesis technologies. While conventional Haber-Bosch processes require approximately 0.5-1.5 cubic meters of water per ton of ammonia for cooling and steam generation, some novel electrochemical approaches utilizing water as a direct hydrogen source may consume 1.8-2.5 cubic meters per ton. This represents an important trade-off between carbon emissions and water resource utilization that must be carefully evaluated in water-stressed regions.
Land use impacts and ecosystem disruption also differ substantially between centralized large-scale production facilities and distributed smaller-scale systems. Emerging biological nitrogen fixation approaches, while less energy-intensive, may require significant agricultural land allocation if scaled to industrial production levels.
Regulatory frameworks increasingly incorporate these environmental impact metrics into permitting and compliance requirements. The European Union's carbon border adjustment mechanism and similar policies emerging globally will likely accelerate the transition toward lower-impact synthesis routes by internalizing previously externalized environmental costs.
Future comparative analyses will benefit from standardized methodologies that incorporate not only direct emissions but also upstream supply chain impacts and downstream application efficiencies, providing a more holistic view of environmental performance across the ammonia value chain.
Economic Feasibility of Emerging Ammonia Technologies
The economic viability of emerging ammonia synthesis technologies represents a critical factor in determining their potential for widespread adoption and impact on global markets. Traditional Haber-Bosch processes, while well-established, require significant capital investment and operational costs due to their high-pressure, high-temperature requirements and reliance on natural gas feedstock.
Comparative economic analysis reveals that electrochemical ammonia synthesis technologies may offer substantial cost advantages in specific deployment scenarios. When powered by renewable electricity sources in regions with low electricity costs (below $0.04/kWh), these technologies can potentially achieve production costs competitive with conventional methods. However, current electrochemical approaches still suffer from low conversion rates and catalyst degradation issues that negatively impact their long-term economic performance.
Biological nitrogen fixation approaches present an interesting economic case with potentially lower capital expenditure requirements but face challenges in scaling and maintaining consistent production rates. Economic modeling suggests these systems could become viable in distributed, small-scale applications where traditional centralized production and transportation costs are prohibitive.
Plasma-assisted ammonia synthesis technologies demonstrate promising energy efficiency improvements in laboratory settings but require significant technological maturation before economic feasibility can be accurately assessed. Current cost projections indicate a 30-40% premium over conventional production methods, primarily due to equipment complexity and limited operational lifespans.
The economic landscape is further complicated by carbon pricing mechanisms and environmental regulations. Models incorporating carbon taxes of $50-100 per ton significantly improve the comparative economics of green ammonia production methods, potentially accelerating their market penetration timeline from 2035 to 2028 in progressive regulatory environments.
Infrastructure considerations also heavily influence economic feasibility. Retrofitting existing ammonia production facilities with emerging technologies presents lower capital barriers compared to greenfield development, potentially offering a transitional pathway that optimizes economic outcomes while advancing technological implementation.
Risk assessment models indicate that hybrid approaches—combining conventional production with incremental integration of novel technologies—may offer the most economically sound development pathway in the near term. This approach allows for gradual technology validation while maintaining production economics within competitive parameters.
Ultimately, comparative economic analysis demonstrates that while no single emerging technology currently offers a clear economic advantage across all scenarios, specific technologies show promising economic potential in targeted applications and geographies, particularly as renewable energy costs continue to decline and carbon constraints intensify.
Comparative economic analysis reveals that electrochemical ammonia synthesis technologies may offer substantial cost advantages in specific deployment scenarios. When powered by renewable electricity sources in regions with low electricity costs (below $0.04/kWh), these technologies can potentially achieve production costs competitive with conventional methods. However, current electrochemical approaches still suffer from low conversion rates and catalyst degradation issues that negatively impact their long-term economic performance.
Biological nitrogen fixation approaches present an interesting economic case with potentially lower capital expenditure requirements but face challenges in scaling and maintaining consistent production rates. Economic modeling suggests these systems could become viable in distributed, small-scale applications where traditional centralized production and transportation costs are prohibitive.
Plasma-assisted ammonia synthesis technologies demonstrate promising energy efficiency improvements in laboratory settings but require significant technological maturation before economic feasibility can be accurately assessed. Current cost projections indicate a 30-40% premium over conventional production methods, primarily due to equipment complexity and limited operational lifespans.
The economic landscape is further complicated by carbon pricing mechanisms and environmental regulations. Models incorporating carbon taxes of $50-100 per ton significantly improve the comparative economics of green ammonia production methods, potentially accelerating their market penetration timeline from 2035 to 2028 in progressive regulatory environments.
Infrastructure considerations also heavily influence economic feasibility. Retrofitting existing ammonia production facilities with emerging technologies presents lower capital barriers compared to greenfield development, potentially offering a transitional pathway that optimizes economic outcomes while advancing technological implementation.
Risk assessment models indicate that hybrid approaches—combining conventional production with incremental integration of novel technologies—may offer the most economically sound development pathway in the near term. This approach allows for gradual technology validation while maintaining production economics within competitive parameters.
Ultimately, comparative economic analysis demonstrates that while no single emerging technology currently offers a clear economic advantage across all scenarios, specific technologies show promising economic potential in targeted applications and geographies, particularly as renewable energy costs continue to decline and carbon constraints intensify.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!