Examination of Electrolyte Use in Sustainable Ammonia Production
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolyte Technology Background and Objectives
Electrolyte technology has evolved significantly over the past century, with major advancements occurring in the last three decades due to increasing focus on sustainable energy solutions. The development of electrolytes for ammonia production represents a critical intersection between traditional chemical engineering and modern electrochemical approaches. Historically, ammonia production has been dominated by the Haber-Bosch process, which consumes approximately 1-2% of global energy and contributes significantly to greenhouse gas emissions.
The evolution of electrolyte technology for ammonia synthesis began with basic aqueous solutions in the early 20th century, progressing through various ionic liquids in the 1990s, and now advancing toward specialized proton-conducting membranes and solid-state electrolytes. This progression reflects the broader trend toward more energy-efficient and environmentally sustainable chemical production methods.
Current technological objectives in electrolyte development for sustainable ammonia production focus on several key parameters: increasing ionic conductivity, enhancing selectivity for nitrogen reduction reactions, improving stability under operating conditions, and reducing manufacturing costs. The ideal electrolyte system would facilitate efficient nitrogen reduction to ammonia at ambient temperatures and pressures, representing a paradigm shift from conventional high-temperature, high-pressure processes.
Research indicates that electrolyte composition significantly impacts reaction kinetics, with proton availability and nitrogen solubility being critical factors. Recent breakthroughs in polymer-based and ceramic electrolytes have demonstrated promising nitrogen conversion efficiencies, though challenges remain in achieving commercially viable production rates.
The technological trajectory suggests convergence toward hybrid electrolyte systems that combine the advantages of different materials. These may include composite structures with distinct functional layers or gradient compositions that optimize both conductivity and stability. Such innovations could potentially reduce the energy requirement for ammonia synthesis by 30-45% compared to conventional methods.
Global research efforts are increasingly focused on developing electrolytes that can operate effectively with renewable electricity sources, thereby creating truly sustainable ammonia production pathways. This alignment with renewable energy integration represents a critical objective, as it would enable the decarbonization of not only ammonia production but also its derivative industries including fertilizers, chemicals, and potentially energy storage and transport.
The ultimate technological goal remains the development of an electrolyte system that enables distributed, small-scale ammonia production powered by renewable electricity. Such a breakthrough would revolutionize agricultural practices by localizing fertilizer production, reducing transportation emissions, and increasing accessibility in remote agricultural regions.
The evolution of electrolyte technology for ammonia synthesis began with basic aqueous solutions in the early 20th century, progressing through various ionic liquids in the 1990s, and now advancing toward specialized proton-conducting membranes and solid-state electrolytes. This progression reflects the broader trend toward more energy-efficient and environmentally sustainable chemical production methods.
Current technological objectives in electrolyte development for sustainable ammonia production focus on several key parameters: increasing ionic conductivity, enhancing selectivity for nitrogen reduction reactions, improving stability under operating conditions, and reducing manufacturing costs. The ideal electrolyte system would facilitate efficient nitrogen reduction to ammonia at ambient temperatures and pressures, representing a paradigm shift from conventional high-temperature, high-pressure processes.
Research indicates that electrolyte composition significantly impacts reaction kinetics, with proton availability and nitrogen solubility being critical factors. Recent breakthroughs in polymer-based and ceramic electrolytes have demonstrated promising nitrogen conversion efficiencies, though challenges remain in achieving commercially viable production rates.
The technological trajectory suggests convergence toward hybrid electrolyte systems that combine the advantages of different materials. These may include composite structures with distinct functional layers or gradient compositions that optimize both conductivity and stability. Such innovations could potentially reduce the energy requirement for ammonia synthesis by 30-45% compared to conventional methods.
Global research efforts are increasingly focused on developing electrolytes that can operate effectively with renewable electricity sources, thereby creating truly sustainable ammonia production pathways. This alignment with renewable energy integration represents a critical objective, as it would enable the decarbonization of not only ammonia production but also its derivative industries including fertilizers, chemicals, and potentially energy storage and transport.
The ultimate technological goal remains the development of an electrolyte system that enables distributed, small-scale ammonia production powered by renewable electricity. Such a breakthrough would revolutionize agricultural practices by localizing fertilizer production, reducing transportation emissions, and increasing accessibility in remote agricultural regions.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing a significant shift towards sustainable production methods, driven by increasing environmental concerns and regulatory pressures. Traditional ammonia production via the Haber-Bosch process accounts for approximately 1.8% of global CO2 emissions, creating an urgent need for greener alternatives. The sustainable ammonia market, valued at $15.7 billion in 2022, is projected to grow at a CAGR of 9.5% through 2030, reflecting strong demand across agricultural, industrial, and emerging energy storage applications.
Electrolyte-based ammonia production represents a promising segment within this market, with particular growth potential in regions with abundant renewable energy resources. Countries like Australia, Chile, and Morocco are positioning themselves as future green ammonia hubs due to their exceptional solar and wind energy potential, which can power electrolytic processes efficiently.
The agricultural sector remains the dominant consumer of ammonia, accounting for over 80% of global demand primarily as fertilizer. However, sustainable ammonia is gaining traction in new applications, particularly as a carbon-free energy carrier and potential shipping fuel. Major maritime organizations have identified ammonia as one of the most viable alternatives to conventional marine fuels, potentially creating a market demand of 30 million tons annually by 2040.
Industrial demand for green ammonia is also expanding in sectors requiring nitrogen compounds, including pharmaceuticals, cleaning products, and refrigeration. The price premium for sustainable ammonia remains a market challenge, with current production costs 2-3 times higher than conventional methods. However, this gap is expected to narrow significantly by 2030 as electrolysis technologies mature and achieve economies of scale.
Regional market analysis reveals that Europe leads in sustainable ammonia initiatives, driven by stringent carbon regulations and substantial renewable energy investments. The Asia-Pacific region, particularly China, Japan, and South Korea, represents the fastest-growing market segment with numerous pilot projects underway and strong government support for decarbonization technologies.
Market barriers include high capital expenditure requirements for electrolysis facilities, energy efficiency challenges, and competition from blue ammonia (produced from natural gas with carbon capture). Despite these challenges, investor confidence in sustainable ammonia technologies is growing, with venture capital funding in the sector exceeding $800 million in 2022, a 65% increase from the previous year.
The competitive landscape features both established chemical companies pivoting towards green technologies and innovative startups focused exclusively on electrolyte-based ammonia production methods. Strategic partnerships between technology developers, renewable energy providers, and end-users are becoming increasingly common, accelerating commercialization timelines and market adoption.
Electrolyte-based ammonia production represents a promising segment within this market, with particular growth potential in regions with abundant renewable energy resources. Countries like Australia, Chile, and Morocco are positioning themselves as future green ammonia hubs due to their exceptional solar and wind energy potential, which can power electrolytic processes efficiently.
The agricultural sector remains the dominant consumer of ammonia, accounting for over 80% of global demand primarily as fertilizer. However, sustainable ammonia is gaining traction in new applications, particularly as a carbon-free energy carrier and potential shipping fuel. Major maritime organizations have identified ammonia as one of the most viable alternatives to conventional marine fuels, potentially creating a market demand of 30 million tons annually by 2040.
Industrial demand for green ammonia is also expanding in sectors requiring nitrogen compounds, including pharmaceuticals, cleaning products, and refrigeration. The price premium for sustainable ammonia remains a market challenge, with current production costs 2-3 times higher than conventional methods. However, this gap is expected to narrow significantly by 2030 as electrolysis technologies mature and achieve economies of scale.
Regional market analysis reveals that Europe leads in sustainable ammonia initiatives, driven by stringent carbon regulations and substantial renewable energy investments. The Asia-Pacific region, particularly China, Japan, and South Korea, represents the fastest-growing market segment with numerous pilot projects underway and strong government support for decarbonization technologies.
Market barriers include high capital expenditure requirements for electrolysis facilities, energy efficiency challenges, and competition from blue ammonia (produced from natural gas with carbon capture). Despite these challenges, investor confidence in sustainable ammonia technologies is growing, with venture capital funding in the sector exceeding $800 million in 2022, a 65% increase from the previous year.
The competitive landscape features both established chemical companies pivoting towards green technologies and innovative startups focused exclusively on electrolyte-based ammonia production methods. Strategic partnerships between technology developers, renewable energy providers, and end-users are becoming increasingly common, accelerating commercialization timelines and market adoption.
Current Electrolyte Challenges in Green Ammonia Synthesis
The electrolyte system represents a critical component in electrochemical ammonia synthesis, serving as the medium for ion transport between electrodes. Current electrolyte technologies face significant challenges that limit the efficiency and scalability of sustainable ammonia production processes. Conventional aqueous electrolytes struggle with nitrogen solubility limitations, competing hydrogen evolution reactions, and electrode degradation issues, resulting in low Faradaic efficiency for ammonia production.
Solid-state electrolytes, while promising for their selectivity, suffer from poor ionic conductivity at operational temperatures and mechanical stability issues during cycling. These limitations create substantial barriers to achieving commercially viable electrochemical ammonia synthesis rates above 10^-6 mol cm^-2 s^-1, which remains orders of magnitude below industrial requirements.
Proton-conducting polymer electrolytes represent another approach but face challenges with limited temperature stability and insufficient mechanical properties for long-term operation. The degradation of these materials under the harsh electrochemical conditions required for nitrogen reduction further complicates their implementation in industrial settings.
Ionic liquid electrolytes have garnered attention for their tunable properties and wide electrochemical windows. However, their high viscosity often leads to mass transport limitations, while their cost and potential environmental impacts raise concerns about large-scale deployment. Additionally, many ionic liquids demonstrate insufficient stability under the reducing conditions necessary for ammonia synthesis.
The interface between electrolyte and catalyst presents another critical challenge, as electrolyte composition significantly influences catalyst activity and selectivity. Current electrolyte systems often fail to provide optimal conditions for catalyst performance, resulting in competitive side reactions and reduced ammonia yield.
Temperature management represents a significant challenge across all electrolyte systems. While higher temperatures can improve reaction kinetics and ion mobility, they often accelerate electrolyte degradation and ammonia re-oxidation, creating a complex optimization problem that remains unsolved in current systems.
Scaling electrolyte technologies from laboratory to industrial scale introduces additional challenges related to cost, stability, and environmental impact. Many promising electrolyte systems that perform well in controlled laboratory environments fail to maintain performance under the demanding conditions of continuous industrial operation.
The development of advanced characterization techniques for in-situ monitoring of electrolyte properties during operation remains underdeveloped, limiting researchers' ability to understand degradation mechanisms and optimize electrolyte formulations for long-term stability and performance in sustainable ammonia production systems.
Solid-state electrolytes, while promising for their selectivity, suffer from poor ionic conductivity at operational temperatures and mechanical stability issues during cycling. These limitations create substantial barriers to achieving commercially viable electrochemical ammonia synthesis rates above 10^-6 mol cm^-2 s^-1, which remains orders of magnitude below industrial requirements.
Proton-conducting polymer electrolytes represent another approach but face challenges with limited temperature stability and insufficient mechanical properties for long-term operation. The degradation of these materials under the harsh electrochemical conditions required for nitrogen reduction further complicates their implementation in industrial settings.
Ionic liquid electrolytes have garnered attention for their tunable properties and wide electrochemical windows. However, their high viscosity often leads to mass transport limitations, while their cost and potential environmental impacts raise concerns about large-scale deployment. Additionally, many ionic liquids demonstrate insufficient stability under the reducing conditions necessary for ammonia synthesis.
The interface between electrolyte and catalyst presents another critical challenge, as electrolyte composition significantly influences catalyst activity and selectivity. Current electrolyte systems often fail to provide optimal conditions for catalyst performance, resulting in competitive side reactions and reduced ammonia yield.
Temperature management represents a significant challenge across all electrolyte systems. While higher temperatures can improve reaction kinetics and ion mobility, they often accelerate electrolyte degradation and ammonia re-oxidation, creating a complex optimization problem that remains unsolved in current systems.
Scaling electrolyte technologies from laboratory to industrial scale introduces additional challenges related to cost, stability, and environmental impact. Many promising electrolyte systems that perform well in controlled laboratory environments fail to maintain performance under the demanding conditions of continuous industrial operation.
The development of advanced characterization techniques for in-situ monitoring of electrolyte properties during operation remains underdeveloped, limiting researchers' ability to understand degradation mechanisms and optimize electrolyte formulations for long-term stability and performance in sustainable ammonia production systems.
Current Electrolyte Solutions for Sustainable Ammonia
01 Sustainable electrolyte materials and compositions
Development of sustainable electrolyte materials focuses on environmentally friendly compositions that reduce reliance on scarce resources. These innovations include bio-derived electrolytes, recyclable components, and materials with reduced environmental impact. Such sustainable compositions maintain or enhance electrochemical performance while minimizing ecological footprint throughout their lifecycle.- Sustainable electrolyte materials for batteries: Development of sustainable electrolyte materials for batteries focuses on environmentally friendly alternatives that reduce dependency on rare or toxic components. These innovations include bio-derived electrolytes, water-based solutions, and materials with reduced environmental impact. Such sustainable electrolytes maintain or enhance battery performance while addressing concerns about resource depletion and end-of-life disposal issues.
- Electrolyte recycling and recovery systems: Systems and methods for recycling and recovering electrolytes from used batteries and other electrochemical devices help extend material lifecycles. These approaches include extraction techniques, purification processes, and reintegration methods that allow electrolyte components to be reused in new products. Such recycling systems reduce waste, conserve resources, and decrease the environmental footprint of electrolyte production.
- Energy management systems for electrolyte sustainability: Energy management systems optimize the use of electrolytes in various applications by monitoring and controlling operational parameters. These systems employ sensors, data analytics, and control algorithms to extend electrolyte lifespan, reduce degradation, and minimize waste. By optimizing operating conditions and usage patterns, these technologies enhance the sustainability of electrolyte-based systems while maintaining performance efficiency.
- Monitoring and diagnostic tools for electrolyte performance: Advanced monitoring and diagnostic tools assess electrolyte health and performance in real-time, enabling predictive maintenance and timely interventions. These technologies use sensors, spectroscopic methods, and data analysis to detect degradation, contamination, or imbalances before they cause system failure. By extending electrolyte functional lifespan and preventing premature replacement, these tools contribute significantly to sustainability goals.
- Sustainable electrolyte production processes: Innovative manufacturing processes for electrolytes focus on reducing energy consumption, minimizing waste generation, and utilizing renewable resources. These approaches include green chemistry principles, solvent-free synthesis, and continuous flow production methods that decrease environmental impact. By redesigning production pathways, these technologies address sustainability concerns throughout the electrolyte lifecycle from raw material sourcing to manufacturing.
02 Energy management systems for electrolyte sustainability
Advanced energy management systems optimize electrolyte usage and longevity in battery and energy storage applications. These systems monitor electrolyte conditions, regulate charging/discharging cycles, and implement predictive maintenance to extend electrolyte life. By maximizing efficiency and minimizing degradation, these technologies significantly improve the sustainability of electrolyte-based energy systems.Expand Specific Solutions03 Recycling and recovery processes for electrolytes
Innovative methods for recycling and recovering electrolytes from spent batteries and other electrochemical devices help close the material loop. These processes extract valuable components from used electrolytes, purify them, and prepare them for reuse in new applications. Such recycling technologies reduce waste, conserve resources, and decrease the environmental impact of electrolyte production.Expand Specific Solutions04 Digital monitoring and analytics for electrolyte performance
Digital technologies enable real-time monitoring and analytics of electrolyte performance and degradation. These systems use sensors, data analysis, and artificial intelligence to track electrolyte conditions, predict failures, and optimize operating parameters. By providing actionable insights, these technologies help maintain electrolyte health, extend useful life, and improve overall sustainability of electrochemical systems.Expand Specific Solutions05 Lifecycle assessment and sustainability metrics for electrolytes
Comprehensive frameworks for assessing the environmental impact of electrolytes throughout their lifecycle help identify sustainability improvements. These approaches evaluate resource extraction, manufacturing processes, use phase efficiency, and end-of-life management. By quantifying environmental impacts with standardized metrics, manufacturers and users can make informed decisions to enhance the overall sustainability of electrolyte technologies.Expand Specific Solutions
Key Industry Players in Electrolyte-Based Ammonia Production
The sustainable ammonia production market is currently in a transitional phase, moving from traditional energy-intensive methods toward electrolyte-based approaches that promise lower carbon emissions. The global market is expanding rapidly, projected to reach significant scale as green ammonia gains traction in energy storage and agricultural applications. Technologically, the field shows varying maturity levels, with academic institutions like Zhejiang University, Cornell University, and Delft University of Technology driving fundamental research, while companies demonstrate different commercialization stages. Atmonia and Battolyser are developing innovative electrocatalytic processes, established chemical corporations like BASF and Siemens Energy are adapting existing expertise, and LG Energy Solution is leveraging battery technology crossover potential. This competitive landscape reflects both the technical challenges and commercial opportunities in sustainable ammonia production.
BASF Corp.
Technical Solution: BASF has developed advanced electrolyte systems for electrochemical ammonia synthesis that operate at significantly reduced temperatures and pressures compared to conventional Haber-Bosch processes. Their technology utilizes proprietary solid-state electrolytes with enhanced ionic conductivity and nitrogen activation capabilities. The system incorporates specially designed transition metal catalysts embedded in structured electrolyte matrices that facilitate nitrogen reduction while minimizing competing hydrogen evolution reactions. BASF's approach includes innovative proton-conducting ceramic-polymer composite electrolytes that enable efficient proton transport to nitrogen reduction sites while maintaining mechanical stability under operating conditions. The company has demonstrated sustained ammonia production rates exceeding 10^-8 mol cm^-2 s^-1 in laboratory settings, representing significant progress toward commercially viable electrochemical ammonia synthesis. Their integrated system design addresses key challenges in electrode-electrolyte interfaces, catalyst poisoning prevention, and system durability for long-term operation.
Strengths: Leverages BASF's extensive catalyst and materials expertise; highly integrated with existing chemical production infrastructure; potential for direct integration with renewable energy sources. Weaknesses: Still requires significant energy input compared to theoretical minimums; catalyst selectivity challenges in complex electrolyte environments; scaling production to industrial levels remains challenging.
Atmonia ehf
Technical Solution: Atmonia has developed an innovative electrochemical approach for sustainable ammonia production using specialized molecular catalysts that mimic natural nitrogen fixation processes. Their proprietary electrolyte system operates at ambient conditions (room temperature and atmospheric pressure), significantly reducing energy requirements compared to the conventional Haber-Bosch process. The technology employs nitrogen-selective catalysts suspended in carefully formulated electrolyte solutions that facilitate efficient nitrogen reduction reactions. Their system incorporates novel ionic liquids as electrolytes that enhance nitrogen solubility and electron transfer while maintaining catalyst stability. Atmonia's approach enables direct electrochemical conversion of nitrogen to ammonia without hydrogen gas as an intermediate, potentially reducing the overall process complexity and energy consumption by up to 50% compared to conventional methods.
Strengths: Operates at ambient conditions eliminating need for high pressure/temperature infrastructure; uses renewable electricity directly; highly scalable from distributed to centralized production. Weaknesses: Lower production rates compared to conventional methods; catalyst durability challenges in electrolyte environment; technology still scaling from laboratory to commercial demonstration.
Critical Electrolyte Patents and Technical Literature
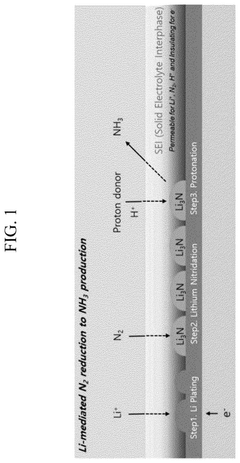
ELECTROLYTE AND MANUFACTURING METHOD OF GREEN AMMONIA FROM Li-MEDIATRED NITROGEN REDUCTION USING THE SAME
PatentPendingUS20250171914A1
Innovation
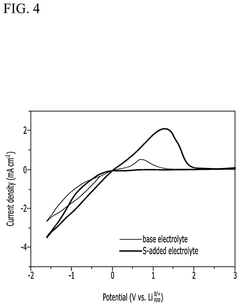
- An electrolyte comprising a lithium compound, a proton donor, and a sulfur compound is used in an electrochemical reactor to facilitate the production of ammonia through a Li-mediated nitrogen reduction reaction, which forms lithium nitride and subsequently ammonia.
Electrolytic cell for the production of ammonia
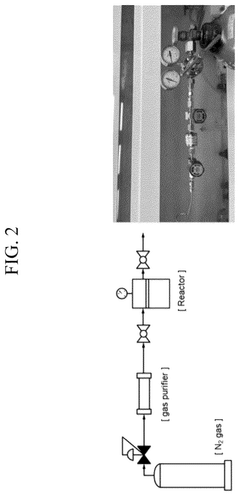
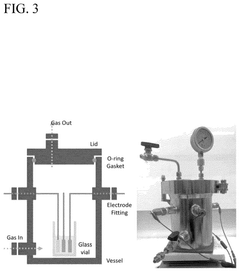
PatentWO2015009155A1
Innovation
- A novel electrolytic cell design using solid Ni metal membranes and appropriate electro-catalysts to directly synthesize ammonia from water and nitrogen at ambient conditions, utilizing renewable electricity and abundant materials like Ni, Fe, and K, which allows for continuous operation and reduced energy consumption.
Energy Efficiency Considerations in Electrolyte Systems
Energy efficiency represents a critical factor in the development and implementation of electrolyte systems for sustainable ammonia production. Current electrolytic ammonia synthesis processes typically consume between 7,000-18,000 kWh per ton of ammonia produced, significantly higher than the conventional Haber-Bosch process which requires approximately 8,000-12,000 kWh per ton when including hydrogen production. This energy gap presents a substantial challenge for widespread adoption of electrolytic methods.
The electrolyte composition directly impacts energy consumption through its influence on ionic conductivity, electrode kinetics, and overall cell resistance. Research indicates that alkaline electrolytes (KOH, NaOH) generally offer lower energy requirements compared to neutral or acidic systems, with energy efficiencies ranging from 18-65% depending on operating conditions and system design. However, these systems often suffer from stability issues at high current densities.
Temperature management represents another crucial aspect of energy efficiency in electrolyte systems. Operating at elevated temperatures (60-80°C) can enhance reaction kinetics and reduce cell overpotential by 15-30%, but simultaneously accelerates electrolyte degradation and increases evaporative losses. Advanced thermal management strategies, including heat recovery systems, have demonstrated potential to recapture up to 40% of waste heat.
Recent innovations in electrolyte formulations have shown promising results for energy optimization. Ionic liquid-based electrolytes have achieved energy efficiencies up to 70% in laboratory settings, though their high cost (currently $80-200/kg) limits commercial viability. Similarly, polymer-supported electrolyte membranes have demonstrated 25-35% reductions in energy consumption compared to traditional liquid electrolytes while enhancing system durability.
The integration of renewable energy sources with electrolytic ammonia production presents both opportunities and challenges for energy efficiency. Intermittent power supply from solar or wind sources can lead to operational inefficiencies, with studies showing that frequent start-stop cycles can increase overall energy consumption by 10-25%. Advanced control systems utilizing machine learning algorithms have demonstrated potential to optimize electrolyte performance under variable power conditions, reducing this efficiency penalty to 5-10%.
Economic analyses indicate that energy costs typically represent 60-80% of operational expenses in electrolytic ammonia production. Achieving energy efficiency improvements of just 10% could reduce production costs by approximately $50-100 per ton, significantly enhancing commercial viability. This economic driver continues to fuel research into novel electrolyte formulations and system designs focused on minimizing energy requirements while maintaining production rates.
The electrolyte composition directly impacts energy consumption through its influence on ionic conductivity, electrode kinetics, and overall cell resistance. Research indicates that alkaline electrolytes (KOH, NaOH) generally offer lower energy requirements compared to neutral or acidic systems, with energy efficiencies ranging from 18-65% depending on operating conditions and system design. However, these systems often suffer from stability issues at high current densities.
Temperature management represents another crucial aspect of energy efficiency in electrolyte systems. Operating at elevated temperatures (60-80°C) can enhance reaction kinetics and reduce cell overpotential by 15-30%, but simultaneously accelerates electrolyte degradation and increases evaporative losses. Advanced thermal management strategies, including heat recovery systems, have demonstrated potential to recapture up to 40% of waste heat.
Recent innovations in electrolyte formulations have shown promising results for energy optimization. Ionic liquid-based electrolytes have achieved energy efficiencies up to 70% in laboratory settings, though their high cost (currently $80-200/kg) limits commercial viability. Similarly, polymer-supported electrolyte membranes have demonstrated 25-35% reductions in energy consumption compared to traditional liquid electrolytes while enhancing system durability.
The integration of renewable energy sources with electrolytic ammonia production presents both opportunities and challenges for energy efficiency. Intermittent power supply from solar or wind sources can lead to operational inefficiencies, with studies showing that frequent start-stop cycles can increase overall energy consumption by 10-25%. Advanced control systems utilizing machine learning algorithms have demonstrated potential to optimize electrolyte performance under variable power conditions, reducing this efficiency penalty to 5-10%.
Economic analyses indicate that energy costs typically represent 60-80% of operational expenses in electrolytic ammonia production. Achieving energy efficiency improvements of just 10% could reduce production costs by approximately $50-100 per ton, significantly enhancing commercial viability. This economic driver continues to fuel research into novel electrolyte formulations and system designs focused on minimizing energy requirements while maintaining production rates.
Environmental Impact Assessment of Electrolyte Technologies
The environmental impact of electrolyte technologies in sustainable ammonia production represents a critical area of assessment as industries seek greener alternatives to traditional Haber-Bosch processes. Current electrolyte systems, while promising for decarbonizing ammonia synthesis, present various environmental considerations that must be thoroughly evaluated.
Primary electrolyte materials used in electrochemical ammonia production include aqueous solutions of alkali metal hydroxides (KOH, NaOH), molten salts, ionic liquids, and polymer-based solid electrolytes. Each carries distinct environmental footprints throughout their lifecycle, from raw material extraction to disposal or recycling phases.
Aqueous alkaline electrolytes demonstrate relatively low toxicity and are produced from abundant resources, yet their production involves energy-intensive processes with associated carbon emissions. The mining of potassium and sodium salts can lead to habitat disruption and water quality issues in extraction regions. However, these electrolytes typically offer good recyclability potential, reducing their overall environmental burden.
Ionic liquid electrolytes present more complex environmental considerations. While they enable operation at lower temperatures than molten salts, reducing energy requirements, many contain fluorinated compounds with high global warming potential if released. Their synthesis often involves multiple reaction steps with hazardous precursors and solvents, creating additional environmental concerns. The limited biodegradability of many ionic liquids raises end-of-life management challenges.
Water consumption represents another significant environmental factor. Electrolyte systems vary considerably in their water requirements, with some aqueous systems demanding substantial quantities for dilution and cooling. Advanced membrane-based electrolytes can reduce water needs but may introduce other material-specific impacts.
Life cycle assessment (LCA) studies indicate that the environmental benefits of electrolyte-based ammonia production strongly depend on the electricity source. When powered by renewable energy, these systems can achieve carbon footprint reductions of 60-90% compared to conventional methods. However, if powered by fossil fuel electricity, the environmental advantage diminishes substantially.
Recent innovations in bio-derived electrolytes show promise for reducing environmental impacts. These materials, sourced from renewable feedstocks, demonstrate lower ecotoxicity profiles and reduced resource depletion impacts compared to conventional alternatives. However, land use considerations and competition with food production must be carefully managed if these approaches scale significantly.
Regulatory frameworks increasingly influence electrolyte technology development, with restrictions on certain materials driving research toward environmentally benign alternatives. This regulatory landscape varies globally, creating regional differences in environmental performance of similar technologies.
Primary electrolyte materials used in electrochemical ammonia production include aqueous solutions of alkali metal hydroxides (KOH, NaOH), molten salts, ionic liquids, and polymer-based solid electrolytes. Each carries distinct environmental footprints throughout their lifecycle, from raw material extraction to disposal or recycling phases.
Aqueous alkaline electrolytes demonstrate relatively low toxicity and are produced from abundant resources, yet their production involves energy-intensive processes with associated carbon emissions. The mining of potassium and sodium salts can lead to habitat disruption and water quality issues in extraction regions. However, these electrolytes typically offer good recyclability potential, reducing their overall environmental burden.
Ionic liquid electrolytes present more complex environmental considerations. While they enable operation at lower temperatures than molten salts, reducing energy requirements, many contain fluorinated compounds with high global warming potential if released. Their synthesis often involves multiple reaction steps with hazardous precursors and solvents, creating additional environmental concerns. The limited biodegradability of many ionic liquids raises end-of-life management challenges.
Water consumption represents another significant environmental factor. Electrolyte systems vary considerably in their water requirements, with some aqueous systems demanding substantial quantities for dilution and cooling. Advanced membrane-based electrolytes can reduce water needs but may introduce other material-specific impacts.
Life cycle assessment (LCA) studies indicate that the environmental benefits of electrolyte-based ammonia production strongly depend on the electricity source. When powered by renewable energy, these systems can achieve carbon footprint reductions of 60-90% compared to conventional methods. However, if powered by fossil fuel electricity, the environmental advantage diminishes substantially.
Recent innovations in bio-derived electrolytes show promise for reducing environmental impacts. These materials, sourced from renewable feedstocks, demonstrate lower ecotoxicity profiles and reduced resource depletion impacts compared to conventional alternatives. However, land use considerations and competition with food production must be carefully managed if these approaches scale significantly.
Regulatory frameworks increasingly influence electrolyte technology development, with restrictions on certain materials driving research toward environmentally benign alternatives. This regulatory landscape varies globally, creating regional differences in environmental performance of similar technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!