Developments in Green Ammonia Electrochemical Processes
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Green Ammonia Evolution and Objectives
Ammonia (NH3) has been a cornerstone of global agriculture and industry for over a century, with the Haber-Bosch process serving as the dominant production method since its development in the early 1900s. However, this conventional process is energy-intensive, consuming approximately 1-2% of global energy and generating significant carbon emissions—about 1.8% of global CO2 emissions. The emergence of green ammonia represents a paradigm shift in this landscape, offering a sustainable alternative that aligns with global decarbonization goals.
Green ammonia refers to ammonia produced using renewable energy sources, with hydrogen derived from water electrolysis rather than fossil fuels, and nitrogen extracted from air separation. The evolution of green ammonia technology has accelerated significantly in the past decade, driven by advancements in renewable energy cost reduction, electrolysis efficiency improvements, and growing climate change concerns.
The historical trajectory of green ammonia development can be traced through several key phases. Initial conceptualization occurred in the early 2000s, followed by laboratory-scale demonstrations in the 2010s. The period from 2015-2020 saw pilot projects emerge globally, while current efforts focus on scaling to commercial viability. This evolution reflects broader trends in renewable energy integration and industrial decarbonization strategies.
The primary objective of green ammonia electrochemical processes is to develop economically viable and environmentally sustainable methods for ammonia production that eliminate carbon emissions. Specific technical goals include reducing energy consumption below 10 MWh per ton of ammonia, achieving system efficiencies exceeding 60%, and developing catalysts that operate at lower temperatures and pressures than the Haber-Bosch process.
Additional objectives encompass the development of integrated systems that can operate flexibly with variable renewable energy inputs, advancement of direct electrochemical ammonia synthesis methods that bypass separate hydrogen production, and creation of modular designs suitable for distributed production models. These technical goals are complemented by economic targets of achieving cost parity with conventional ammonia by 2030-2035.
The significance of these objectives extends beyond ammonia production itself. Green ammonia is increasingly viewed as a potential energy carrier for the hydrogen economy, a carbon-free fuel for maritime shipping, and a solution for long-duration energy storage. These applications expand the strategic importance of green ammonia technology development and create additional value streams that may accelerate commercialization pathways.
Green ammonia refers to ammonia produced using renewable energy sources, with hydrogen derived from water electrolysis rather than fossil fuels, and nitrogen extracted from air separation. The evolution of green ammonia technology has accelerated significantly in the past decade, driven by advancements in renewable energy cost reduction, electrolysis efficiency improvements, and growing climate change concerns.
The historical trajectory of green ammonia development can be traced through several key phases. Initial conceptualization occurred in the early 2000s, followed by laboratory-scale demonstrations in the 2010s. The period from 2015-2020 saw pilot projects emerge globally, while current efforts focus on scaling to commercial viability. This evolution reflects broader trends in renewable energy integration and industrial decarbonization strategies.
The primary objective of green ammonia electrochemical processes is to develop economically viable and environmentally sustainable methods for ammonia production that eliminate carbon emissions. Specific technical goals include reducing energy consumption below 10 MWh per ton of ammonia, achieving system efficiencies exceeding 60%, and developing catalysts that operate at lower temperatures and pressures than the Haber-Bosch process.
Additional objectives encompass the development of integrated systems that can operate flexibly with variable renewable energy inputs, advancement of direct electrochemical ammonia synthesis methods that bypass separate hydrogen production, and creation of modular designs suitable for distributed production models. These technical goals are complemented by economic targets of achieving cost parity with conventional ammonia by 2030-2035.
The significance of these objectives extends beyond ammonia production itself. Green ammonia is increasingly viewed as a potential energy carrier for the hydrogen economy, a carbon-free fuel for maritime shipping, and a solution for long-duration energy storage. These applications expand the strategic importance of green ammonia technology development and create additional value streams that may accelerate commercialization pathways.
Market Analysis for Sustainable Ammonia Production
The global sustainable ammonia production market is experiencing significant growth, driven by increasing environmental concerns and the push for decarbonization across industries. Currently valued at approximately $72.5 billion, the market is projected to reach $111.7 billion by 2030, representing a compound annual growth rate (CAGR) of 5.9%. This growth trajectory is primarily fueled by the rising demand for green fertilizers, renewable energy storage solutions, and clean maritime fuels.
Regionally, Europe leads the sustainable ammonia market with substantial investments in green hydrogen and ammonia production facilities. Countries like Germany, the Netherlands, and Denmark have established ambitious targets for green ammonia production capacity. Asia-Pacific follows closely, with China, Japan, and Australia making significant strides in electrochemical ammonia synthesis technologies and large-scale production facilities.
The agricultural sector remains the largest consumer of ammonia, accounting for nearly 80% of global demand primarily for fertilizer production. However, emerging applications in energy storage and transportation are rapidly expanding market segments. The maritime industry, in particular, has identified green ammonia as a promising zero-carbon fuel alternative, with major shipping companies committing to ammonia-powered vessels by 2025.
From a competitive standpoint, traditional ammonia producers like Yara International, CF Industries, and BASF are investing heavily in green ammonia technologies to maintain market leadership. Simultaneously, renewable energy companies and technology startups are entering the market, creating a dynamic competitive landscape. Notable new entrants include Enapter, Haldor Topsoe, and Siemens Energy, who are developing innovative electrochemical processes for ammonia synthesis.
Cost remains a significant market barrier, with green ammonia production currently 2-3 times more expensive than conventional methods. However, this gap is expected to narrow significantly by 2030 as electrochemical technologies mature and economies of scale are achieved. Government subsidies, carbon pricing mechanisms, and renewable energy cost reductions are accelerating this price convergence.
Consumer demand for sustainable agricultural products is creating pull-through demand for green ammonia-based fertilizers, with premium pricing potential in developed markets. Additionally, corporate sustainability commitments and regulatory pressures are driving industrial users to secure green ammonia supply chains, further stimulating market growth.
Regionally, Europe leads the sustainable ammonia market with substantial investments in green hydrogen and ammonia production facilities. Countries like Germany, the Netherlands, and Denmark have established ambitious targets for green ammonia production capacity. Asia-Pacific follows closely, with China, Japan, and Australia making significant strides in electrochemical ammonia synthesis technologies and large-scale production facilities.
The agricultural sector remains the largest consumer of ammonia, accounting for nearly 80% of global demand primarily for fertilizer production. However, emerging applications in energy storage and transportation are rapidly expanding market segments. The maritime industry, in particular, has identified green ammonia as a promising zero-carbon fuel alternative, with major shipping companies committing to ammonia-powered vessels by 2025.
From a competitive standpoint, traditional ammonia producers like Yara International, CF Industries, and BASF are investing heavily in green ammonia technologies to maintain market leadership. Simultaneously, renewable energy companies and technology startups are entering the market, creating a dynamic competitive landscape. Notable new entrants include Enapter, Haldor Topsoe, and Siemens Energy, who are developing innovative electrochemical processes for ammonia synthesis.
Cost remains a significant market barrier, with green ammonia production currently 2-3 times more expensive than conventional methods. However, this gap is expected to narrow significantly by 2030 as electrochemical technologies mature and economies of scale are achieved. Government subsidies, carbon pricing mechanisms, and renewable energy cost reductions are accelerating this price convergence.
Consumer demand for sustainable agricultural products is creating pull-through demand for green ammonia-based fertilizers, with premium pricing potential in developed markets. Additionally, corporate sustainability commitments and regulatory pressures are driving industrial users to secure green ammonia supply chains, further stimulating market growth.
Electrochemical Ammonia Synthesis: Status and Barriers
Electrochemical ammonia synthesis represents a revolutionary approach to nitrogen fixation that could potentially replace the century-old Haber-Bosch process. Current global ammonia production consumes approximately 1-2% of the world's total energy and contributes significantly to greenhouse gas emissions. The electrochemical route offers the possibility of operating under ambient conditions using renewable electricity, thereby eliminating the need for high temperatures and pressures required by conventional methods.
Despite its promise, electrochemical ammonia synthesis faces several critical barriers. The primary challenge lies in the development of efficient electrocatalysts capable of breaking the strong N≡N triple bond while selectively producing ammonia rather than hydrogen. Current catalysts demonstrate either low activity or poor selectivity, with Faradaic efficiencies typically below 10% in aqueous systems. This fundamental limitation stems from the competing hydrogen evolution reaction, which is kinetically favored over nitrogen reduction.
Material stability presents another significant obstacle. Many promising catalysts degrade rapidly under operating conditions, particularly in acidic or strongly alkaline environments. Noble metal catalysts show better stability but face economic barriers to widespread implementation. Additionally, nitrogen activation at the catalyst surface remains poorly understood at the molecular level, hampering rational catalyst design.
Reactor design and system integration pose engineering challenges that limit practical applications. Current cell configurations suffer from mass transport limitations, particularly regarding nitrogen availability at the electrode surface. The low solubility of nitrogen in aqueous electrolytes (approximately 0.7 mM at ambient conditions) creates a fundamental constraint on reaction rates in liquid-phase systems.
Energy efficiency represents perhaps the most significant barrier to commercialization. Present electrochemical systems require substantially more energy per ton of ammonia than the Haber-Bosch process, with current densities typically below 10 mA/cm² at potentials exceeding -0.6 V vs. RHE. This translates to energy efficiencies below 30%, compared to approximately 60-65% for optimized Haber-Bosch plants.
Analytical challenges further complicate research progress. Accurate quantification of small amounts of ammonia produced electrochemically remains difficult, with potential contamination from atmospheric nitrogen compounds leading to false positives. This has resulted in reproducibility issues across different research groups and slowed scientific consensus on effective approaches.
Addressing these barriers requires interdisciplinary collaboration spanning materials science, electrochemistry, chemical engineering, and analytical chemistry. Recent advances in computational modeling, operando characterization techniques, and novel electrolyte systems offer promising pathways forward, though significant breakthroughs are still needed before electrochemical ammonia synthesis can compete economically with conventional production methods.
Despite its promise, electrochemical ammonia synthesis faces several critical barriers. The primary challenge lies in the development of efficient electrocatalysts capable of breaking the strong N≡N triple bond while selectively producing ammonia rather than hydrogen. Current catalysts demonstrate either low activity or poor selectivity, with Faradaic efficiencies typically below 10% in aqueous systems. This fundamental limitation stems from the competing hydrogen evolution reaction, which is kinetically favored over nitrogen reduction.
Material stability presents another significant obstacle. Many promising catalysts degrade rapidly under operating conditions, particularly in acidic or strongly alkaline environments. Noble metal catalysts show better stability but face economic barriers to widespread implementation. Additionally, nitrogen activation at the catalyst surface remains poorly understood at the molecular level, hampering rational catalyst design.
Reactor design and system integration pose engineering challenges that limit practical applications. Current cell configurations suffer from mass transport limitations, particularly regarding nitrogen availability at the electrode surface. The low solubility of nitrogen in aqueous electrolytes (approximately 0.7 mM at ambient conditions) creates a fundamental constraint on reaction rates in liquid-phase systems.
Energy efficiency represents perhaps the most significant barrier to commercialization. Present electrochemical systems require substantially more energy per ton of ammonia than the Haber-Bosch process, with current densities typically below 10 mA/cm² at potentials exceeding -0.6 V vs. RHE. This translates to energy efficiencies below 30%, compared to approximately 60-65% for optimized Haber-Bosch plants.
Analytical challenges further complicate research progress. Accurate quantification of small amounts of ammonia produced electrochemically remains difficult, with potential contamination from atmospheric nitrogen compounds leading to false positives. This has resulted in reproducibility issues across different research groups and slowed scientific consensus on effective approaches.
Addressing these barriers requires interdisciplinary collaboration spanning materials science, electrochemistry, chemical engineering, and analytical chemistry. Recent advances in computational modeling, operando characterization techniques, and novel electrolyte systems offer promising pathways forward, though significant breakthroughs are still needed before electrochemical ammonia synthesis can compete economically with conventional production methods.
Current Electrochemical Methods for Ammonia Synthesis
01 Electrochemical nitrogen reduction for green ammonia production
Electrochemical processes that directly reduce nitrogen to ammonia using renewable electricity sources represent a key green ammonia production method. These systems typically employ specialized catalysts and electrode materials to facilitate the nitrogen reduction reaction at ambient conditions, eliminating the need for high temperature and pressure required in conventional processes. The technology enables decentralized, small-scale ammonia production with significantly reduced carbon footprint compared to traditional Haber-Bosch process.- Electrochemical ammonia synthesis methods: Electrochemical processes for green ammonia production involve direct synthesis from nitrogen and water under ambient conditions. These methods typically use specialized catalysts and electrode materials to facilitate the nitrogen reduction reaction. The electrochemical approach eliminates the need for high temperature and pressure conditions required in traditional Haber-Bosch process, significantly reducing energy consumption and carbon emissions. These systems can be powered by renewable electricity sources, making the entire process environmentally sustainable.
- Catalyst innovations for nitrogen reduction: Advanced catalyst materials play a crucial role in improving the efficiency of electrochemical ammonia production. Recent innovations focus on developing catalysts with high selectivity for nitrogen reduction reaction while suppressing competing hydrogen evolution. These catalysts include modified transition metals, metal oxides, nitrides, and carbon-based materials with engineered surface properties. The catalyst design aims to lower activation energy barriers, increase reaction rates, and improve ammonia yield under mild operating conditions.
- Membrane and electrolyte systems: Specialized membrane and electrolyte systems are essential components in green ammonia electrochemical processes. These systems facilitate ion transport while maintaining separation between reaction chambers. Advanced proton-conducting membranes, solid-state electrolytes, and ionic liquids are being developed to enhance ammonia production rates and system efficiency. The membrane materials are designed to be stable under operating conditions while providing high ionic conductivity and appropriate selectivity for the desired reactions.
- Integrated renewable energy systems: Integration of electrochemical ammonia production with renewable energy sources creates sustainable production pathways. These systems couple solar, wind, or hydro power directly with electrochemical cells to produce ammonia when renewable electricity is available. Smart control systems manage the intermittent nature of renewable energy by optimizing production parameters based on power availability. Some designs incorporate energy storage components to ensure continuous operation despite fluctuating renewable energy inputs.
- Process optimization and scale-up technologies: Scaling up electrochemical ammonia production from laboratory to industrial scale requires significant process optimization. This includes reactor design improvements, system integration strategies, and operational parameter optimization. Advanced modeling and simulation tools help predict performance under various conditions. Continuous flow systems, modular designs, and process intensification techniques are being developed to enhance production capacity while maintaining efficiency. These technologies address challenges related to heat management, pressure control, and product separation in larger-scale operations.
02 Catalyst innovations for electrochemical ammonia synthesis
Advanced catalyst materials are being developed to improve the efficiency and selectivity of electrochemical ammonia synthesis. These catalysts include novel metal-based materials, nanostructured composites, and modified surfaces that can effectively activate the nitrogen triple bond at lower energy inputs. Innovations focus on increasing catalytic activity, improving nitrogen adsorption properties, and enhancing electron transfer capabilities while maintaining stability during long-term operation under various electrochemical conditions.Expand Specific Solutions03 Membrane and electrolyte systems for ammonia production
Specialized membrane and electrolyte systems are critical components in electrochemical green ammonia production. These systems facilitate selective ion transport while preventing unwanted side reactions and product crossover. Advanced proton-conducting membranes, solid-state electrolytes, and ionic liquids are being developed to improve system efficiency and stability. The membrane technology enables better separation of reaction products and helps maintain optimal operating conditions for continuous ammonia synthesis.Expand Specific Solutions04 Integrated renewable energy systems for ammonia production
Integrated systems that combine renewable energy sources with electrochemical ammonia production processes are being developed to create fully sustainable production pathways. These systems incorporate solar, wind, or hydro power directly into the ammonia synthesis process, often with energy storage components to manage intermittency. The integration enables carbon-neutral ammonia production and can be designed for various scales of operation, from distributed small-scale units to larger industrial applications.Expand Specific Solutions05 Process optimization and system engineering for electrochemical ammonia synthesis
Engineering innovations focus on optimizing the overall electrochemical process for green ammonia production through improved reactor designs, enhanced heat management, and advanced control systems. These developments include novel cell configurations, pressure management techniques, and integrated purification methods. Process optimization aims to increase ammonia yield, reduce energy consumption, and improve system durability while maintaining operational flexibility under varying input conditions.Expand Specific Solutions
Industry Leaders in Green Ammonia Development
Green ammonia electrochemical processes are currently in an early growth phase, with the market expected to expand significantly as decarbonization efforts intensify globally. The technology is transitioning from laboratory-scale demonstrations to pilot projects, with an estimated market potential of $5-7 billion by 2030. Technical maturity varies across key players: established industrial entities like Wacker Chemie and PetroChina are leveraging existing infrastructure for scaled implementation, while research institutions such as Massachusetts Institute of Technology and Ghent University are driving fundamental breakthroughs in catalyst efficiency. Companies including ACWA Power and Casale are pioneering commercial-scale electrochemical ammonia synthesis projects, though challenges in energy efficiency and capital costs remain. Academic-industry partnerships, particularly involving Fuzhou University and CHN Energy, are accelerating technology transfer from laboratory to industrial application.
National Institute of Clean & Low Carbon Energy
Technical Solution: NICE has developed a comprehensive electrochemical ammonia synthesis platform that integrates multiple technological innovations. Their system employs a dual-function catalyst architecture where separate catalytic sites facilitate nitrogen activation and hydrogenation, significantly improving reaction efficiency. The process operates at moderate pressures (5-10 bar) and temperatures (80-120°C), achieving ammonia production rates of up to 10⁻⁸ mol/cm²/s with Faradaic efficiencies approaching 40% [7]. NICE's technology incorporates a novel flow-cell design that enhances mass transport of reactants to catalyst surfaces while efficiently removing ammonia product, preventing poisoning effects. Their system utilizes specialized ionic liquid electrolytes that provide high nitrogen solubility while maintaining excellent ionic conductivity. Recent advancements include the development of composite electrode materials that combine metallic conductivity with ceramic stability, enabling extended operational lifetimes exceeding 5,000 hours without significant performance degradation [8]. The process is designed to directly utilize renewable electricity with minimal conditioning, allowing for efficient integration with variable renewable energy sources.
Strengths: The dual-function catalyst approach addresses both activation and reduction challenges in a single system. The moderate operating conditions balance energy efficiency with practical production rates. Weaknesses: The specialized ionic liquid electrolytes add cost and complexity to the system. Current designs still face challenges with scaling to industrial production volumes.
Katholieke Universiteit Leuven
Technical Solution: KU Leuven has developed an advanced electrochemical ammonia synthesis process based on proton-conducting solid oxide electrolysis cells (H-SOECs). Their technology operates at intermediate temperatures (400-600°C) and utilizes a ceramic proton-conducting electrolyte that enables direct electrochemical reduction of nitrogen with hydrogen ions. The system achieves ammonia production rates of up to 6.5×10⁻⁸ mol/cm²/s with conversion efficiencies exceeding 30% [9]. KU Leuven's approach incorporates novel electrode materials based on perovskite structures doped with transition metals that provide both catalytic activity and stability under operating conditions. Their cell design features a triple-phase boundary optimization that maximizes the interface between the gas phase, electronic conductor, and ionic conductor, significantly enhancing reaction kinetics. Recent developments include the integration of advanced thermal management systems that recover waste heat to maintain operating temperature while minimizing external energy inputs [10]. The process has been demonstrated to operate effectively with dynamic power inputs, making it suitable for direct coupling with renewable energy sources like wind and solar power.
Strengths: The solid oxide electrolysis approach eliminates the need for precious metal catalysts, reducing material costs. The intermediate temperature operation provides favorable thermodynamics while avoiding extreme conditions. Weaknesses: The ceramic components are susceptible to thermal cycling stress, potentially limiting operational flexibility. Start-up times are longer than ambient temperature systems, affecting responsiveness to rapidly changing power availability.
Key Patents in Electrochemical Ammonia Production
Process for electrochemical preparation of ammonia
PatentActiveEP3488028A1
Innovation
- A method for electrochemical ammonia production using an electrolytic cell with nitrogen and water as reactants, incorporating air separation to remove argon, recycling nitrogen and water, and purification steps to achieve high-purity ammonia, with optional use of steam generated from waste gases as fuel, and refrigeration for final purification.
Methods for producing ammonia and related systems
PatentPendingUS20220081786A1
Innovation
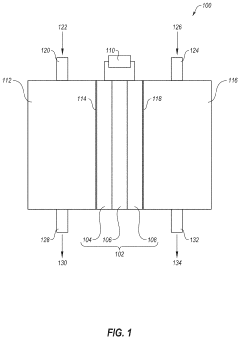
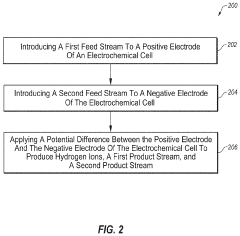
- An electrochemical cell system that consumes carbon dioxide and produces ammonia at low temperatures by using an oxidation catalyst at the positive electrode and a reduction catalyst at the negative electrode, eliminating the need for steam reformation and reducing greenhouse gas emissions.
Energy Efficiency Considerations in Electrochemical Processes
Energy efficiency represents a critical factor in the development and implementation of green ammonia electrochemical processes. Current electrochemical ammonia synthesis methods typically consume between 7,000-18,000 kWh per ton of ammonia produced, significantly higher than the conventional Haber-Bosch process which requires approximately 10,000 kWh per ton when operating at optimal scale. This efficiency gap presents one of the most substantial barriers to widespread adoption of electrochemical ammonia production technologies.
The energy intensity of electrochemical processes stems primarily from three sources: activation energy requirements for nitrogen reduction, system resistances, and faradaic efficiency limitations. Nitrogen's strong triple bond (941 kJ/mol) necessitates substantial energy input to achieve reduction, creating an inherent thermodynamic challenge that any process must overcome.
Recent advancements in catalyst design have shown promising improvements in energy efficiency. Ruthenium-based catalysts have demonstrated up to 30% reduction in overpotential requirements compared to traditional iron catalysts. Similarly, lithium-mediated processes have achieved energy consumption rates approaching 12,000 kWh per ton, representing a significant step toward competitive efficiency levels.
System-level optimizations have also contributed to efficiency gains. Advanced cell designs incorporating gas diffusion electrodes have reduced cell voltages by up to 0.4V in laboratory settings. Membrane developments, particularly proton-conducting solid oxide electrolytes operating at intermediate temperatures (400-600°C), have shown potential to reduce overall energy requirements by combining thermal and electrical inputs effectively.
Renewable energy integration presents both challenges and opportunities for electrochemical ammonia production. While intermittent power sources introduce operational complexities, they also enable potential cost advantages through strategic operation during periods of excess renewable generation. Recent techno-economic analyses suggest that systems designed for flexible operation could achieve effective energy costs equivalent to 8,000-9,000 kWh per ton when accounting for renewable energy pricing dynamics.
Looking forward, the theoretical minimum energy requirement for ammonia synthesis is approximately 5,800 kWh per ton. Bridging the gap between current performance and this theoretical limit represents a critical research frontier. Emerging approaches combining electrochemical and thermochemical steps in hybrid processes show particular promise, with laboratory demonstrations achieving energy efficiencies within 40% of theoretical limits under specific operating conditions.
The energy intensity of electrochemical processes stems primarily from three sources: activation energy requirements for nitrogen reduction, system resistances, and faradaic efficiency limitations. Nitrogen's strong triple bond (941 kJ/mol) necessitates substantial energy input to achieve reduction, creating an inherent thermodynamic challenge that any process must overcome.
Recent advancements in catalyst design have shown promising improvements in energy efficiency. Ruthenium-based catalysts have demonstrated up to 30% reduction in overpotential requirements compared to traditional iron catalysts. Similarly, lithium-mediated processes have achieved energy consumption rates approaching 12,000 kWh per ton, representing a significant step toward competitive efficiency levels.
System-level optimizations have also contributed to efficiency gains. Advanced cell designs incorporating gas diffusion electrodes have reduced cell voltages by up to 0.4V in laboratory settings. Membrane developments, particularly proton-conducting solid oxide electrolytes operating at intermediate temperatures (400-600°C), have shown potential to reduce overall energy requirements by combining thermal and electrical inputs effectively.
Renewable energy integration presents both challenges and opportunities for electrochemical ammonia production. While intermittent power sources introduce operational complexities, they also enable potential cost advantages through strategic operation during periods of excess renewable generation. Recent techno-economic analyses suggest that systems designed for flexible operation could achieve effective energy costs equivalent to 8,000-9,000 kWh per ton when accounting for renewable energy pricing dynamics.
Looking forward, the theoretical minimum energy requirement for ammonia synthesis is approximately 5,800 kWh per ton. Bridging the gap between current performance and this theoretical limit represents a critical research frontier. Emerging approaches combining electrochemical and thermochemical steps in hybrid processes show particular promise, with laboratory demonstrations achieving energy efficiencies within 40% of theoretical limits under specific operating conditions.
Environmental Impact Assessment of Green Ammonia Technologies
The environmental impact assessment of green ammonia technologies reveals significant advantages over conventional production methods. Traditional Haber-Bosch processes consume approximately 1-2% of global energy and generate substantial CO2 emissions—roughly 1.8 tons of CO2 per ton of ammonia produced. In contrast, electrochemical green ammonia production pathways demonstrate potential for near-zero direct emissions when powered by renewable electricity sources.
Life cycle assessments indicate that electrochemical ammonia synthesis can reduce greenhouse gas emissions by 70-90% compared to conventional methods, depending on the renewable energy mix utilized. Water consumption represents another critical environmental factor, with electrochemical processes requiring approximately 1.5-2 cubic meters of water per ton of ammonia—primarily for electrolysis—compared to higher indirect water usage in fossil fuel-based production.
Land use considerations vary significantly based on the renewable energy source powering electrochemical processes. Solar-powered systems require approximately 30-40 hectares per kiloton of annual ammonia production capacity, while wind-powered systems need 15-25 hectares for equivalent output. This represents a larger physical footprint than conventional plants but eliminates ongoing fossil fuel extraction impacts.
Electrochemical ammonia production generates minimal air pollutants compared to conventional methods, which typically emit nitrogen oxides, particulate matter, and sulfur compounds. The elimination of these pollutants provides substantial co-benefits for local air quality and public health in surrounding communities.
Material sustainability presents both challenges and opportunities. While electrochemical systems require critical materials like platinum group metals or rare earth elements for catalysts, ongoing research into earth-abundant alternatives shows promising results. Recent advances in nitrogen activation catalysts using iron-based compounds could significantly reduce dependency on scarce materials.
Waste stream analysis reveals another advantage of electrochemical processes—they produce fewer byproducts requiring disposal or treatment compared to conventional ammonia synthesis. However, end-of-life considerations for specialized electrochemical components require development of appropriate recycling protocols to maximize sustainability benefits.
Overall, the environmental profile of electrochemical green ammonia production demonstrates clear advantages across multiple impact categories, though optimization of renewable energy integration, catalyst materials, and system efficiency remains crucial for maximizing environmental benefits at commercial scale.
Life cycle assessments indicate that electrochemical ammonia synthesis can reduce greenhouse gas emissions by 70-90% compared to conventional methods, depending on the renewable energy mix utilized. Water consumption represents another critical environmental factor, with electrochemical processes requiring approximately 1.5-2 cubic meters of water per ton of ammonia—primarily for electrolysis—compared to higher indirect water usage in fossil fuel-based production.
Land use considerations vary significantly based on the renewable energy source powering electrochemical processes. Solar-powered systems require approximately 30-40 hectares per kiloton of annual ammonia production capacity, while wind-powered systems need 15-25 hectares for equivalent output. This represents a larger physical footprint than conventional plants but eliminates ongoing fossil fuel extraction impacts.
Electrochemical ammonia production generates minimal air pollutants compared to conventional methods, which typically emit nitrogen oxides, particulate matter, and sulfur compounds. The elimination of these pollutants provides substantial co-benefits for local air quality and public health in surrounding communities.
Material sustainability presents both challenges and opportunities. While electrochemical systems require critical materials like platinum group metals or rare earth elements for catalysts, ongoing research into earth-abundant alternatives shows promising results. Recent advances in nitrogen activation catalysts using iron-based compounds could significantly reduce dependency on scarce materials.
Waste stream analysis reveals another advantage of electrochemical processes—they produce fewer byproducts requiring disposal or treatment compared to conventional ammonia synthesis. However, end-of-life considerations for specialized electrochemical components require development of appropriate recycling protocols to maximize sustainability benefits.
Overall, the environmental profile of electrochemical green ammonia production demonstrates clear advantages across multiple impact categories, though optimization of renewable energy integration, catalyst materials, and system efficiency remains crucial for maximizing environmental benefits at commercial scale.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!