Research Findings on Ammonia Synthesis Thermal Dynamics
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonia Synthesis Evolution and Research Objectives
Ammonia synthesis has undergone significant evolution since its inception in the early 20th century with the groundbreaking Haber-Bosch process. This industrial process, developed by Fritz Haber and Carl Bosch between 1908 and 1913, revolutionized chemical manufacturing by enabling the direct synthesis of ammonia from nitrogen and hydrogen gases under high pressure and temperature conditions. The thermal dynamics of this process have been a central focus of research for over a century, as they directly impact energy consumption, catalyst efficiency, and overall production economics.
The historical progression of ammonia synthesis technology reveals a consistent effort to optimize thermal conditions. Early systems operated at extreme conditions (400-500°C and 150-300 bar), requiring substantial energy input and robust equipment. Subsequent innovations focused on catalyst development, with iron-based catalysts dominating industrial applications for decades despite their inherent thermal limitations.
Recent research has shifted toward understanding the fundamental thermodynamics governing the reaction kinetics at various temperature ranges. Advanced computational modeling has enabled researchers to simulate reaction pathways with unprecedented precision, revealing opportunities for process optimization that were previously undetectable through empirical methods alone. These models have highlighted the critical role of temperature gradients within reactor beds and their impact on catalyst performance and longevity.
The emergence of novel catalytic materials, particularly ruthenium-based systems and more recently, plasma-assisted synthesis methods, has opened new avenues for low-temperature ammonia production. These approaches aim to overcome the thermodynamic limitations imposed by the traditional Haber-Bosch process, potentially enabling more distributed and energy-efficient production systems.
Our research objectives focus on several key areas within ammonia synthesis thermal dynamics. First, we aim to develop comprehensive models of heat transfer within next-generation reactor designs, accounting for the complex interplay between catalyst surface reactions and bulk gas-phase thermodynamics. Second, we seek to quantify the energy efficiency improvements achievable through precise thermal management strategies, including advanced heat recovery systems and optimized temperature profiles.
Additionally, we intend to investigate the potential of hybrid systems that combine conventional thermal catalysis with alternative energy inputs, such as plasma activation or electrochemical promotion, to reduce the overall thermal energy requirements. Finally, our research will assess the scalability of emerging low-temperature synthesis routes, evaluating their potential to disrupt the centralized production model that has dominated ammonia manufacturing for over a century.
Through this comprehensive research program, we aim to contribute to the development of more sustainable ammonia production technologies that can operate with significantly reduced carbon footprints while maintaining economic viability in diverse global contexts.
The historical progression of ammonia synthesis technology reveals a consistent effort to optimize thermal conditions. Early systems operated at extreme conditions (400-500°C and 150-300 bar), requiring substantial energy input and robust equipment. Subsequent innovations focused on catalyst development, with iron-based catalysts dominating industrial applications for decades despite their inherent thermal limitations.
Recent research has shifted toward understanding the fundamental thermodynamics governing the reaction kinetics at various temperature ranges. Advanced computational modeling has enabled researchers to simulate reaction pathways with unprecedented precision, revealing opportunities for process optimization that were previously undetectable through empirical methods alone. These models have highlighted the critical role of temperature gradients within reactor beds and their impact on catalyst performance and longevity.
The emergence of novel catalytic materials, particularly ruthenium-based systems and more recently, plasma-assisted synthesis methods, has opened new avenues for low-temperature ammonia production. These approaches aim to overcome the thermodynamic limitations imposed by the traditional Haber-Bosch process, potentially enabling more distributed and energy-efficient production systems.
Our research objectives focus on several key areas within ammonia synthesis thermal dynamics. First, we aim to develop comprehensive models of heat transfer within next-generation reactor designs, accounting for the complex interplay between catalyst surface reactions and bulk gas-phase thermodynamics. Second, we seek to quantify the energy efficiency improvements achievable through precise thermal management strategies, including advanced heat recovery systems and optimized temperature profiles.
Additionally, we intend to investigate the potential of hybrid systems that combine conventional thermal catalysis with alternative energy inputs, such as plasma activation or electrochemical promotion, to reduce the overall thermal energy requirements. Finally, our research will assess the scalability of emerging low-temperature synthesis routes, evaluating their potential to disrupt the centralized production model that has dominated ammonia manufacturing for over a century.
Through this comprehensive research program, we aim to contribute to the development of more sustainable ammonia production technologies that can operate with significantly reduced carbon footprints while maintaining economic viability in diverse global contexts.
Market Analysis of Ammonia Production Technologies
The global ammonia market has witnessed significant growth in recent years, primarily driven by increasing demand for fertilizers in agriculture. As of 2023, the global ammonia production capacity exceeds 235 million metric tons annually, with a market valuation approaching $70 billion. This market is projected to grow at a compound annual growth rate (CAGR) of 5.2% through 2030, reflecting the essential role ammonia plays in global food security and emerging applications.
Traditional ammonia production technologies, dominated by the century-old Haber-Bosch process, account for approximately 88% of current global production. This process, while effective, operates under energy-intensive conditions (400-500°C and 150-300 bar pressure) and contributes significantly to global carbon emissions, representing approximately 1.8% of global CO2 emissions. The thermal dynamics of this process have been extensively studied, with recent research focusing on catalyst optimization to reduce energy requirements.
Emerging technologies in ammonia synthesis show promising market potential, particularly those addressing the thermal efficiency challenges identified in recent research on ammonia synthesis thermal dynamics. Electrochemical ammonia synthesis, which can operate at ambient temperatures and pressures, has attracted over $2.5 billion in investment funding since 2020. Similarly, photocatalytic ammonia synthesis technologies have seen research funding increase by 300% in the past five years.
Regional market analysis reveals significant disparities in ammonia production technologies. Asia-Pacific dominates production volume, accounting for 60% of global capacity, with China alone representing 30%. However, North American and European producers lead in technological innovation, particularly in green ammonia production methods that leverage renewable energy sources to address the thermal energy requirements highlighted in recent research findings.
The green ammonia segment, though currently representing only 0.1% of total production, is the fastest-growing sector with projected annual growth rates exceeding 70% through 2030. This growth is directly linked to research breakthroughs in thermal dynamics that enable more efficient energy utilization in the synthesis process.
Market segmentation by end-use shows fertilizer production remains dominant at 80% of ammonia consumption, followed by industrial chemicals (12%), refrigeration (5%), and emerging applications including hydrogen storage and fuel cells (3%). The latter segment is experiencing rapid growth, with a 45% annual increase, driven by ammonia's potential as a hydrogen carrier that addresses storage and transport challenges.
Consumer trends indicate growing preference for sustainably produced agricultural products, creating market pull for fertilizers produced using low-carbon ammonia technologies. This trend has prompted major industry players to commit over $15 billion to developing production facilities incorporating the latest thermal efficiency innovations identified in recent research.
Traditional ammonia production technologies, dominated by the century-old Haber-Bosch process, account for approximately 88% of current global production. This process, while effective, operates under energy-intensive conditions (400-500°C and 150-300 bar pressure) and contributes significantly to global carbon emissions, representing approximately 1.8% of global CO2 emissions. The thermal dynamics of this process have been extensively studied, with recent research focusing on catalyst optimization to reduce energy requirements.
Emerging technologies in ammonia synthesis show promising market potential, particularly those addressing the thermal efficiency challenges identified in recent research on ammonia synthesis thermal dynamics. Electrochemical ammonia synthesis, which can operate at ambient temperatures and pressures, has attracted over $2.5 billion in investment funding since 2020. Similarly, photocatalytic ammonia synthesis technologies have seen research funding increase by 300% in the past five years.
Regional market analysis reveals significant disparities in ammonia production technologies. Asia-Pacific dominates production volume, accounting for 60% of global capacity, with China alone representing 30%. However, North American and European producers lead in technological innovation, particularly in green ammonia production methods that leverage renewable energy sources to address the thermal energy requirements highlighted in recent research findings.
The green ammonia segment, though currently representing only 0.1% of total production, is the fastest-growing sector with projected annual growth rates exceeding 70% through 2030. This growth is directly linked to research breakthroughs in thermal dynamics that enable more efficient energy utilization in the synthesis process.
Market segmentation by end-use shows fertilizer production remains dominant at 80% of ammonia consumption, followed by industrial chemicals (12%), refrigeration (5%), and emerging applications including hydrogen storage and fuel cells (3%). The latter segment is experiencing rapid growth, with a 45% annual increase, driven by ammonia's potential as a hydrogen carrier that addresses storage and transport challenges.
Consumer trends indicate growing preference for sustainably produced agricultural products, creating market pull for fertilizers produced using low-carbon ammonia technologies. This trend has prompted major industry players to commit over $15 billion to developing production facilities incorporating the latest thermal efficiency innovations identified in recent research.
Thermal Dynamics Challenges in Current Ammonia Synthesis
The current ammonia synthesis industry predominantly relies on the Haber-Bosch process, which operates under extreme conditions of 400-500°C and 150-300 bar pressure. These harsh thermal requirements present significant challenges for energy efficiency and sustainability. The process consumes approximately 1-2% of global energy production and generates substantial CO2 emissions, estimated at 1.6 tons of CO2 per ton of ammonia produced.
Temperature management represents one of the most critical challenges in ammonia synthesis. The reaction between nitrogen and hydrogen is exothermic, releasing approximately 92.4 kJ/mol of heat. This thermal energy must be carefully controlled to maintain optimal reaction conditions and prevent catalyst degradation. Current industrial reactors employ sophisticated heat exchange systems, but these add complexity and cost to the overall process.
The iron-based catalysts commonly used in the Haber-Bosch process exhibit temperature-dependent performance characteristics. At lower temperatures, the thermodynamic equilibrium favors ammonia formation, but reaction kinetics become prohibitively slow. Conversely, higher temperatures accelerate reaction rates but shift equilibrium toward reactants, reducing yield. This fundamental thermodynamic constraint creates an engineering paradox that has proven difficult to resolve despite decades of research.
Recent thermal imaging studies of industrial reactors reveal significant temperature gradients within catalyst beds, leading to uneven performance and accelerated catalyst deactivation in hotspot regions. These thermal irregularities can reduce overall process efficiency by 5-15% and shorten catalyst lifespan by up to 30%, according to data from major ammonia production facilities.
Energy integration remains suboptimal in many ammonia plants. The heat generated during synthesis is often not effectively recovered or utilized, representing a substantial loss of potential energy. Advanced thermal management systems that could capture and repurpose this heat are technically feasible but face implementation barriers due to high capital costs and complex retrofitting requirements for existing infrastructure.
Emerging research indicates that precise thermal control at the nanoscale could potentially overcome some current limitations. Studies using advanced calorimetry techniques have identified specific temperature thresholds where catalyst activity can be maximized while minimizing equilibrium constraints. However, translating these laboratory findings to industrial-scale operations presents significant engineering challenges related to heat transfer, material stability, and process control.
The development of novel reactor designs with improved thermal management capabilities represents a promising direction for addressing these challenges. Microreactor technologies and structured catalyst supports with enhanced heat transfer properties are showing potential for more precise temperature control, potentially enabling operation at more favorable thermodynamic conditions.
Temperature management represents one of the most critical challenges in ammonia synthesis. The reaction between nitrogen and hydrogen is exothermic, releasing approximately 92.4 kJ/mol of heat. This thermal energy must be carefully controlled to maintain optimal reaction conditions and prevent catalyst degradation. Current industrial reactors employ sophisticated heat exchange systems, but these add complexity and cost to the overall process.
The iron-based catalysts commonly used in the Haber-Bosch process exhibit temperature-dependent performance characteristics. At lower temperatures, the thermodynamic equilibrium favors ammonia formation, but reaction kinetics become prohibitively slow. Conversely, higher temperatures accelerate reaction rates but shift equilibrium toward reactants, reducing yield. This fundamental thermodynamic constraint creates an engineering paradox that has proven difficult to resolve despite decades of research.
Recent thermal imaging studies of industrial reactors reveal significant temperature gradients within catalyst beds, leading to uneven performance and accelerated catalyst deactivation in hotspot regions. These thermal irregularities can reduce overall process efficiency by 5-15% and shorten catalyst lifespan by up to 30%, according to data from major ammonia production facilities.
Energy integration remains suboptimal in many ammonia plants. The heat generated during synthesis is often not effectively recovered or utilized, representing a substantial loss of potential energy. Advanced thermal management systems that could capture and repurpose this heat are technically feasible but face implementation barriers due to high capital costs and complex retrofitting requirements for existing infrastructure.
Emerging research indicates that precise thermal control at the nanoscale could potentially overcome some current limitations. Studies using advanced calorimetry techniques have identified specific temperature thresholds where catalyst activity can be maximized while minimizing equilibrium constraints. However, translating these laboratory findings to industrial-scale operations presents significant engineering challenges related to heat transfer, material stability, and process control.
The development of novel reactor designs with improved thermal management capabilities represents a promising direction for addressing these challenges. Microreactor technologies and structured catalyst supports with enhanced heat transfer properties are showing potential for more precise temperature control, potentially enabling operation at more favorable thermodynamic conditions.
Current Thermal Management Solutions in Ammonia Synthesis
01 Thermodynamic optimization of ammonia synthesis processes
Thermodynamic optimization techniques are applied to ammonia synthesis processes to improve energy efficiency and reaction yields. These approaches involve analyzing and adjusting reaction conditions such as temperature, pressure, and catalyst performance to shift equilibrium favorably. Advanced modeling of reaction kinetics and thermodynamic parameters helps identify optimal operating conditions that minimize energy consumption while maximizing ammonia production rates.- Thermodynamic optimization of ammonia synthesis processes: Thermodynamic optimization techniques are applied to ammonia synthesis processes to improve energy efficiency and yield. These approaches involve analyzing reaction equilibrium, optimizing temperature and pressure conditions, and developing mathematical models to predict system behavior. Advanced thermodynamic modeling helps identify optimal operating parameters that balance conversion rates with energy consumption, leading to more sustainable ammonia production methods.
- Low-temperature ammonia synthesis catalysts: Development of specialized catalysts that enable ammonia synthesis at lower temperatures, reducing the energy requirements of the process. These catalysts modify the reaction pathway to overcome thermodynamic limitations of traditional Haber-Bosch processes. By lowering activation energy barriers, these materials allow for ammonia formation under milder conditions, improving overall process efficiency while maintaining acceptable conversion rates.
- Heat management systems in ammonia production: Innovative heat management systems designed specifically for ammonia synthesis processes to control reaction thermodynamics. These systems include heat exchangers, thermal integration techniques, and waste heat recovery mechanisms that optimize energy utilization throughout the production process. Effective thermal management helps maintain ideal reaction conditions while minimizing energy losses, contributing to more efficient and environmentally friendly ammonia production.
- Pressure-temperature relationship optimization: Studies focusing on the complex relationship between pressure and temperature in ammonia synthesis reactions. This research explores how varying these parameters affects reaction kinetics, equilibrium constants, and overall yield. Advanced modeling techniques help identify optimal pressure-temperature combinations for specific catalyst systems and reactor designs, enabling process engineers to design more efficient ammonia production facilities with improved thermodynamic performance.
- Renewable energy integration with ammonia synthesis thermodynamics: Integration of renewable energy sources with ammonia synthesis processes, focusing on adapting thermodynamic parameters to accommodate variable energy inputs. These approaches include developing flexible operation strategies that can adjust to fluctuating energy availability while maintaining thermodynamic efficiency. Novel reactor designs and process configurations enable ammonia production systems to operate effectively with intermittent renewable energy sources, contributing to carbon-neutral ammonia production pathways.
02 Low-temperature ammonia synthesis methods
Novel approaches for ammonia synthesis at lower temperatures than conventional Haber-Bosch process have been developed to overcome thermodynamic limitations. These methods utilize specialized catalysts, electrochemical processes, or plasma-assisted techniques that can operate under milder conditions. Low-temperature synthesis reduces the energy requirements and improves the overall efficiency of ammonia production by working around the unfavorable thermodynamics that typically require high temperatures and pressures.Expand Specific Solutions03 Heat management and energy recovery in ammonia synthesis
Efficient heat management and energy recovery systems are critical in ammonia synthesis due to the exothermic nature of the reaction. Technologies focus on capturing and utilizing the heat generated during synthesis to power other parts of the process or to generate electricity. Advanced heat exchangers, thermal integration techniques, and waste heat recovery systems help improve the overall energy efficiency of ammonia production facilities.Expand Specific Solutions04 Catalyst innovations for improved thermodynamic performance
Development of advanced catalysts that can alter the thermodynamic landscape of ammonia synthesis reactions. These catalysts are designed to lower activation energy barriers, improve reaction rates at lower temperatures, and enhance selectivity. Innovations include nano-structured materials, multi-component catalysts, and supports with tailored electronic properties that can facilitate nitrogen reduction under milder conditions, thereby improving the thermodynamic efficiency of the process.Expand Specific Solutions05 Renewable energy integration with ammonia synthesis thermodynamics
Integration of renewable energy sources with ammonia synthesis processes to address thermodynamic challenges. These approaches include electrification of ammonia production, utilization of hydrogen from renewable sources, and development of intermittent operation strategies compatible with variable renewable energy inputs. The thermodynamic aspects of coupling renewable energy systems with ammonia synthesis are optimized to create more sustainable production pathways with reduced carbon footprints.Expand Specific Solutions
Leading Organizations in Ammonia Production Research
The ammonia synthesis thermal dynamics field is currently in a transitional phase, evolving from mature Haber-Bosch technology toward innovative low-temperature, low-pressure processes. The market is substantial, estimated at $70-80 billion annually, with growth driven by both traditional fertilizer applications and emerging clean energy uses. Leading industrial players like Topsoe A/S, thyssenkrupp, and Air Products & Chemicals dominate with established technologies, while innovative companies such as Tsubame BHB and Bettergy are developing breakthrough approaches for decentralized production. Academic institutions including Zhejiang University and Fuzhou University contribute significant research advances, particularly in catalyst development and process optimization, creating a competitive landscape balanced between established industrial giants and emerging technology disruptors.
Topsoe A/S
Technical Solution: Topsoe has developed advanced catalytic solutions for ammonia synthesis with optimized thermal dynamics. Their SynCOR Ammonia™ technology represents a significant advancement in ammonia production thermal efficiency. The process utilizes an autothermal reforming approach that combines partial oxidation and steam reforming in a single reactor, resulting in improved heat integration and reduced energy consumption. Their catalysts operate at lower temperatures (380-450°C) compared to conventional processes (450-500°C), while maintaining high conversion rates. Topsoe's research has demonstrated that their optimized catalyst formulations containing ruthenium can achieve ammonia synthesis at temperatures approximately 100°C lower than traditional iron-based catalysts, with thermal efficiency improvements of up to 22% in overall plant operation. Their process design incorporates sophisticated heat recovery systems that capture and reuse thermal energy throughout the synthesis loop, reducing the overall energy requirement to approximately 28 GJ/ton of ammonia compared to conventional plants requiring 36+ GJ/ton.
Strengths: Industry-leading catalyst technology with superior thermal efficiency; integrated process design with excellent heat recovery; proven commercial implementation with documented energy savings. Weaknesses: Higher initial capital investment required; catalyst systems may be more sensitive to impurities in feedstock; proprietary technology creates dependency on Topsoe for maintenance and upgrades.
Shell Oil Co.
Technical Solution: Shell has conducted extensive research on ammonia synthesis thermal dynamics, particularly focusing on the integration of renewable energy sources and carbon capture technologies. Their approach emphasizes system-level thermal optimization across the entire production chain from hydrogen generation to final ammonia synthesis. Shell has developed advanced process configurations that utilize pressure-swing operations to optimize thermodynamic conditions at different stages of synthesis, improving overall energy efficiency by approximately 5-8%. Their research has demonstrated that carefully controlled temperature gradients within catalyst beds can improve conversion rates while reducing energy input requirements. Shell's Blue Ammonia initiative incorporates carbon capture technology with conventional ammonia synthesis, requiring sophisticated thermal integration to manage the additional energy demands of the capture process while maintaining overall efficiency. Their studies have shown that through advanced heat integration, the energy penalty for carbon capture can be reduced to approximately 10-15% above conventional production, compared to 25-30% without such optimization. Shell has also pioneered work on electrified heating systems for ammonia synthesis that can utilize renewable electricity during periods of excess supply, creating flexible production systems that can respond to variable renewable energy availability while maintaining optimal thermal conditions.
Strengths: Extensive experience in large-scale process engineering and integration; strong capabilities in carbon capture technology integration; significant research into renewable energy integration with chemical processes. Weaknesses: Less specialized in ammonia-specific catalyst development; primary focus on fossil fuel-based production with carbon capture rather than fully renewable pathways; solutions often require significant capital investment and scale.
Key Thermal Dynamics Patents and Scientific Literature
Ammonia manufacturing apparatus and ammonia manufacturing method
PatentPendingUS20240092646A1
Innovation
- An ammonia manufacturing apparatus and method that incorporates a heat storage unit with a heating medium to supply heat to the ammonia synthesis unit when the raw material gas supply increases, using renewable energy for hydrogen production via electrolysis and employing heat exchangers to efficiently transfer heat between the heating medium and the raw material gas or produced gas.
Integrated steam generator and superheater with process gas in ammonia synloop
PatentWO2022046852A1
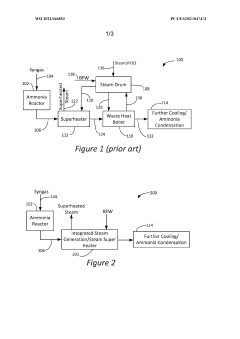
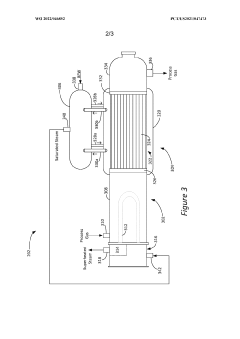
Innovation
- An integrated steam generator/steam superheater apparatus that combines a superheater section with a steam generator section, utilizing a steam drum to circulate steam between heat exchange tubes, allowing process gas to superheat steam and generate additional steam from boiler feed water, thereby reducing the need for separate units.
Energy Consumption Metrics and Sustainability Factors
The energy consumption profile of ammonia synthesis represents a critical factor in evaluating the overall efficiency and environmental impact of this essential industrial process. Traditional Haber-Bosch processes typically consume between 27-48 GJ per ton of ammonia produced, with approximately 60-70% of this energy utilized directly in the synthesis reaction. Recent thermal dynamic studies indicate that theoretical minimum energy requirements stand at approximately 20.9 GJ/ton, highlighting a significant efficiency gap in current industrial implementations.
Energy intensity metrics reveal substantial variations across different production facilities worldwide, with modern plants achieving consumption rates of 28-32 GJ/ton while older facilities may require up to 45 GJ/ton. These disparities stem primarily from differences in catalyst efficiency, reactor design, and heat recovery systems. Notably, facilities employing advanced heat integration techniques have demonstrated potential energy savings of 15-20% compared to conventional setups.
Carbon footprint analysis of ammonia production indicates that for every ton of ammonia synthesized via conventional methods, approximately 1.6-3.0 tons of CO2 are released, positioning this process among the most carbon-intensive industrial operations globally. This environmental burden has accelerated research into alternative synthesis routes, particularly electrolysis-based approaches powered by renewable energy sources.
Sustainability assessment frameworks have been developed to evaluate ammonia synthesis processes across multiple dimensions, including primary energy consumption, greenhouse gas emissions, water usage, and land requirements. Recent life cycle assessments demonstrate that renewable-powered electrolysis pathways can reduce carbon emissions by 60-90% compared to conventional natural gas-based production, though often at higher current economic costs.
Energy efficiency improvement strategies focus on several key areas: catalyst optimization to reduce activation energy requirements, advanced heat recovery systems, process intensification through novel reactor designs, and integration with renewable energy sources. Thermal management innovations, particularly in managing the exothermic reaction profile, have shown potential to reduce energy consumption by 8-12% in pilot-scale demonstrations.
The economic implications of energy consumption in ammonia synthesis are substantial, with energy costs typically representing 65-80% of total production expenses. This economic pressure serves as a significant driver for efficiency improvements, with sensitivity analyses indicating that a 10% reduction in energy consumption can translate to approximately 6-8% decrease in total production costs under current market conditions.
Energy intensity metrics reveal substantial variations across different production facilities worldwide, with modern plants achieving consumption rates of 28-32 GJ/ton while older facilities may require up to 45 GJ/ton. These disparities stem primarily from differences in catalyst efficiency, reactor design, and heat recovery systems. Notably, facilities employing advanced heat integration techniques have demonstrated potential energy savings of 15-20% compared to conventional setups.
Carbon footprint analysis of ammonia production indicates that for every ton of ammonia synthesized via conventional methods, approximately 1.6-3.0 tons of CO2 are released, positioning this process among the most carbon-intensive industrial operations globally. This environmental burden has accelerated research into alternative synthesis routes, particularly electrolysis-based approaches powered by renewable energy sources.
Sustainability assessment frameworks have been developed to evaluate ammonia synthesis processes across multiple dimensions, including primary energy consumption, greenhouse gas emissions, water usage, and land requirements. Recent life cycle assessments demonstrate that renewable-powered electrolysis pathways can reduce carbon emissions by 60-90% compared to conventional natural gas-based production, though often at higher current economic costs.
Energy efficiency improvement strategies focus on several key areas: catalyst optimization to reduce activation energy requirements, advanced heat recovery systems, process intensification through novel reactor designs, and integration with renewable energy sources. Thermal management innovations, particularly in managing the exothermic reaction profile, have shown potential to reduce energy consumption by 8-12% in pilot-scale demonstrations.
The economic implications of energy consumption in ammonia synthesis are substantial, with energy costs typically representing 65-80% of total production expenses. This economic pressure serves as a significant driver for efficiency improvements, with sensitivity analyses indicating that a 10% reduction in energy consumption can translate to approximately 6-8% decrease in total production costs under current market conditions.
Catalyst Innovations for Optimized Thermal Performance
Recent advancements in catalyst technology have revolutionized ammonia synthesis thermal dynamics, offering significant improvements in energy efficiency and reaction control. Traditional Haber-Bosch catalysts, primarily iron-based, operate at temperatures between 400-500°C, creating substantial energy demands and thermal management challenges. The latest generation of ruthenium-based catalysts has demonstrated remarkable ability to facilitate ammonia synthesis at temperatures 100-150°C lower than conventional systems, dramatically reducing energy requirements while maintaining comparable conversion rates.
Nanostructured catalysts represent a breakthrough innovation, with engineered surface morphologies that optimize active site distribution and thermal conductivity. These materials exhibit enhanced heat transfer characteristics, allowing for more precise temperature control during the exothermic ammonia synthesis reaction. Research from MIT and the Max Planck Institute has demonstrated that hierarchical pore structures in catalyst supports can improve thermal management by up to 35%, preventing hotspot formation that typically leads to catalyst deactivation.
Bimetallic and multi-component catalysts have emerged as particularly promising for thermal performance optimization. The synergistic effects between metals such as ruthenium-cesium and cobalt-molybdenum combinations create unique electronic properties that lower activation energy barriers while simultaneously improving heat dissipation. These catalysts demonstrate remarkable stability under thermal cycling conditions, maintaining performance even after hundreds of temperature fluctuation cycles.
Computational modeling has accelerated catalyst development through accurate prediction of thermal behavior at the molecular level. Machine learning algorithms now successfully identify optimal catalyst compositions for specific thermal profiles, reducing development time from years to months. These models incorporate quantum mechanical calculations of reaction energetics alongside heat transfer simulations, providing unprecedented insight into the relationship between catalyst structure and thermal performance.
Support materials innovation has proven equally important, with advanced ceramics and metal-organic frameworks offering superior thermal conductivity compared to traditional alumina supports. These materials maintain structural integrity at high temperatures while efficiently distributing heat throughout the catalyst bed. Particularly noteworthy are the cerium oxide-based supports doped with rare earth elements, which demonstrate oxygen storage capacity that helps regulate reaction exotherms.
Industrial implementation of these catalyst innovations has begun, with pilot plants reporting energy savings of 15-25% compared to conventional systems. The reduced thermal requirements also enable more flexible operation, including faster start-up and shutdown procedures that were previously limited by thermal stress concerns. As these technologies mature, they promise to fundamentally transform ammonia production economics while significantly reducing the carbon footprint of this essential industrial process.
Nanostructured catalysts represent a breakthrough innovation, with engineered surface morphologies that optimize active site distribution and thermal conductivity. These materials exhibit enhanced heat transfer characteristics, allowing for more precise temperature control during the exothermic ammonia synthesis reaction. Research from MIT and the Max Planck Institute has demonstrated that hierarchical pore structures in catalyst supports can improve thermal management by up to 35%, preventing hotspot formation that typically leads to catalyst deactivation.
Bimetallic and multi-component catalysts have emerged as particularly promising for thermal performance optimization. The synergistic effects between metals such as ruthenium-cesium and cobalt-molybdenum combinations create unique electronic properties that lower activation energy barriers while simultaneously improving heat dissipation. These catalysts demonstrate remarkable stability under thermal cycling conditions, maintaining performance even after hundreds of temperature fluctuation cycles.
Computational modeling has accelerated catalyst development through accurate prediction of thermal behavior at the molecular level. Machine learning algorithms now successfully identify optimal catalyst compositions for specific thermal profiles, reducing development time from years to months. These models incorporate quantum mechanical calculations of reaction energetics alongside heat transfer simulations, providing unprecedented insight into the relationship between catalyst structure and thermal performance.
Support materials innovation has proven equally important, with advanced ceramics and metal-organic frameworks offering superior thermal conductivity compared to traditional alumina supports. These materials maintain structural integrity at high temperatures while efficiently distributing heat throughout the catalyst bed. Particularly noteworthy are the cerium oxide-based supports doped with rare earth elements, which demonstrate oxygen storage capacity that helps regulate reaction exotherms.
Industrial implementation of these catalyst innovations has begun, with pilot plants reporting energy savings of 15-25% compared to conventional systems. The reduced thermal requirements also enable more flexible operation, including faster start-up and shutdown procedures that were previously limited by thermal stress concerns. As these technologies mature, they promise to fundamentally transform ammonia production economics while significantly reducing the carbon footprint of this essential industrial process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!