Methods for Assessing Electrode Efficiency in Ammonia Synthesis
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonia Synthesis Electrode Technology Background and Objectives
Ammonia synthesis represents one of the most significant industrial processes of the 20th century, with the Haber-Bosch process enabling large-scale nitrogen fixation that revolutionized agricultural productivity worldwide. The evolution of ammonia synthesis technology spans over a century, beginning with Fritz Haber's laboratory demonstrations in 1909 and Carl Bosch's subsequent industrial implementation. Traditional ammonia production relies on high-temperature (400-500°C) and high-pressure (150-300 bar) conditions with iron-based catalysts, consuming approximately 1-2% of global energy production annually.
The technological trajectory has shifted dramatically in recent years toward electrochemical ammonia synthesis methods, which offer potential for decentralized production under ambient conditions. This paradigm shift is driven by increasing environmental concerns, energy efficiency demands, and the need for sustainable fertilizer production pathways. Electrochemical approaches potentially enable ammonia synthesis directly from nitrogen and water using renewable electricity, representing a carbon-neutral alternative to the energy-intensive Haber-Bosch process.
Electrode efficiency assessment in ammonia synthesis has emerged as a critical research focus, as electrode materials and structures fundamentally determine reaction kinetics, selectivity, and overall system performance. The development of standardized methodologies for evaluating electrode efficiency is essential for meaningful comparison between different catalyst systems and reactor designs across the research community.
Current technical objectives in this field center on developing reliable, reproducible metrics for quantifying electrode performance. These include Faradaic efficiency (measuring what percentage of applied current results in ammonia formation), ammonia yield rate (typically reported in μg h⁻¹ mg⁻¹cat or μmol h⁻¹ cm⁻²), electrode stability over extended operation periods, and selectivity against competing reactions such as hydrogen evolution.
The scientific community faces significant challenges in establishing standardized protocols, particularly regarding product detection and quantification. Conventional methods like spectrophotometric assays (indophenol blue method) have detection limits that sometimes approach the production rates of experimental systems, creating uncertainty in reported results. Advanced techniques including nuclear magnetic resonance spectroscopy (NMR), ion chromatography, and isotope labeling studies are increasingly employed to verify ammonia production and distinguish it from potential contaminants.
Looking forward, the field aims to develop in-situ and operando characterization techniques that can monitor electrode performance in real-time, providing deeper insights into reaction mechanisms and degradation pathways. Additionally, there is growing recognition of the need for standardized testing protocols and reporting frameworks to facilitate meaningful comparison between different research efforts and accelerate progress toward commercially viable electrochemical ammonia synthesis technologies.
The technological trajectory has shifted dramatically in recent years toward electrochemical ammonia synthesis methods, which offer potential for decentralized production under ambient conditions. This paradigm shift is driven by increasing environmental concerns, energy efficiency demands, and the need for sustainable fertilizer production pathways. Electrochemical approaches potentially enable ammonia synthesis directly from nitrogen and water using renewable electricity, representing a carbon-neutral alternative to the energy-intensive Haber-Bosch process.
Electrode efficiency assessment in ammonia synthesis has emerged as a critical research focus, as electrode materials and structures fundamentally determine reaction kinetics, selectivity, and overall system performance. The development of standardized methodologies for evaluating electrode efficiency is essential for meaningful comparison between different catalyst systems and reactor designs across the research community.
Current technical objectives in this field center on developing reliable, reproducible metrics for quantifying electrode performance. These include Faradaic efficiency (measuring what percentage of applied current results in ammonia formation), ammonia yield rate (typically reported in μg h⁻¹ mg⁻¹cat or μmol h⁻¹ cm⁻²), electrode stability over extended operation periods, and selectivity against competing reactions such as hydrogen evolution.
The scientific community faces significant challenges in establishing standardized protocols, particularly regarding product detection and quantification. Conventional methods like spectrophotometric assays (indophenol blue method) have detection limits that sometimes approach the production rates of experimental systems, creating uncertainty in reported results. Advanced techniques including nuclear magnetic resonance spectroscopy (NMR), ion chromatography, and isotope labeling studies are increasingly employed to verify ammonia production and distinguish it from potential contaminants.
Looking forward, the field aims to develop in-situ and operando characterization techniques that can monitor electrode performance in real-time, providing deeper insights into reaction mechanisms and degradation pathways. Additionally, there is growing recognition of the need for standardized testing protocols and reporting frameworks to facilitate meaningful comparison between different research efforts and accelerate progress toward commercially viable electrochemical ammonia synthesis technologies.
Market Analysis of Ammonia Production Technologies
The global ammonia market is experiencing significant growth, with production reaching approximately 235 million metric tons in 2022 and projected to exceed 290 million tons by 2030. This growth is primarily driven by increasing demand for fertilizers, which currently accounts for about 80% of ammonia consumption worldwide. The remaining 20% is distributed across various industrial applications including refrigeration, water purification, pharmaceuticals, and increasingly, as a potential carbon-free energy carrier.
Traditional ammonia production technologies dominate the current market landscape, with the Haber-Bosch process accounting for over 90% of global production. This century-old technology, while well-established, is energy-intensive and responsible for approximately 1.8% of global CO2 emissions, consuming 1-2% of the world's total energy production.
The market for electrode-based ammonia synthesis technologies is emerging as a promising alternative, particularly as global sustainability initiatives gain momentum. Electrochemical ammonia synthesis methods offer the potential for decentralized production using renewable electricity, potentially reducing the carbon footprint by up to 80% compared to conventional methods when powered by renewable energy sources.
Market analysis indicates that the electrochemical ammonia synthesis segment is growing at a compound annual growth rate (CAGR) of approximately 9.5%, significantly outpacing the overall ammonia market growth rate of 3.2%. This accelerated growth is attributed to increasing investment in green ammonia technologies, with global investments reaching $5.4 billion in 2022, a threefold increase from 2018 levels.
Regional analysis shows Asia-Pacific dominating the conventional ammonia production market with approximately 45% market share, led by China and India. However, Europe and North America are leading in electrochemical ammonia technology development, with significant research initiatives and pilot projects underway in Germany, Denmark, the United States, and Japan.
The competitive landscape is evolving rapidly, with traditional chemical companies like BASF, Yara, and CF Industries investing heavily in electrochemical ammonia technologies. Simultaneously, technology startups such as Starfire Energy, Jupiter Ionics, and Nitricity are gaining traction with innovative electrode designs and catalysts that promise improved efficiency and selectivity.
Price sensitivity remains a critical market factor, as conventional ammonia production costs range from $400-600 per ton, while electrochemical methods currently average $900-1,200 per ton. However, this gap is expected to narrow as electrode efficiency improves and renewable electricity costs continue to decline, with some projections suggesting price parity could be achieved by 2030 in regions with abundant renewable energy resources.
Traditional ammonia production technologies dominate the current market landscape, with the Haber-Bosch process accounting for over 90% of global production. This century-old technology, while well-established, is energy-intensive and responsible for approximately 1.8% of global CO2 emissions, consuming 1-2% of the world's total energy production.
The market for electrode-based ammonia synthesis technologies is emerging as a promising alternative, particularly as global sustainability initiatives gain momentum. Electrochemical ammonia synthesis methods offer the potential for decentralized production using renewable electricity, potentially reducing the carbon footprint by up to 80% compared to conventional methods when powered by renewable energy sources.
Market analysis indicates that the electrochemical ammonia synthesis segment is growing at a compound annual growth rate (CAGR) of approximately 9.5%, significantly outpacing the overall ammonia market growth rate of 3.2%. This accelerated growth is attributed to increasing investment in green ammonia technologies, with global investments reaching $5.4 billion in 2022, a threefold increase from 2018 levels.
Regional analysis shows Asia-Pacific dominating the conventional ammonia production market with approximately 45% market share, led by China and India. However, Europe and North America are leading in electrochemical ammonia technology development, with significant research initiatives and pilot projects underway in Germany, Denmark, the United States, and Japan.
The competitive landscape is evolving rapidly, with traditional chemical companies like BASF, Yara, and CF Industries investing heavily in electrochemical ammonia technologies. Simultaneously, technology startups such as Starfire Energy, Jupiter Ionics, and Nitricity are gaining traction with innovative electrode designs and catalysts that promise improved efficiency and selectivity.
Price sensitivity remains a critical market factor, as conventional ammonia production costs range from $400-600 per ton, while electrochemical methods currently average $900-1,200 per ton. However, this gap is expected to narrow as electrode efficiency improves and renewable electricity costs continue to decline, with some projections suggesting price parity could be achieved by 2030 in regions with abundant renewable energy resources.
Current Electrode Assessment Methods and Challenges
The assessment of electrode efficiency in ammonia synthesis requires sophisticated methodologies to accurately evaluate performance metrics. Currently, electrochemical techniques dominate the evaluation landscape, with cyclic voltammetry (CV) standing as the cornerstone method. CV provides critical insights into electrode reaction kinetics, electron transfer processes, and catalytic activity by measuring current responses to voltage sweeps. The resulting voltammograms reveal reduction peaks that directly correlate with nitrogen reduction reaction (NRR) efficiency.
Linear sweep voltammetry (LSV) complements CV by offering detailed information about electrode polarization behavior and overpotential requirements. This technique is particularly valuable for comparing different catalyst materials under standardized conditions, allowing researchers to quantify activation barriers and reaction rates across various electrode compositions.
Chronoamperometry represents another essential assessment tool, enabling the measurement of current stability over extended operation periods. This long-duration testing reveals degradation patterns and provides critical data on electrode durability—a parameter equally important as initial efficiency in practical applications. The stability curves generated through chronoamperometry help identify catalyst poisoning, structural changes, and activity losses that may not be apparent in short-term evaluations.
Electrochemical impedance spectroscopy (EIS) has emerged as a powerful non-destructive technique for characterizing electrode-electrolyte interfaces. By applying small-amplitude AC signals across a frequency spectrum, EIS generates Nyquist plots that reveal charge transfer resistances, double-layer capacitances, and diffusion limitations. These parameters provide mechanistic insights into reaction pathways and rate-limiting steps.
Despite these advanced methodologies, significant challenges persist in electrode assessment. The primary difficulty lies in distinguishing true ammonia production from contamination sources, as trace ammonia from the environment or nitrogen-containing reagents can lead to false positives. This necessitates rigorous control experiments and multiple verification techniques to validate results.
Standardization represents another major challenge, as variations in testing protocols, reference electrodes, electrolyte compositions, and cell configurations make direct comparisons between research groups problematic. The field currently lacks universally accepted benchmarking procedures, hampering collaborative progress and technology transfer.
Additionally, the scalability gap between laboratory assessments and industrial requirements creates significant uncertainty in performance predictions. Most evaluations occur in small-scale setups with idealized conditions, failing to account for mass transport limitations, heat management issues, and mechanical stresses present in scaled systems. This disconnect between laboratory metrics and practical performance remains a critical barrier to commercial implementation.
Linear sweep voltammetry (LSV) complements CV by offering detailed information about electrode polarization behavior and overpotential requirements. This technique is particularly valuable for comparing different catalyst materials under standardized conditions, allowing researchers to quantify activation barriers and reaction rates across various electrode compositions.
Chronoamperometry represents another essential assessment tool, enabling the measurement of current stability over extended operation periods. This long-duration testing reveals degradation patterns and provides critical data on electrode durability—a parameter equally important as initial efficiency in practical applications. The stability curves generated through chronoamperometry help identify catalyst poisoning, structural changes, and activity losses that may not be apparent in short-term evaluations.
Electrochemical impedance spectroscopy (EIS) has emerged as a powerful non-destructive technique for characterizing electrode-electrolyte interfaces. By applying small-amplitude AC signals across a frequency spectrum, EIS generates Nyquist plots that reveal charge transfer resistances, double-layer capacitances, and diffusion limitations. These parameters provide mechanistic insights into reaction pathways and rate-limiting steps.
Despite these advanced methodologies, significant challenges persist in electrode assessment. The primary difficulty lies in distinguishing true ammonia production from contamination sources, as trace ammonia from the environment or nitrogen-containing reagents can lead to false positives. This necessitates rigorous control experiments and multiple verification techniques to validate results.
Standardization represents another major challenge, as variations in testing protocols, reference electrodes, electrolyte compositions, and cell configurations make direct comparisons between research groups problematic. The field currently lacks universally accepted benchmarking procedures, hampering collaborative progress and technology transfer.
Additionally, the scalability gap between laboratory assessments and industrial requirements creates significant uncertainty in performance predictions. Most evaluations occur in small-scale setups with idealized conditions, failing to account for mass transport limitations, heat management issues, and mechanical stresses present in scaled systems. This disconnect between laboratory metrics and practical performance remains a critical barrier to commercial implementation.
State-of-the-Art Electrode Efficiency Evaluation Approaches
01 Metal-based catalytic electrodes
Metal-based electrodes, particularly those containing iron, ruthenium, or nickel, serve as effective catalysts for ammonia synthesis. These metals provide active sites for nitrogen reduction, enhancing the efficiency of the electrochemical process. The catalytic performance can be further improved by controlling the morphology, particle size, and surface area of the metal components, leading to higher ammonia yield rates and energy efficiency.- Metal-based catalytic electrodes: Metal-based electrodes, particularly those containing transition metals like iron, nickel, and ruthenium, serve as effective catalysts for ammonia synthesis. These electrodes provide active sites for nitrogen reduction and hydrogen activation, which are crucial steps in the ammonia synthesis process. The catalytic activity can be enhanced by controlling the morphology, crystal structure, and surface properties of the metal electrodes, leading to improved efficiency in ammonia production under milder conditions than traditional Haber-Bosch process.
- Nanostructured electrode materials: Nanostructured materials offer enhanced performance for ammonia synthesis electrodes due to their high surface area and abundant active sites. These materials include nanoparticles, nanowires, and nanosheets that can be engineered to optimize nitrogen adsorption and electron transfer. The nanoscale architecture allows for better mass transport and reaction kinetics, resulting in higher ammonia production rates at lower energy inputs. Additionally, the controlled synthesis of these nanostructures enables precise tuning of their electrocatalytic properties.
- Composite and supported electrode materials: Composite electrodes combining multiple active materials or supported catalysts on conductive substrates demonstrate superior performance in electrochemical ammonia synthesis. These electrodes typically feature catalytically active components dispersed on high-surface-area supports like carbon materials, metal oxides, or conductive polymers. The synergistic effects between the components enhance nitrogen activation and electron transfer efficiency. The support materials also improve stability and durability of the catalysts under operating conditions, while allowing for reduced noble metal loading and lower overall costs.
- Electrode design and architecture optimization: The physical design and architecture of electrodes significantly impact ammonia synthesis efficiency. Factors such as porosity, thickness, and geometric structure affect mass transport, reaction kinetics, and overall performance. Advanced electrode designs incorporate hierarchical structures with optimized macro, meso, and micropores to facilitate reactant diffusion and product removal. Three-dimensional electrode architectures provide increased active surface area while maintaining good electrical conductivity and mechanical stability. Innovative manufacturing techniques like 3D printing and template-assisted synthesis enable precise control over electrode architecture.
- Operating conditions and system integration: The efficiency of electrodes for ammonia synthesis is heavily influenced by operating conditions and system integration factors. Parameters such as temperature, pressure, electrolyte composition, applied potential, and current density must be optimized for specific electrode materials. Advanced electrochemical cell designs incorporate improved mass transport, thermal management, and electrical contact systems. Integration with renewable energy sources enables sustainable ammonia production, while continuous flow systems and improved separation techniques enhance overall process efficiency. Systematic approaches to electrode testing and performance evaluation help identify optimal operating windows.
02 Nanostructured electrode materials
Nanostructured materials offer enhanced performance for ammonia synthesis electrodes due to their high surface area and abundant active sites. These materials include nanowires, nanoparticles, and nanoporous structures that facilitate nitrogen adsorption and reduction. The controlled synthesis of these nanostructures allows for optimized electron transfer pathways and improved catalytic activity, resulting in higher ammonia production rates at lower energy inputs.Expand Specific Solutions03 Composite and hybrid electrode materials
Composite electrodes combining multiple functional materials demonstrate superior performance in ammonia synthesis. These hybrid structures typically integrate catalytic metals with conductive supports such as carbon-based materials or metal oxides. The synergistic effects between components enhance electron transfer, nitrogen activation, and proton supply, leading to improved reaction kinetics and overall efficiency of the electrochemical ammonia synthesis process.Expand Specific Solutions04 Advanced electrode fabrication techniques
Innovative fabrication methods significantly impact electrode performance for ammonia synthesis. Techniques such as atomic layer deposition, electrodeposition, and plasma treatment enable precise control over electrode structure and composition. These advanced manufacturing approaches allow for the creation of defect-rich surfaces, optimized porosity, and tailored interfaces that enhance catalytic activity and stability, resulting in more efficient ammonia production systems.Expand Specific Solutions05 Electrolyte and operating condition optimization
The efficiency of ammonia synthesis electrodes is heavily influenced by electrolyte composition and operating conditions. Factors such as pH, temperature, pressure, and applied potential significantly affect the reaction pathways and selectivity. Optimizing these parameters, along with electrode design, can suppress competing reactions like hydrogen evolution, enhance nitrogen reduction, and improve faradaic efficiency, leading to more energy-efficient ammonia production processes.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The ammonia synthesis electrode efficiency assessment field is currently in a growth phase, characterized by significant academic and industrial collaboration. The market is expanding rapidly due to increasing demand for sustainable ammonia production methods, with an estimated global market value exceeding $70 billion. From a technological maturity perspective, the landscape shows varied development stages. Academic institutions like Monash University, Zhejiang University, and Technical University of Denmark are pioneering fundamental research, while companies including GenCell, BASF, and Siemens are advancing practical applications. Notably, LG Energy Solution and Toshiba Energy Systems are developing commercial-scale implementations, while specialized players like Atmonia are creating breakthrough electrocatalytic processes. This competitive environment reflects the technology's transition from laboratory research to industrial application, with significant innovation occurring at the academic-industry interface.
BASF Corp.
Technical Solution: BASF has developed a comprehensive electrode efficiency assessment framework for ammonia synthesis that bridges fundamental research and industrial application. Their methodology integrates high-throughput experimentation with advanced in-situ characterization techniques to evaluate electrode materials under realistic operating conditions. BASF employs specialized flow reactors with precise temperature and pressure control to simulate industrial conditions while enabling accurate product quantification. Their approach incorporates membrane-electrode assembly (MEA) testing protocols that evaluate not only catalytic activity but also long-term stability and resistance to common contaminants[3]. BASF has pioneered the use of operando X-ray absorption spectroscopy and environmental transmission electron microscopy to monitor structural changes in electrodes during ammonia synthesis. Their assessment methodology includes standardized protocols for calculating energy efficiency that account for both electrical input and thermal management requirements, providing a holistic view of electrode performance in practical applications[6].
Strengths: Industrial relevance with testing under realistic operating conditions; comprehensive evaluation incorporating activity, selectivity, stability and scalability; standardized protocols enabling direct comparison between different catalyst systems. Weaknesses: Proprietary nature of some assessment techniques limits broader scientific adoption; focus on industrial viability may overlook promising early-stage materials; significant infrastructure requirements for comprehensive assessment.
Atmonia ehf
Technical Solution: Atmonia has developed a proprietary electrode efficiency assessment methodology specifically tailored for their biomimetic catalyst approach to ammonia synthesis. Their assessment framework centers on molecular-level characterization of nitrogen activation at catalyst active sites, using a combination of electrochemical techniques and spectroscopic methods. Atmonia employs cyclic voltammetry with rotating disk electrode configurations to precisely measure kinetic parameters and distinguish between different reaction pathways[1]. Their methodology incorporates isotope-labeled experiments using 15N2 to conclusively verify ammonia production and calculate exact conversion efficiencies. Atmonia has pioneered the use of specialized flow-cell designs that enable long-duration stability testing under industrially relevant conditions while maintaining precise control over reactant delivery and product collection. Their assessment protocol includes comprehensive impedance analysis to characterize mass transport limitations and charge transfer resistances at the electrode-electrolyte interface[5].
Strengths: Highly specialized for biomimetic catalyst systems; integrated approach connecting molecular mechanisms to overall efficiency; robust verification protocols eliminating false positives. Weaknesses: Methodology optimized for Atmonia's specific catalyst systems may have limited applicability to other approaches; resource-intensive assessment procedures; primarily focused on laboratory-scale demonstration rather than industrial implementation.
Key Scientific Breakthroughs in Electrode Assessment
Electrochemical ammonia synthesis
PatentPendingUS20230086549A1
Innovation
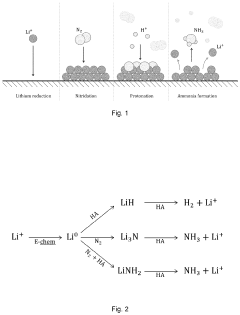
- Implementing a pulsed cathode potential and pulsed cathodic current load, cycling between the lithium reduction potential and a less negative potential, and using a source of mediating cations such as lithium ions, helps maintain cathode stability and efficiency by regenerating the cathode and optimizing ammonia synthesis.
Electrochemical synthesis of ammonia using separation membrane and ionic liquid
PatentInactiveUS20230073509A1
Innovation
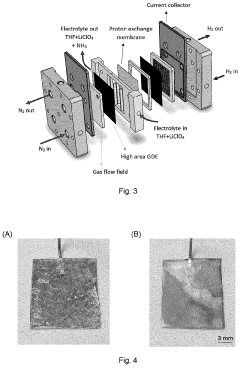
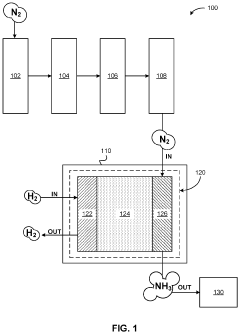
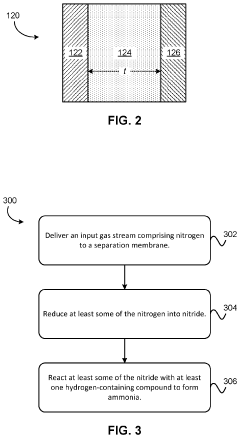
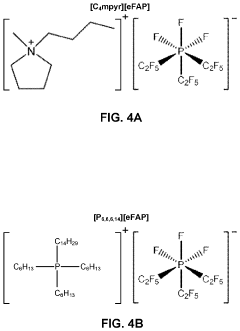
- A system utilizing a separation membrane with an anode, cathode, and porous support material, along with ionic liquids, to reduce nitrogen into nitride ions and facilitate their hydrogenation to form ammonia at room temperature, avoiding high energy requirements and parasitic hydrogen evolution.
Environmental Impact and Sustainability Considerations
The environmental impact of electrode-based ammonia synthesis processes represents a critical dimension in evaluating their overall efficiency and viability. Traditional Haber-Bosch ammonia production consumes approximately 1-2% of global energy and generates significant carbon emissions—approximately 1.8 tons of CO2 per ton of ammonia produced. Electrochemical ammonia synthesis methods offer promising alternatives with potentially lower environmental footprints, but comprehensive assessment frameworks are essential to verify these benefits.
When assessing electrode efficiency in ammonia synthesis, life cycle assessment (LCA) methodologies provide valuable insights into environmental impacts across the entire production chain. These assessments must consider raw material extraction, electrode manufacturing processes, operational energy requirements, and end-of-life disposal or recycling pathways. Particularly important is the source of electricity powering electrochemical processes, as this significantly influences the overall carbon footprint.
Electrode materials present substantial sustainability challenges. Many high-performance electrodes incorporate precious metals like ruthenium or platinum, raising concerns about resource scarcity and extraction impacts. Assessment methodologies must therefore incorporate metrics for material criticality, recyclability potential, and substitution possibilities. Recent research has focused on developing efficient electrodes using earth-abundant materials such as iron-based catalysts and nitrogen-doped carbon structures, which should be prioritized in environmental assessments.
Water consumption represents another crucial environmental consideration in electrode efficiency evaluation. While electrochemical processes typically require less water than conventional methods, the quality of wastewater discharge and potential contamination from catalyst leaching must be carefully monitored. Standardized protocols for measuring water footprints specific to electrochemical ammonia synthesis are currently underdeveloped and require further refinement.
Energy efficiency metrics must extend beyond simple conversion rates to include considerations of renewable energy integration potential. Electrochemical ammonia synthesis offers unique advantages in its compatibility with intermittent renewable energy sources, potentially enabling distributed production systems that minimize transportation impacts. Assessment methodologies should therefore incorporate flexibility parameters that evaluate how effectively electrode systems can operate under variable power conditions typical of renewable energy sources.
Regulatory frameworks and sustainability certification systems are increasingly influencing technology adoption pathways. Comprehensive electrode efficiency assessments must therefore align with emerging standards such as the EU Taxonomy for Sustainable Activities and similar frameworks in other regions. This alignment ensures that technological development proceeds in harmony with broader sustainability objectives and facilitates market acceptance of innovative ammonia synthesis technologies.
When assessing electrode efficiency in ammonia synthesis, life cycle assessment (LCA) methodologies provide valuable insights into environmental impacts across the entire production chain. These assessments must consider raw material extraction, electrode manufacturing processes, operational energy requirements, and end-of-life disposal or recycling pathways. Particularly important is the source of electricity powering electrochemical processes, as this significantly influences the overall carbon footprint.
Electrode materials present substantial sustainability challenges. Many high-performance electrodes incorporate precious metals like ruthenium or platinum, raising concerns about resource scarcity and extraction impacts. Assessment methodologies must therefore incorporate metrics for material criticality, recyclability potential, and substitution possibilities. Recent research has focused on developing efficient electrodes using earth-abundant materials such as iron-based catalysts and nitrogen-doped carbon structures, which should be prioritized in environmental assessments.
Water consumption represents another crucial environmental consideration in electrode efficiency evaluation. While electrochemical processes typically require less water than conventional methods, the quality of wastewater discharge and potential contamination from catalyst leaching must be carefully monitored. Standardized protocols for measuring water footprints specific to electrochemical ammonia synthesis are currently underdeveloped and require further refinement.
Energy efficiency metrics must extend beyond simple conversion rates to include considerations of renewable energy integration potential. Electrochemical ammonia synthesis offers unique advantages in its compatibility with intermittent renewable energy sources, potentially enabling distributed production systems that minimize transportation impacts. Assessment methodologies should therefore incorporate flexibility parameters that evaluate how effectively electrode systems can operate under variable power conditions typical of renewable energy sources.
Regulatory frameworks and sustainability certification systems are increasingly influencing technology adoption pathways. Comprehensive electrode efficiency assessments must therefore align with emerging standards such as the EU Taxonomy for Sustainable Activities and similar frameworks in other regions. This alignment ensures that technological development proceeds in harmony with broader sustainability objectives and facilitates market acceptance of innovative ammonia synthesis technologies.
Standardization and Benchmarking Protocols
The development of standardized protocols for evaluating electrode performance in ammonia synthesis represents a critical need in the field. Currently, researchers employ diverse methodologies, equipment configurations, and reporting metrics, making direct comparisons between studies challenging and potentially misleading. Establishing universal benchmarking standards would significantly accelerate progress by enabling meaningful cross-laboratory validation and technology assessment.
A comprehensive standardization framework should address multiple dimensions of electrode evaluation. Reaction conditions must be precisely defined, including temperature ranges (typically 25-400°C), pressure parameters (1-100 atm), electrolyte compositions, and feed gas ratios. These parameters dramatically influence performance metrics and must be controlled to ensure reproducibility across different research environments.
Performance metrics require particular attention in standardization efforts. Faradaic efficiency calculations, ammonia yield rates, and energy efficiency assessments should follow consistent methodologies. The scientific community would benefit from adopting unified equations for calculating key parameters such as turnover frequency (TOF), current density normalization approaches, and specific energy consumption metrics (kWh/kg NH3).
Analytical validation protocols represent another crucial standardization component. Methods for quantifying produced ammonia—including colorimetric techniques (indophenol blue method), ion chromatography, and NMR spectroscopy—should be standardized with defined detection limits, calibration procedures, and quality control measures. Cross-validation using multiple detection methods should become standard practice to eliminate false positives.
Durability testing protocols constitute an often overlooked yet essential aspect of electrode assessment. Standardized stability tests should specify minimum operational timeframes (e.g., 100+ hours), cycling conditions, and degradation rate calculation methodologies. Accelerated aging protocols could provide valuable insights into long-term performance while reducing testing timeframes.
International collaboration between academic institutions, industry stakeholders, and standards organizations will be necessary to develop and implement these protocols. Organizations such as IUPAC, ISO, and ASTM could provide the institutional framework for formalizing these standards. The establishment of round-robin testing programs across multiple laboratories would validate protocol robustness and identify areas requiring refinement.
Digital platforms for sharing standardized experimental data would further enhance benchmarking capabilities, allowing researchers to compare their results against established reference electrodes and systems. This approach would accelerate the identification of truly promising materials and configurations while reducing research redundancy and resource expenditure.
A comprehensive standardization framework should address multiple dimensions of electrode evaluation. Reaction conditions must be precisely defined, including temperature ranges (typically 25-400°C), pressure parameters (1-100 atm), electrolyte compositions, and feed gas ratios. These parameters dramatically influence performance metrics and must be controlled to ensure reproducibility across different research environments.
Performance metrics require particular attention in standardization efforts. Faradaic efficiency calculations, ammonia yield rates, and energy efficiency assessments should follow consistent methodologies. The scientific community would benefit from adopting unified equations for calculating key parameters such as turnover frequency (TOF), current density normalization approaches, and specific energy consumption metrics (kWh/kg NH3).
Analytical validation protocols represent another crucial standardization component. Methods for quantifying produced ammonia—including colorimetric techniques (indophenol blue method), ion chromatography, and NMR spectroscopy—should be standardized with defined detection limits, calibration procedures, and quality control measures. Cross-validation using multiple detection methods should become standard practice to eliminate false positives.
Durability testing protocols constitute an often overlooked yet essential aspect of electrode assessment. Standardized stability tests should specify minimum operational timeframes (e.g., 100+ hours), cycling conditions, and degradation rate calculation methodologies. Accelerated aging protocols could provide valuable insights into long-term performance while reducing testing timeframes.
International collaboration between academic institutions, industry stakeholders, and standards organizations will be necessary to develop and implement these protocols. Organizations such as IUPAC, ISO, and ASTM could provide the institutional framework for formalizing these standards. The establishment of round-robin testing programs across multiple laboratories would validate protocol robustness and identify areas requiring refinement.
Digital platforms for sharing standardized experimental data would further enhance benchmarking capabilities, allowing researchers to compare their results against established reference electrodes and systems. This approach would accelerate the identification of truly promising materials and configurations while reducing research redundancy and resource expenditure.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!