Study of Catalytic Surface Reactions in Ammonia Production
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonia Catalysis Background and Objectives
Ammonia synthesis represents one of the most significant industrial chemical processes developed in the 20th century, with the Haber-Bosch process standing as a cornerstone of modern agriculture and chemical manufacturing. Since its development in the early 1900s by Fritz Haber and Carl Bosch, this catalytic process has enabled the large-scale production of ammonia from atmospheric nitrogen and hydrogen, fundamentally transforming global food production capabilities through nitrogen-based fertilizers.
The evolution of ammonia synthesis catalysis has progressed through several distinct phases, beginning with the original iron-based catalysts developed by Alwin Mittasch, which remarkably remain in widespread industrial use today. Subsequent advancements have explored ruthenium-based systems, which offer improved activity at lower temperatures and pressures, though economic considerations have limited their commercial adoption.
Current technical objectives in ammonia catalysis research focus on addressing the inherent energy intensity of the traditional Haber-Bosch process, which consumes approximately 1-2% of global energy production. This energy demand stems from the harsh reaction conditions required (400-500°C and 150-300 bar) to overcome the kinetic barriers of nitrogen triple-bond activation.
The field is now witnessing a paradigm shift toward more sustainable ammonia production pathways. Emerging research directions include electrochemical and photocatalytic ammonia synthesis, which aim to operate under ambient conditions using renewable electricity. Biological inspiration from nitrogenase enzymes has also prompted exploration of biomimetic catalysts capable of nitrogen fixation under mild conditions.
A critical technical goal involves developing catalysts with improved nitrogen adsorption and activation properties while maintaining resistance to common catalyst poisons such as oxygen, water, and sulfur compounds. Surface science techniques have become instrumental in understanding the atomic-level interactions at catalyst surfaces, enabling rational catalyst design rather than empirical approaches.
The ultimate objective of contemporary research is to establish economically viable pathways for decentralized, small-scale ammonia production that can operate efficiently using intermittent renewable energy sources. This would represent a transformative development, potentially enabling localized fertilizer production and creating new opportunities for ammonia as an energy carrier in a hydrogen economy.
Understanding the fundamental surface chemistry of nitrogen activation remains the cornerstone challenge, with particular focus on identifying novel catalyst compositions and structures that can lower the activation energy barrier while maintaining sufficient stability for industrial implementation.
The evolution of ammonia synthesis catalysis has progressed through several distinct phases, beginning with the original iron-based catalysts developed by Alwin Mittasch, which remarkably remain in widespread industrial use today. Subsequent advancements have explored ruthenium-based systems, which offer improved activity at lower temperatures and pressures, though economic considerations have limited their commercial adoption.
Current technical objectives in ammonia catalysis research focus on addressing the inherent energy intensity of the traditional Haber-Bosch process, which consumes approximately 1-2% of global energy production. This energy demand stems from the harsh reaction conditions required (400-500°C and 150-300 bar) to overcome the kinetic barriers of nitrogen triple-bond activation.
The field is now witnessing a paradigm shift toward more sustainable ammonia production pathways. Emerging research directions include electrochemical and photocatalytic ammonia synthesis, which aim to operate under ambient conditions using renewable electricity. Biological inspiration from nitrogenase enzymes has also prompted exploration of biomimetic catalysts capable of nitrogen fixation under mild conditions.
A critical technical goal involves developing catalysts with improved nitrogen adsorption and activation properties while maintaining resistance to common catalyst poisons such as oxygen, water, and sulfur compounds. Surface science techniques have become instrumental in understanding the atomic-level interactions at catalyst surfaces, enabling rational catalyst design rather than empirical approaches.
The ultimate objective of contemporary research is to establish economically viable pathways for decentralized, small-scale ammonia production that can operate efficiently using intermittent renewable energy sources. This would represent a transformative development, potentially enabling localized fertilizer production and creating new opportunities for ammonia as an energy carrier in a hydrogen economy.
Understanding the fundamental surface chemistry of nitrogen activation remains the cornerstone challenge, with particular focus on identifying novel catalyst compositions and structures that can lower the activation energy barrier while maintaining sufficient stability for industrial implementation.
Market Analysis of Ammonia Production Technologies
The global ammonia market is experiencing significant growth, with a current market value estimated at $70 billion and projected to reach $110 billion by 2030. This growth is primarily driven by increasing demand for fertilizers, which account for approximately 80% of ammonia consumption worldwide. The agricultural sector's expansion, particularly in developing regions like Asia-Pacific and Latin America, continues to fuel this demand as global food production requirements rise with population growth.
Industrial applications represent the second-largest market segment, with ammonia being utilized in the production of explosives, pharmaceuticals, textiles, and cleaning products. This segment is expected to grow at a compound annual growth rate of 5.2% through 2028, outpacing the overall market growth rate of 4.7%.
The ammonia production technology landscape is currently dominated by the traditional Haber-Bosch process, which accounts for over 90% of global production capacity. However, this century-old technology faces significant challenges due to its high energy consumption and substantial carbon footprint, with ammonia production responsible for approximately 1.8% of global CO2 emissions.
Emerging green ammonia technologies are gaining traction in response to increasing environmental regulations and corporate sustainability commitments. These technologies utilize renewable energy sources and alternative catalytic processes to reduce or eliminate carbon emissions. The green ammonia market segment, though currently representing less than 1% of total production, is projected to grow at over 70% annually through 2030.
Regional analysis reveals that Asia-Pacific dominates ammonia production, with China accounting for approximately 30% of global capacity. North America and Europe follow with 15% and 12% respectively, while the Middle East is rapidly expanding its production capabilities due to advantageous natural gas pricing.
Price volatility remains a significant market challenge, with ammonia prices fluctuating between $250-800 per ton over the past five years. This volatility is primarily driven by natural gas price variations, which account for 70-80% of production costs in conventional processes. This economic pressure is accelerating research into catalytic innovations that can improve energy efficiency and reduce production costs.
Market consolidation is evident, with the top ten producers controlling approximately 55% of global capacity. Major players are increasingly investing in research and development of advanced catalytic technologies to maintain competitive advantages, with annual R&D expenditures in ammonia production technologies exceeding $2 billion globally.
Industrial applications represent the second-largest market segment, with ammonia being utilized in the production of explosives, pharmaceuticals, textiles, and cleaning products. This segment is expected to grow at a compound annual growth rate of 5.2% through 2028, outpacing the overall market growth rate of 4.7%.
The ammonia production technology landscape is currently dominated by the traditional Haber-Bosch process, which accounts for over 90% of global production capacity. However, this century-old technology faces significant challenges due to its high energy consumption and substantial carbon footprint, with ammonia production responsible for approximately 1.8% of global CO2 emissions.
Emerging green ammonia technologies are gaining traction in response to increasing environmental regulations and corporate sustainability commitments. These technologies utilize renewable energy sources and alternative catalytic processes to reduce or eliminate carbon emissions. The green ammonia market segment, though currently representing less than 1% of total production, is projected to grow at over 70% annually through 2030.
Regional analysis reveals that Asia-Pacific dominates ammonia production, with China accounting for approximately 30% of global capacity. North America and Europe follow with 15% and 12% respectively, while the Middle East is rapidly expanding its production capabilities due to advantageous natural gas pricing.
Price volatility remains a significant market challenge, with ammonia prices fluctuating between $250-800 per ton over the past five years. This volatility is primarily driven by natural gas price variations, which account for 70-80% of production costs in conventional processes. This economic pressure is accelerating research into catalytic innovations that can improve energy efficiency and reduce production costs.
Market consolidation is evident, with the top ten producers controlling approximately 55% of global capacity. Major players are increasingly investing in research and development of advanced catalytic technologies to maintain competitive advantages, with annual R&D expenditures in ammonia production technologies exceeding $2 billion globally.
Current Catalytic Surface Reaction Challenges
The current landscape of catalytic surface reactions in ammonia production faces several significant challenges despite over a century of industrial implementation. The Haber-Bosch process, while revolutionary, operates under energy-intensive conditions requiring temperatures of 400-500°C and pressures of 150-300 bar, resulting in substantial energy consumption—approximately 1-2% of global energy production.
A primary technical challenge lies in the nitrogen activation step, which involves breaking the strong N≡N triple bond. Current iron-based catalysts require high temperatures to overcome this activation barrier, creating an inherent energy efficiency limitation. This challenge is compounded by the thermodynamic constraints of the reaction, where higher temperatures favor the reverse reaction according to Le Chatelier's principle.
Catalyst deactivation presents another persistent issue. Industrial catalysts suffer from sintering, poisoning by oxygen and sulfur compounds, and coking, all of which reduce active site availability and necessitate costly catalyst regeneration or replacement. The iron-based catalysts, while economically viable, exhibit lower intrinsic activity compared to ruthenium-based alternatives, creating a trade-off between cost and performance.
Selectivity challenges also exist, particularly in managing the competing hydrogen evolution reaction that consumes valuable hydrogen without producing ammonia. Current catalysts struggle to achieve optimal nitrogen adsorption while simultaneously facilitating N-H bond formation, resulting in reduced reaction efficiency and yield.
Scale-up issues persist when transitioning from laboratory discoveries to industrial implementation. Many promising catalytic materials that demonstrate excellent performance in controlled laboratory environments fail to maintain their activity under industrial conditions or cannot be manufactured cost-effectively at scale.
The development of sustainable ammonia production faces additional hurdles in integrating renewable energy sources. Intermittent renewable electricity supply creates operational challenges for traditional catalytic systems designed for continuous operation. Current catalysts are not optimized for the dynamic operating conditions necessary for renewable energy integration.
Recent research has focused on electrocatalytic and photocatalytic ammonia synthesis as alternatives to thermal catalysis, but these approaches face their own challenges in achieving practical reaction rates and selectivity under ambient conditions. The fundamental understanding of reaction mechanisms at the molecular level remains incomplete, hampering rational catalyst design efforts.
A primary technical challenge lies in the nitrogen activation step, which involves breaking the strong N≡N triple bond. Current iron-based catalysts require high temperatures to overcome this activation barrier, creating an inherent energy efficiency limitation. This challenge is compounded by the thermodynamic constraints of the reaction, where higher temperatures favor the reverse reaction according to Le Chatelier's principle.
Catalyst deactivation presents another persistent issue. Industrial catalysts suffer from sintering, poisoning by oxygen and sulfur compounds, and coking, all of which reduce active site availability and necessitate costly catalyst regeneration or replacement. The iron-based catalysts, while economically viable, exhibit lower intrinsic activity compared to ruthenium-based alternatives, creating a trade-off between cost and performance.
Selectivity challenges also exist, particularly in managing the competing hydrogen evolution reaction that consumes valuable hydrogen without producing ammonia. Current catalysts struggle to achieve optimal nitrogen adsorption while simultaneously facilitating N-H bond formation, resulting in reduced reaction efficiency and yield.
Scale-up issues persist when transitioning from laboratory discoveries to industrial implementation. Many promising catalytic materials that demonstrate excellent performance in controlled laboratory environments fail to maintain their activity under industrial conditions or cannot be manufactured cost-effectively at scale.
The development of sustainable ammonia production faces additional hurdles in integrating renewable energy sources. Intermittent renewable electricity supply creates operational challenges for traditional catalytic systems designed for continuous operation. Current catalysts are not optimized for the dynamic operating conditions necessary for renewable energy integration.
Recent research has focused on electrocatalytic and photocatalytic ammonia synthesis as alternatives to thermal catalysis, but these approaches face their own challenges in achieving practical reaction rates and selectivity under ambient conditions. The fundamental understanding of reaction mechanisms at the molecular level remains incomplete, hampering rational catalyst design efforts.
State-of-the-Art Catalytic Surface Reaction Methods
01 Catalytic surface reactions in chemical synthesis
Catalytic surface reactions play a crucial role in chemical synthesis processes. These reactions involve the use of catalytic surfaces to facilitate chemical transformations, enhancing reaction rates and selectivity. The catalytic surfaces provide active sites where reactants can adsorb, interact, and form desired products under milder conditions than would be required without catalysts. This approach is particularly valuable in industrial applications where efficiency and product specificity are essential.- Catalytic surface reactions in chemical synthesis: Catalytic surface reactions play a crucial role in chemical synthesis processes. These reactions involve the use of catalytic surfaces to facilitate chemical transformations, enhancing reaction rates and selectivity. The catalytic surfaces provide active sites where reactants can adsorb, interact, and form products more efficiently than in homogeneous reactions. This approach is particularly valuable in industrial applications where high yield and purity of products are essential.
- Heterogeneous catalysis for environmental applications: Heterogeneous catalytic surface reactions are employed in environmental applications to reduce pollutants and harmful emissions. These catalytic systems utilize solid surfaces that interact with gaseous or liquid reactants to convert environmentally harmful substances into benign products. Applications include catalytic converters in automobiles, air purification systems, and water treatment technologies. The efficiency of these catalytic processes depends on factors such as surface area, catalyst composition, and reaction conditions.
- Novel catalyst materials and surface modifications: Innovations in catalyst materials and surface modifications enhance the performance of catalytic surface reactions. These advancements include the development of nanostructured catalysts, composite materials, and surface-modified catalysts with improved activity, selectivity, and stability. Techniques such as doping, alloying, and creating specific surface structures are employed to optimize catalytic properties. These novel materials expand the range of possible catalytic transformations and improve the efficiency of existing processes.
- Reactor design for catalytic surface reactions: Specialized reactor designs optimize catalytic surface reactions by enhancing contact between reactants and catalytic surfaces. These designs consider factors such as flow patterns, temperature distribution, pressure conditions, and catalyst arrangement to maximize reaction efficiency. Innovations include fluidized bed reactors, fixed bed reactors with optimized geometry, microreactors, and membrane reactors. Proper reactor design is crucial for scaling up laboratory processes to industrial production while maintaining catalytic performance.
- Mechanisms and kinetics of catalytic surface reactions: Understanding the mechanisms and kinetics of catalytic surface reactions is essential for optimizing catalytic processes. Research in this area focuses on identifying reaction pathways, intermediate species, rate-determining steps, and the influence of reaction conditions on catalytic performance. Advanced analytical techniques and computational methods are employed to study adsorption phenomena, surface diffusion, and the electronic properties of catalytic surfaces. This fundamental knowledge guides the rational design of more efficient catalytic systems.
02 Metal-based catalytic surfaces for reaction enhancement
Metal-based materials serve as effective catalytic surfaces for various chemical reactions. These surfaces, often comprising noble metals or transition metal compounds, provide unique electronic and structural properties that facilitate bond breaking and formation. The metal catalysts can be used in pure form, as alloys, or supported on various substrates to maximize surface area and catalytic efficiency. Their ability to lower activation energy barriers makes them particularly valuable in industrial processes requiring high throughput and energy efficiency.Expand Specific Solutions03 Novel reactor designs for catalytic surface reactions
Innovative reactor designs enhance the performance of catalytic surface reactions by optimizing contact between reactants and catalytic surfaces. These designs focus on parameters such as flow dynamics, temperature distribution, and catalyst arrangement to maximize reaction efficiency. Advanced reactor configurations may incorporate features like fluidized beds, fixed catalyst layers, or microreactor channels that increase surface area exposure while maintaining optimal reaction conditions. Such engineering approaches significantly improve conversion rates, selectivity, and overall process economics.Expand Specific Solutions04 Surface modification techniques for enhanced catalytic activity
Various surface modification techniques can be employed to enhance the catalytic activity of reaction surfaces. These methods include physical treatments like etching and plasma processing, chemical modifications through functionalization, and the deposition of active species onto support materials. By tailoring surface properties such as roughness, porosity, and chemical composition, researchers can create catalytic surfaces with improved reactivity, selectivity, and stability. These modified surfaces often demonstrate superior performance in challenging reaction environments.Expand Specific Solutions05 Environmental applications of catalytic surface reactions
Catalytic surface reactions have significant applications in environmental protection and remediation technologies. These include processes for air pollution control, wastewater treatment, and conversion of harmful substances into benign compounds. Catalytic surfaces can facilitate the breakdown of pollutants like volatile organic compounds, nitrogen oxides, and carbon monoxide into less harmful substances. The development of efficient, durable catalytic materials for these applications continues to be an important area of research with substantial environmental benefits.Expand Specific Solutions
Leading Companies and Research Institutions in Ammonia Catalysis
The catalytic surface reactions in ammonia production field is currently in a mature development stage, with a global market size exceeding $70 billion annually. The technology has reached high maturity levels, with established players like Topsoe A/S and Air Liquide leading conventional catalytic processes. However, innovation is accelerating with research institutions (Max Planck Society, CNRS, MIT) and corporations (Toyota, SABIC) developing next-generation catalysts. Emerging companies like Atmonia are pioneering electro-catalytic processes for sustainable ammonia synthesis. The competitive landscape shows a balance between traditional industrial giants optimizing existing processes and research-driven entities exploring novel catalytic mechanisms to address efficiency and sustainability challenges in ammonia production.
Topsoe A/S
Technical Solution: Topsoe has developed advanced catalytic technologies for ammonia synthesis focusing on improved energy efficiency and reduced carbon footprint. Their TITAN series catalysts feature optimized iron-based formulations with structural promoters that enhance active site availability and stability. The company has pioneered the integration of ruthenium-based catalysts that operate at lower pressures (40-100 bar) compared to conventional Haber-Bosch processes (150-300 bar), reducing energy requirements by up to 20%. Topsoe's SynCOR Ammonia™ technology combines innovative catalysts with process optimization, utilizing a single-train design that improves heat integration and reduces CO2 emissions by approximately 3-5% compared to conventional plants[1]. Their latest development includes magnetite-based catalysts with controlled porosity and surface area exceeding 15 m²/g, allowing for enhanced mass transfer and reaction kinetics at the catalyst surface.
Strengths: Industry-leading expertise in catalyst development with over 80 years of experience; integrated solutions combining catalysts with process technology; strong focus on sustainability with lower energy consumption designs. Weaknesses: Higher initial investment costs for their advanced catalyst systems; technology primarily optimized for large-scale production facilities rather than distributed manufacturing.
Atmonia ehf
Technical Solution: Atmonia has developed an electrochemical approach to ammonia synthesis that fundamentally reimagines catalytic surface reactions. Their technology employs non-noble metal nitride catalysts that facilitate nitrogen reduction at ambient conditions, bypassing the high-pressure, high-temperature requirements of traditional Haber-Bosch processes. The catalyst system features nanostructured transition metal nitrides with engineered defect sites that lower the activation energy for N₂ bond cleavage. These catalysts are integrated into a membrane electrode assembly where nitrogen is reduced at the cathode while water is oxidized at the anode. The company's proprietary catalyst preparation method involves controlled nitridation processes that create specific crystal facets with optimal binding energies for reaction intermediates. Atmonia's catalysts demonstrate faradaic efficiencies of 10-15% for ammonia production at ambient pressure, with current densities reaching 50-100 mA/cm²[2]. Their system incorporates in-situ regeneration mechanisms to address catalyst poisoning issues that typically plague low-temperature ammonia synthesis.
Strengths: Operates at ambient temperature and pressure, dramatically reducing energy requirements; eliminates need for hydrogen feedstock by using water as hydrogen source; modular design suitable for distributed production and renewable energy integration. Weaknesses: Lower production rates compared to conventional processes; catalyst stability and longevity issues under continuous operation; currently at lower technology readiness level compared to established industrial processes.
Key Patents and Scientific Breakthroughs in Ammonia Catalysis
Improvements in or relating to catalytic materials adapted for use in the synthesis of ammonia
PatentInactiveGB153254A
Innovation
- A method involving melting iron with a jet of oxygen, collecting the molten material in a magnesia crucible, and subjecting it to further oxygen peroxidation while stirring, resulting in a higher oxygen oxide catalytic material, which is then enhanced with lime addition to extend activity and longevity.
Electrochemical synthesis of ammonia
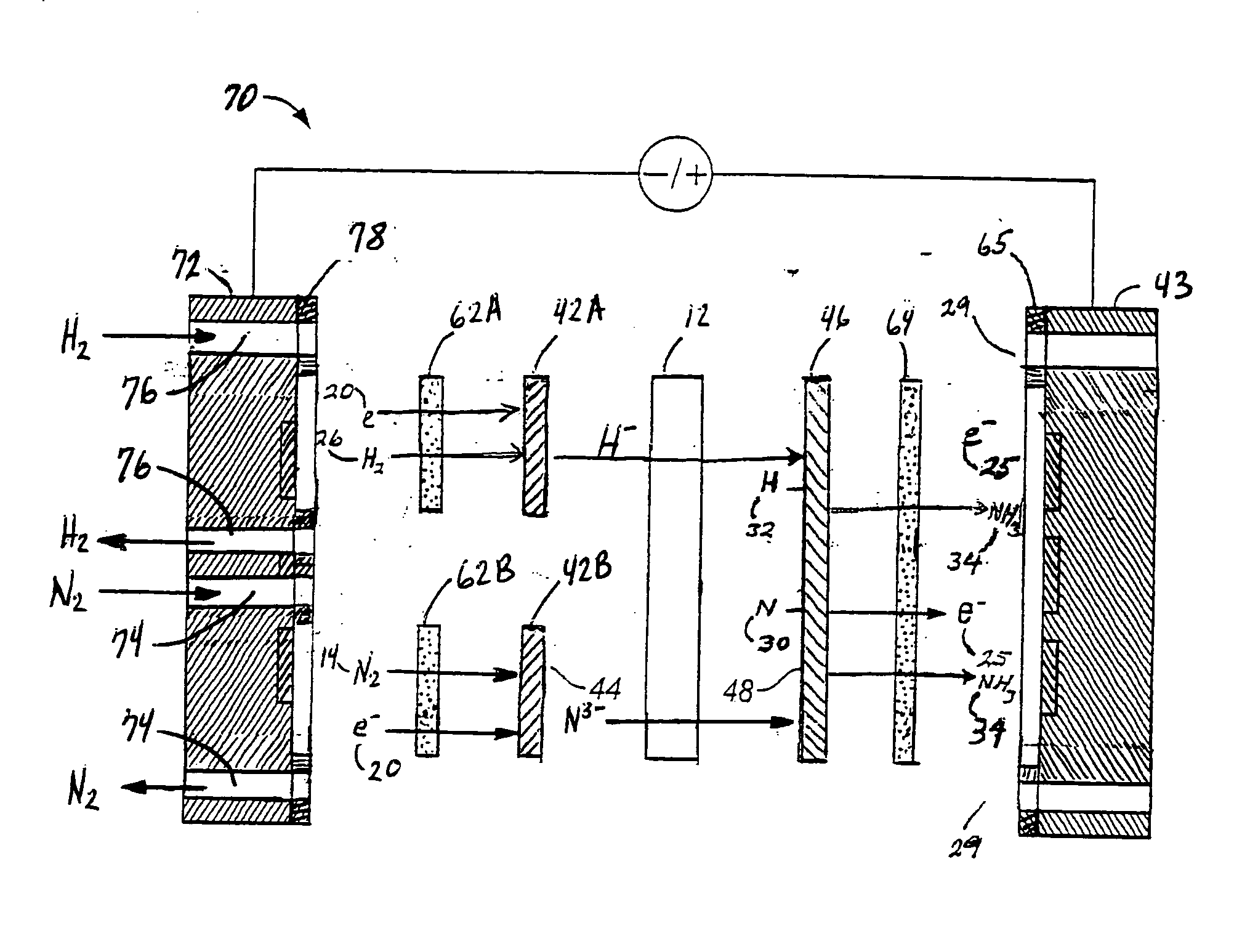
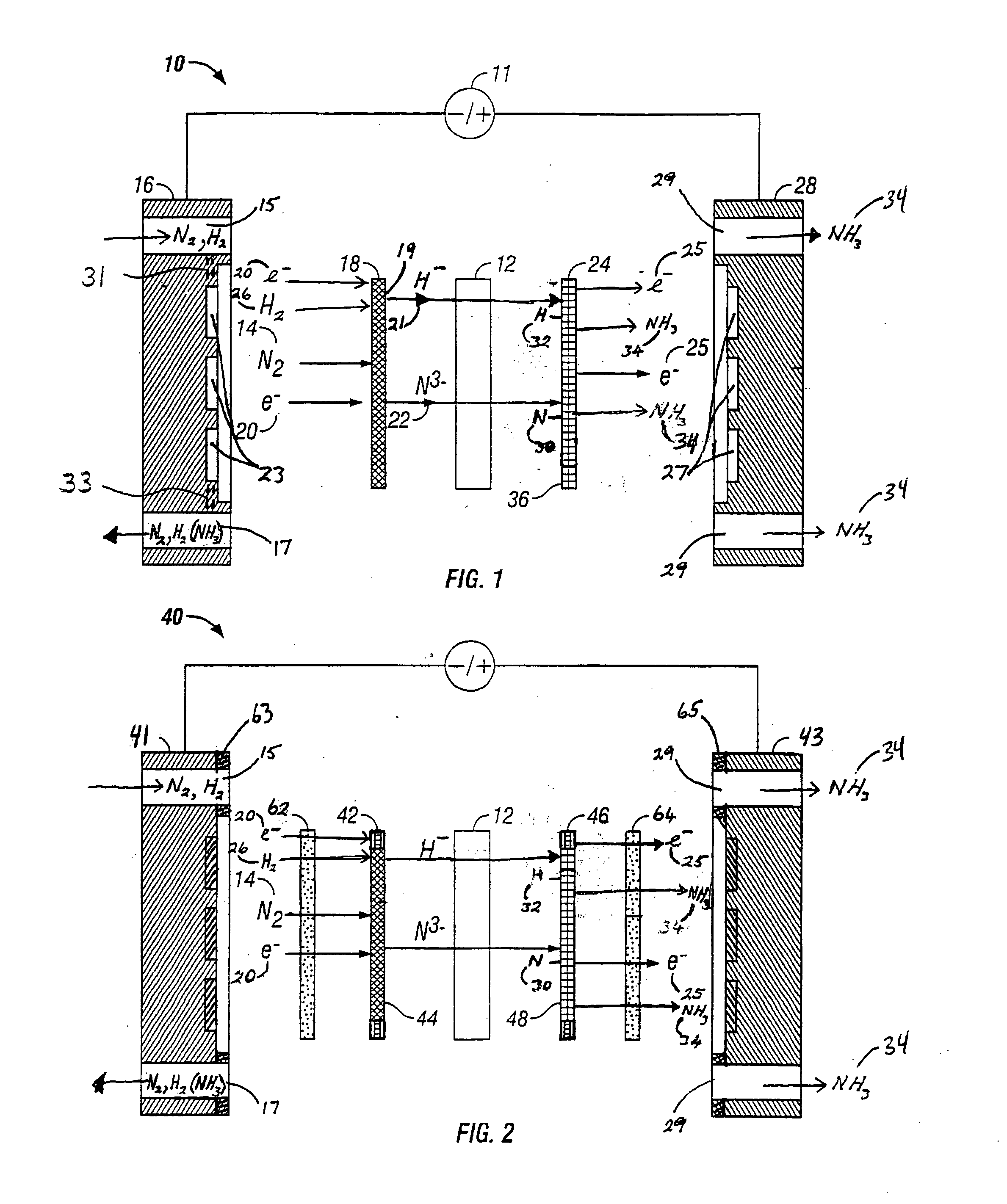
PatentInactiveUS20060049063A1
Innovation
- An anodic electrochemical method using molten salts with dissolved nitride ions and a porous anode structure to oxidize nitride ions and react them with adsorbed hydrogen atoms to form ammonia, allowing for the production of ammonia at lower temperatures and pressures with improved conversion efficiencies.
Environmental Impact and Sustainability Considerations
Ammonia production, particularly through the Haber-Bosch process, presents significant environmental challenges that must be addressed for sustainable industrial development. The catalytic surface reactions involved in this process currently consume approximately 1-2% of global energy production and contribute substantially to greenhouse gas emissions, with estimates suggesting that ammonia synthesis accounts for approximately 1.4% of global CO2 emissions. The high-pressure, high-temperature conditions required for traditional catalytic processes represent a substantial environmental burden.
Recent advancements in catalyst design have demonstrated potential for reducing the environmental footprint of ammonia production. Novel catalysts operating at lower temperatures and pressures could reduce energy requirements by up to 20-30%, according to research from leading institutions. These improvements would directly translate to reduced carbon emissions and more sustainable production methods. Additionally, the development of ruthenium-based catalysts has shown promise in increasing reaction efficiency while requiring less extreme operating conditions.
Water consumption represents another critical environmental consideration in ammonia synthesis. Traditional production methods require significant water inputs for cooling and steam generation, with estimates suggesting that producing one ton of ammonia requires approximately 20-30 cubic meters of water. Emerging technologies incorporating water recycling systems and more efficient heat exchange mechanisms have demonstrated potential water savings of up to 40% in pilot implementations.
The environmental impact extends beyond production to the entire lifecycle of ammonia-based products. Nitrogen runoff from agricultural applications of ammonia-derived fertilizers contributes to water eutrophication and ecosystem disruption. Catalytic technologies that enable more precise ammonia delivery systems or controlled-release fertilizers could significantly reduce these downstream environmental impacts while maintaining agricultural productivity.
Renewable energy integration presents perhaps the most promising pathway toward sustainable ammonia production. Electrocatalytic ammonia synthesis powered by renewable electricity sources could potentially eliminate direct carbon emissions from the production process. Several demonstration projects have successfully implemented solar and wind-powered ammonia synthesis, though challenges remain in scaling these technologies to industrial levels. The theoretical energy efficiency of these systems suggests potential energy savings of 30-50% compared to conventional methods.
Regulatory frameworks increasingly recognize the environmental impact of traditional ammonia production methods. Carbon pricing mechanisms and emissions regulations in major manufacturing regions are creating economic incentives for developing and implementing more sustainable catalytic technologies. Industry leaders have responded by establishing sustainability targets that specifically address improvements in catalytic efficiency and environmental performance metrics.
Recent advancements in catalyst design have demonstrated potential for reducing the environmental footprint of ammonia production. Novel catalysts operating at lower temperatures and pressures could reduce energy requirements by up to 20-30%, according to research from leading institutions. These improvements would directly translate to reduced carbon emissions and more sustainable production methods. Additionally, the development of ruthenium-based catalysts has shown promise in increasing reaction efficiency while requiring less extreme operating conditions.
Water consumption represents another critical environmental consideration in ammonia synthesis. Traditional production methods require significant water inputs for cooling and steam generation, with estimates suggesting that producing one ton of ammonia requires approximately 20-30 cubic meters of water. Emerging technologies incorporating water recycling systems and more efficient heat exchange mechanisms have demonstrated potential water savings of up to 40% in pilot implementations.
The environmental impact extends beyond production to the entire lifecycle of ammonia-based products. Nitrogen runoff from agricultural applications of ammonia-derived fertilizers contributes to water eutrophication and ecosystem disruption. Catalytic technologies that enable more precise ammonia delivery systems or controlled-release fertilizers could significantly reduce these downstream environmental impacts while maintaining agricultural productivity.
Renewable energy integration presents perhaps the most promising pathway toward sustainable ammonia production. Electrocatalytic ammonia synthesis powered by renewable electricity sources could potentially eliminate direct carbon emissions from the production process. Several demonstration projects have successfully implemented solar and wind-powered ammonia synthesis, though challenges remain in scaling these technologies to industrial levels. The theoretical energy efficiency of these systems suggests potential energy savings of 30-50% compared to conventional methods.
Regulatory frameworks increasingly recognize the environmental impact of traditional ammonia production methods. Carbon pricing mechanisms and emissions regulations in major manufacturing regions are creating economic incentives for developing and implementing more sustainable catalytic technologies. Industry leaders have responded by establishing sustainability targets that specifically address improvements in catalytic efficiency and environmental performance metrics.
Energy Efficiency Optimization in Ammonia Production
Energy efficiency optimization represents a critical frontier in modern ammonia production processes. The Haber-Bosch process, while revolutionary, consumes approximately 1-2% of global energy production, creating an urgent need for efficiency improvements. Current industrial ammonia synthesis typically operates at high temperatures (400-500°C) and pressures (150-300 bar), conditions that demand substantial energy input and contribute significantly to production costs and environmental impact.
Recent advancements in catalytic surface engineering have demonstrated promising pathways toward reducing these energy requirements. Ruthenium-based catalysts have shown potential to operate at lower temperatures and pressures than traditional iron-based systems, potentially reducing energy consumption by 20-30%. Additionally, structured catalysts with optimized surface morphologies have demonstrated improved ammonia yield per unit energy input, with some experimental designs achieving efficiency gains of 15-25% compared to conventional catalysts.
Process integration innovations represent another significant avenue for energy optimization. Heat recovery systems that capture and repurpose thermal energy from reaction stages have been implemented in next-generation ammonia plants, reducing overall energy requirements by up to 15%. Advanced process control systems utilizing machine learning algorithms have further optimized reaction conditions in real-time, leading to documented energy savings of 5-10% in pilot implementations.
Renewable energy integration presents perhaps the most transformative opportunity for energy efficiency in ammonia production. Electrolysis-based ammonia synthesis powered by renewable electricity eliminates the energy-intensive steam reforming step traditionally used to produce hydrogen feedstock. Several demonstration projects have achieved carbon emission reductions of 60-90% compared to conventional processes, though challenges in scaling and intermittency management remain significant barriers to widespread adoption.
Membrane reactor technology represents an emerging approach with substantial efficiency potential. These systems combine reaction and separation processes, shifting reaction equilibrium favorably while reducing energy-intensive separation steps. Laboratory-scale membrane reactors have demonstrated theoretical energy savings of up to 25%, though commercial-scale implementation faces materials durability challenges under industrial conditions.
The economic implications of these efficiency improvements are substantial. Analysis indicates that a 20% reduction in energy consumption could reduce production costs by 15-18%, potentially transforming market dynamics in regions with higher energy costs. Furthermore, enhanced energy efficiency directly correlates with reduced carbon emissions, positioning optimized ammonia production processes favorably within increasingly stringent regulatory frameworks governing industrial emissions.
Recent advancements in catalytic surface engineering have demonstrated promising pathways toward reducing these energy requirements. Ruthenium-based catalysts have shown potential to operate at lower temperatures and pressures than traditional iron-based systems, potentially reducing energy consumption by 20-30%. Additionally, structured catalysts with optimized surface morphologies have demonstrated improved ammonia yield per unit energy input, with some experimental designs achieving efficiency gains of 15-25% compared to conventional catalysts.
Process integration innovations represent another significant avenue for energy optimization. Heat recovery systems that capture and repurpose thermal energy from reaction stages have been implemented in next-generation ammonia plants, reducing overall energy requirements by up to 15%. Advanced process control systems utilizing machine learning algorithms have further optimized reaction conditions in real-time, leading to documented energy savings of 5-10% in pilot implementations.
Renewable energy integration presents perhaps the most transformative opportunity for energy efficiency in ammonia production. Electrolysis-based ammonia synthesis powered by renewable electricity eliminates the energy-intensive steam reforming step traditionally used to produce hydrogen feedstock. Several demonstration projects have achieved carbon emission reductions of 60-90% compared to conventional processes, though challenges in scaling and intermittency management remain significant barriers to widespread adoption.
Membrane reactor technology represents an emerging approach with substantial efficiency potential. These systems combine reaction and separation processes, shifting reaction equilibrium favorably while reducing energy-intensive separation steps. Laboratory-scale membrane reactors have demonstrated theoretical energy savings of up to 25%, though commercial-scale implementation faces materials durability challenges under industrial conditions.
The economic implications of these efficiency improvements are substantial. Analysis indicates that a 20% reduction in energy consumption could reduce production costs by 15-18%, potentially transforming market dynamics in regions with higher energy costs. Furthermore, enhanced energy efficiency directly correlates with reduced carbon emissions, positioning optimized ammonia production processes favorably within increasingly stringent regulatory frameworks governing industrial emissions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!