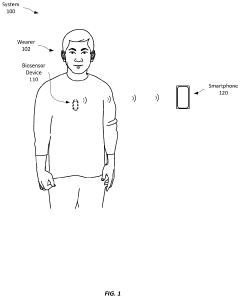
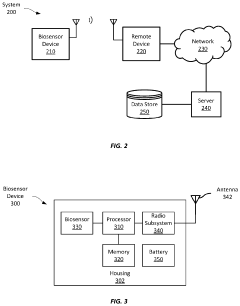
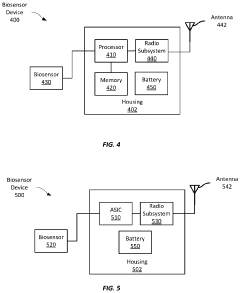
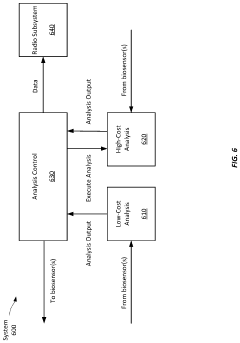
Comparison of Performance Metrics in Wearable Biosensors
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Wearable Biosensor Evolution and Objectives
Wearable biosensors have evolved significantly over the past two decades, transforming from simple pedometers and heart rate monitors to sophisticated multi-parameter sensing platforms capable of continuous health monitoring. The evolution began in the early 2000s with basic activity trackers, followed by the integration of photoplethysmography (PPG) sensors around 2010, which enabled heart rate monitoring in consumer devices. By 2015, electrochemical sensors for sweat analysis and continuous glucose monitoring represented a significant technological leap.
The current generation of wearable biosensors incorporates multiple sensing modalities, including optical, electrochemical, and impedance-based technologies. These advancements have been driven by miniaturization of electronics, improvements in battery technology, and the development of flexible, stretchable materials that conform to the human body. The integration of artificial intelligence and machine learning algorithms has further enhanced the analytical capabilities of these devices, enabling more accurate interpretation of physiological signals.
Performance metrics for wearable biosensors have traditionally focused on technical specifications such as sensitivity, specificity, and accuracy. However, as these devices transition from consumer wellness products to clinical-grade monitoring tools, the evaluation framework has expanded to include reliability under various environmental conditions, long-term stability, and validation against gold standard clinical measurements.
The primary objective in the field is to establish standardized performance metrics that can effectively compare different biosensing technologies across various application scenarios. This standardization is crucial as the industry moves toward medical-grade applications where regulatory approval requires rigorous validation. Current challenges include the lack of consensus on testing protocols and the difficulty in comparing sensors that operate on different physical principles.
Another key goal is to develop metrics that balance technical performance with user-centric factors such as comfort, battery life, and ease of use. The ideal wearable biosensor must not only provide accurate measurements but also integrate seamlessly into users' daily lives to ensure consistent long-term usage and data collection.
Looking forward, the field aims to establish performance benchmarks that address emerging capabilities such as multi-analyte detection, real-time data processing, and integration with telehealth systems. These metrics will need to evaluate not just the sensing capabilities but also data security, interoperability with healthcare systems, and the ability to derive actionable insights from collected physiological data.
The current generation of wearable biosensors incorporates multiple sensing modalities, including optical, electrochemical, and impedance-based technologies. These advancements have been driven by miniaturization of electronics, improvements in battery technology, and the development of flexible, stretchable materials that conform to the human body. The integration of artificial intelligence and machine learning algorithms has further enhanced the analytical capabilities of these devices, enabling more accurate interpretation of physiological signals.
Performance metrics for wearable biosensors have traditionally focused on technical specifications such as sensitivity, specificity, and accuracy. However, as these devices transition from consumer wellness products to clinical-grade monitoring tools, the evaluation framework has expanded to include reliability under various environmental conditions, long-term stability, and validation against gold standard clinical measurements.
The primary objective in the field is to establish standardized performance metrics that can effectively compare different biosensing technologies across various application scenarios. This standardization is crucial as the industry moves toward medical-grade applications where regulatory approval requires rigorous validation. Current challenges include the lack of consensus on testing protocols and the difficulty in comparing sensors that operate on different physical principles.
Another key goal is to develop metrics that balance technical performance with user-centric factors such as comfort, battery life, and ease of use. The ideal wearable biosensor must not only provide accurate measurements but also integrate seamlessly into users' daily lives to ensure consistent long-term usage and data collection.
Looking forward, the field aims to establish performance benchmarks that address emerging capabilities such as multi-analyte detection, real-time data processing, and integration with telehealth systems. These metrics will need to evaluate not just the sensing capabilities but also data security, interoperability with healthcare systems, and the ability to derive actionable insights from collected physiological data.
Market Analysis of Wearable Biosensor Applications
The wearable biosensor market has experienced exponential growth over the past decade, expanding from primarily fitness-focused devices to comprehensive health monitoring solutions. Current market valuations place the global wearable biosensor industry at approximately 25 billion USD in 2023, with projections indicating growth to reach 67 billion USD by 2028, representing a compound annual growth rate (CAGR) of 21.8%. This remarkable expansion is driven by increasing health consciousness among consumers and the growing prevalence of chronic diseases requiring continuous monitoring.
Consumer applications currently dominate the market, accounting for roughly 65% of total revenue. Fitness tracking remains the largest segment, though medical-grade monitoring devices are showing the fastest growth rate at 27% annually. Regionally, North America leads with approximately 40% market share, followed by Europe (25%) and Asia-Pacific (22%), with the latter demonstrating the highest growth potential due to increasing healthcare expenditure and technological adoption.
The healthcare sector represents the most promising vertical for wearable biosensors, particularly in remote patient monitoring, which has seen adoption rates increase by 38% since the COVID-19 pandemic. Hospitals and clinics are increasingly integrating wearable data into electronic health records, with 47% of healthcare providers now utilizing some form of wearable biosensor data in patient care protocols.
Key market drivers include aging populations in developed economies, rising healthcare costs creating demand for preventative solutions, and increasing consumer interest in personalized health insights. The integration of artificial intelligence for data analysis has enhanced the value proposition of these devices, with 78% of new biosensor products featuring some form of AI-powered analytics.
Market challenges include data privacy concerns, with 62% of potential users citing privacy as a primary adoption barrier. Regulatory hurdles also impact market growth, as medical-grade sensors face stringent approval processes that can delay market entry by 18-24 months compared to consumer-grade alternatives. Battery life limitations and sensor accuracy in real-world conditions remain technical challenges affecting user satisfaction and retention.
Emerging opportunities include the integration of biosensors with telehealth platforms, expansion into workplace wellness programs, and development of specialized sensors for underserved medical conditions such as neurological disorders and respiratory diseases. The market for mental health monitoring through biosensors is projected to grow at 32% annually, representing a significant new frontier for application development.
Consumer applications currently dominate the market, accounting for roughly 65% of total revenue. Fitness tracking remains the largest segment, though medical-grade monitoring devices are showing the fastest growth rate at 27% annually. Regionally, North America leads with approximately 40% market share, followed by Europe (25%) and Asia-Pacific (22%), with the latter demonstrating the highest growth potential due to increasing healthcare expenditure and technological adoption.
The healthcare sector represents the most promising vertical for wearable biosensors, particularly in remote patient monitoring, which has seen adoption rates increase by 38% since the COVID-19 pandemic. Hospitals and clinics are increasingly integrating wearable data into electronic health records, with 47% of healthcare providers now utilizing some form of wearable biosensor data in patient care protocols.
Key market drivers include aging populations in developed economies, rising healthcare costs creating demand for preventative solutions, and increasing consumer interest in personalized health insights. The integration of artificial intelligence for data analysis has enhanced the value proposition of these devices, with 78% of new biosensor products featuring some form of AI-powered analytics.
Market challenges include data privacy concerns, with 62% of potential users citing privacy as a primary adoption barrier. Regulatory hurdles also impact market growth, as medical-grade sensors face stringent approval processes that can delay market entry by 18-24 months compared to consumer-grade alternatives. Battery life limitations and sensor accuracy in real-world conditions remain technical challenges affecting user satisfaction and retention.
Emerging opportunities include the integration of biosensors with telehealth platforms, expansion into workplace wellness programs, and development of specialized sensors for underserved medical conditions such as neurological disorders and respiratory diseases. The market for mental health monitoring through biosensors is projected to grow at 32% annually, representing a significant new frontier for application development.
Current Performance Metrics Landscape and Challenges
The current landscape of performance metrics for wearable biosensors is characterized by a diverse array of evaluation criteria that vary significantly across different application domains. Accuracy remains the cornerstone metric, typically measured through mean absolute error (MAE), root mean square error (RMSE), and correlation coefficients when comparing biosensor readings against clinical gold standards. However, the benchmarking methodologies lack standardization across manufacturers, making direct comparisons between competing technologies challenging.
Sensitivity and specificity metrics have gained prominence, particularly for biosensors designed for medical diagnostics and health monitoring. These metrics evaluate a sensor's ability to correctly identify positive cases while minimizing false positives. For continuous monitoring devices, additional metrics such as signal-to-noise ratio (SNR), drift characteristics, and baseline stability have become increasingly important as they directly impact long-term reliability.
Power efficiency metrics present a significant challenge in the current landscape. While battery life is commonly reported, standardized testing protocols that account for various usage scenarios and environmental conditions remain underdeveloped. This inconsistency creates difficulties for end-users attempting to make informed decisions based on real-world performance expectations.
Response time and sampling frequency metrics vary widely across the industry, with some manufacturers prioritizing rapid detection for critical health parameters while others focus on consistent long-term monitoring capabilities. The trade-offs between these approaches are rarely quantified in standardized ways, creating confusion in the marketplace.
Form factor and wearability metrics represent another challenging area, as they involve subjective user experience aspects alongside objective measurements like weight, size, and flexibility. Current evaluation frameworks struggle to integrate these qualitative and quantitative dimensions effectively.
Interoperability metrics have emerged as a critical concern as healthcare ecosystems become increasingly connected. Standards for data format compatibility, API accessibility, and integration capabilities with electronic health records are developing but remain fragmented across different regions and healthcare systems.
Data security and privacy metrics have gained prominence following increased regulatory scrutiny, yet standardized evaluation frameworks for assessing biosensor vulnerability to data breaches or unauthorized access remain in early development stages. This creates significant challenges for organizations attempting to implement these technologies in compliance-sensitive environments.
The most pressing challenge in the current performance metrics landscape is the lack of context-specific evaluation frameworks that acknowledge the varying requirements across different use cases, from clinical applications to consumer wellness tracking.
Sensitivity and specificity metrics have gained prominence, particularly for biosensors designed for medical diagnostics and health monitoring. These metrics evaluate a sensor's ability to correctly identify positive cases while minimizing false positives. For continuous monitoring devices, additional metrics such as signal-to-noise ratio (SNR), drift characteristics, and baseline stability have become increasingly important as they directly impact long-term reliability.
Power efficiency metrics present a significant challenge in the current landscape. While battery life is commonly reported, standardized testing protocols that account for various usage scenarios and environmental conditions remain underdeveloped. This inconsistency creates difficulties for end-users attempting to make informed decisions based on real-world performance expectations.
Response time and sampling frequency metrics vary widely across the industry, with some manufacturers prioritizing rapid detection for critical health parameters while others focus on consistent long-term monitoring capabilities. The trade-offs between these approaches are rarely quantified in standardized ways, creating confusion in the marketplace.
Form factor and wearability metrics represent another challenging area, as they involve subjective user experience aspects alongside objective measurements like weight, size, and flexibility. Current evaluation frameworks struggle to integrate these qualitative and quantitative dimensions effectively.
Interoperability metrics have emerged as a critical concern as healthcare ecosystems become increasingly connected. Standards for data format compatibility, API accessibility, and integration capabilities with electronic health records are developing but remain fragmented across different regions and healthcare systems.
Data security and privacy metrics have gained prominence following increased regulatory scrutiny, yet standardized evaluation frameworks for assessing biosensor vulnerability to data breaches or unauthorized access remain in early development stages. This creates significant challenges for organizations attempting to implement these technologies in compliance-sensitive environments.
The most pressing challenge in the current performance metrics landscape is the lack of context-specific evaluation frameworks that acknowledge the varying requirements across different use cases, from clinical applications to consumer wellness tracking.
Standardized Evaluation Frameworks for Biosensors
01 Accuracy and reliability metrics for wearable biosensors
Wearable biosensors require specific performance metrics to evaluate their accuracy and reliability. These metrics include sensitivity, specificity, precision, and measurement error rates. The accuracy of biosensors is critical for providing reliable health data and can be assessed through comparison with gold standard medical devices. Performance validation protocols often include testing under various environmental conditions and user activities to ensure consistent readings.- Accuracy and reliability metrics for wearable biosensors: Wearable biosensors require specific performance metrics to evaluate their accuracy and reliability. These metrics include sensitivity, specificity, precision, and error rates. The accuracy of biosensor measurements is critical for medical applications and health monitoring. Performance evaluation frameworks help in assessing how well these devices capture and process physiological data under various conditions, ensuring reliable health insights for users.
- Power efficiency and battery life optimization: Energy consumption is a critical performance metric for wearable biosensors. Optimizing power efficiency extends battery life, allowing for longer continuous monitoring periods. This includes implementing power management algorithms, low-power sensing modes, and efficient data transmission protocols. Performance metrics in this category measure energy consumption per sensing operation, standby power usage, and overall operational duration on a single charge.
- Data processing and analytics performance: Performance metrics for data processing capabilities in wearable biosensors include computational efficiency, algorithm accuracy, and real-time processing capabilities. These metrics evaluate how quickly and accurately biosensor data can be processed, analyzed, and transformed into actionable health insights. This includes measuring latency in data processing, accuracy of pattern recognition algorithms, and efficiency of machine learning models implemented within the biosensor system.
- User experience and comfort metrics: Wearable biosensor performance is also measured through user experience metrics, including comfort during extended wear, ease of use, and user interface responsiveness. These metrics assess how well the device integrates into daily life without causing discomfort or disruption. Performance in this category includes evaluation of form factor, weight, skin irritation potential, and overall user satisfaction with the device's functionality and wearability over time.
- Connectivity and interoperability performance: Connectivity performance metrics evaluate how effectively wearable biosensors communicate with other devices and systems. These metrics include data transmission rates, connection stability, interoperability with various platforms, and security of data exchange. Performance in this category measures the reliability of wireless connections, latency in data transmission, compatibility with healthcare information systems, and adherence to data exchange standards and protocols.
02 Power efficiency and battery life optimization
Power consumption is a critical performance metric for wearable biosensors, directly impacting usability and adoption. Metrics include battery life under various usage scenarios, power consumption rates during active sensing versus standby modes, and energy efficiency of data processing algorithms. Advanced power management techniques such as adaptive sampling rates and selective sensor activation help optimize battery performance while maintaining data quality and sensor functionality.Expand Specific Solutions03 Data processing and analytics performance
Performance metrics for data processing capabilities in wearable biosensors include computational efficiency, algorithm accuracy, and real-time processing capabilities. These metrics evaluate how quickly and accurately biosensor data can be processed, analyzed, and transformed into actionable health insights. Advanced analytics techniques such as machine learning algorithms can improve the interpretation of biosensor data, while edge computing capabilities reduce latency and enable faster response times for critical health monitoring applications.Expand Specific Solutions04 User experience and comfort metrics
User-centric performance metrics evaluate the wearability, comfort, and overall user experience of biosensors. These include metrics for device weight, size, flexibility, skin irritation levels, and ease of use. User compliance rates and long-term adherence to wearing the devices are important indicators of successful design. Ergonomic considerations and adaptability to different body types and activities significantly impact user acceptance and the quality of data collected during real-world use.Expand Specific Solutions05 Connectivity and interoperability standards
Connectivity performance metrics assess how effectively wearable biosensors communicate with other devices and systems. These include data transmission rates, connection stability, range, latency, and compatibility with various platforms and protocols. Interoperability standards ensure that biosensor data can be seamlessly integrated with healthcare systems, mobile applications, and other digital health platforms. Security and privacy metrics evaluate the protection of sensitive health data during transmission and storage.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The wearable biosensor market is experiencing rapid growth, currently in its early maturity phase with significant innovation potential. Market size is expanding substantially as healthcare monitoring shifts toward continuous, non-invasive solutions. Technologically, the field shows varying maturity levels across different sensing modalities. Leading players like Samsung Electronics, Google (through Verily Life Sciences), and Polar Electro are driving commercial adoption with advanced sensor technologies, while academic institutions including MIT, Caltech, and University of California are pioneering next-generation sensing capabilities. Research collaborations between companies like Logitech, Toshiba, and Honeywell with academic partners are accelerating development of more accurate, power-efficient biosensors with enhanced signal processing capabilities, particularly for continuous health monitoring applications.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed advanced wearable biosensor technology through their Samsung Health platform and Galaxy Watch series. Their approach integrates multiple biosensors including photoplethysmography (PPG), electrocardiogram (ECG), bioelectrical impedance analysis (BIA), and temperature sensors within compact wearable form factors. Samsung's performance metrics framework emphasizes power efficiency through their proprietary BioActive Sensor that combines multiple sensing functions into a single chip. Their biosensors achieve sampling rates of up to 60Hz for continuous heart rate monitoring while maintaining battery life of 40+ hours. Samsung has implemented adaptive sampling algorithms that dynamically adjust measurement frequency based on user activity levels, reducing power consumption by approximately 30% compared to constant sampling approaches. Their comparative metrics focus on signal-to-noise ratio optimization, with their latest sensors achieving SNR improvements of approximately 20% over previous generations through advanced signal processing techniques.
Strengths: Comprehensive integration of multiple biosensor types in compact form factors; industry-leading power efficiency through hardware optimization; robust signal processing algorithms for noise reduction. Weaknesses: Closed ecosystem limits research accessibility; performance metrics primarily optimized for consumer applications rather than clinical validation; proprietary nature of their technology creates barriers for standardized comparison.
Google LLC
Technical Solution: Google's approach to wearable biosensor performance metrics is centered around their Fitbit acquisition and Google Fit platform. Their technical solution emphasizes long-term continuous monitoring with standardized performance metrics across diverse sensor types. Google has developed a comprehensive framework for evaluating biosensor performance that includes accuracy validation against gold standard medical devices, with published correlation coefficients exceeding 0.95 for heart rate measurements during various activity levels. Their biosensor technology incorporates multi-wavelength PPG sensors operating at sampling frequencies of 25-100Hz depending on activity context, with proprietary algorithms that compensate for motion artifacts and skin tone variations. Google's performance metrics system includes power consumption optimization through their custom low-power processing units that enable continuous monitoring while maintaining 7+ days of battery life in some implementations. Their comparative analysis framework incorporates machine learning models that continuously improve sensor accuracy through large-scale data collection, with documented improvements in blood oxygen measurement accuracy by approximately 15% through algorithm refinements based on population-level data analysis.
Strengths: Extensive data collection capabilities enabling continuous algorithm improvement; strong focus on inclusivity with performance metrics accounting for diverse skin tones and body types; robust validation against clinical standards. Weaknesses: Fragmented approach across multiple product lines with inconsistent performance metrics; privacy concerns potentially limiting comprehensive data utilization; relatively recent entry into advanced biosensor hardware development compared to competitors.
Critical Performance Parameters and Benchmarking Methods
Wearable biosensor devices with adaptive power consumption
PatentActiveUS20220183574A1
Innovation
- Implementing adaptive power management strategies, where the device dynamically adjusts sensor data analysis complexity and sampling rates based on received data, and only activates wireless transmission for urgent events, thereby reducing unnecessary power consumption and extending the device's operational life.
System and method for determining performance capacity
PatentActiveUS20170127957A1
Innovation
- A system that includes wearable biosensors and motion sensors, coupled with processors, to generate biometric and activity data, create response profiles based on HRV and fatigue scores, and use models to predict user responses to training loads, allowing for personalized training load determination.
Regulatory Standards and Compliance Requirements
The regulatory landscape for wearable biosensors is complex and multifaceted, requiring manufacturers to navigate various standards across different jurisdictions. In the United States, the Food and Drug Administration (FDA) classifies wearable biosensors based on their intended use and risk level. Class I devices, which include basic activity trackers, face minimal regulatory requirements, while Class II devices such as continuous glucose monitors require 510(k) clearance. Class III devices, which may include implantable biosensors, undergo the most rigorous premarket approval process.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which introduced more stringent requirements for clinical evidence, post-market surveillance, and unique device identification. Wearable biosensors that make medical claims must comply with these regulations, demonstrating safety and performance through clinical validation studies.
International standards such as ISO 13485 for quality management systems and IEC 60601 for electrical medical equipment safety provide frameworks for ensuring consistent performance metrics across wearable biosensors. Additionally, ISO 14971 guides manufacturers in risk management processes, which is crucial for devices collecting sensitive biometric data.
Data privacy regulations significantly impact wearable biosensor development and deployment. The General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US establish requirements for securing personal health information. Manufacturers must implement robust data encryption, user consent mechanisms, and transparent data handling policies to achieve compliance.
Performance metrics for wearable biosensors must be validated according to specific regulatory guidelines. The Clinical and Laboratory Standards Institute (CLSI) provides protocols for analytical performance validation, including accuracy, precision, linearity, and detection limits. These standardized approaches enable meaningful comparison between different biosensor technologies and ensure reliable clinical decision-making.
Emerging regulatory considerations include interoperability standards such as IEEE 11073 for personal health devices, which facilitate data exchange between different systems. Additionally, regulatory bodies are developing frameworks for software as a medical device (SaMD) and artificial intelligence/machine learning (AI/ML) algorithms that may be incorporated into advanced biosensors for data analysis and interpretation.
Compliance with these diverse regulatory requirements necessitates comprehensive documentation, including technical files, clinical evidence, risk management reports, and post-market surveillance plans. Manufacturers must establish performance metrics that align with regulatory expectations while demonstrating the clinical utility and safety of their wearable biosensor technologies.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which introduced more stringent requirements for clinical evidence, post-market surveillance, and unique device identification. Wearable biosensors that make medical claims must comply with these regulations, demonstrating safety and performance through clinical validation studies.
International standards such as ISO 13485 for quality management systems and IEC 60601 for electrical medical equipment safety provide frameworks for ensuring consistent performance metrics across wearable biosensors. Additionally, ISO 14971 guides manufacturers in risk management processes, which is crucial for devices collecting sensitive biometric data.
Data privacy regulations significantly impact wearable biosensor development and deployment. The General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US establish requirements for securing personal health information. Manufacturers must implement robust data encryption, user consent mechanisms, and transparent data handling policies to achieve compliance.
Performance metrics for wearable biosensors must be validated according to specific regulatory guidelines. The Clinical and Laboratory Standards Institute (CLSI) provides protocols for analytical performance validation, including accuracy, precision, linearity, and detection limits. These standardized approaches enable meaningful comparison between different biosensor technologies and ensure reliable clinical decision-making.
Emerging regulatory considerations include interoperability standards such as IEEE 11073 for personal health devices, which facilitate data exchange between different systems. Additionally, regulatory bodies are developing frameworks for software as a medical device (SaMD) and artificial intelligence/machine learning (AI/ML) algorithms that may be incorporated into advanced biosensors for data analysis and interpretation.
Compliance with these diverse regulatory requirements necessitates comprehensive documentation, including technical files, clinical evidence, risk management reports, and post-market surveillance plans. Manufacturers must establish performance metrics that align with regulatory expectations while demonstrating the clinical utility and safety of their wearable biosensor technologies.
Cross-Platform Interoperability Considerations
In the rapidly evolving landscape of wearable biosensors, cross-platform interoperability represents a critical challenge that significantly impacts the utility and adoption of these technologies. Current wearable biosensor ecosystems often operate as isolated islands, with proprietary data formats, communication protocols, and APIs creating substantial barriers to seamless integration across different platforms and devices.
The fragmentation of standards across major wearable technology providers—including Apple Health, Google Fit, Samsung Health, and Fitbit—creates significant compatibility issues when comparing performance metrics. Each platform implements unique data processing algorithms and calibration methodologies, resulting in inconsistent measurements even when the underlying sensor technologies are similar. This discrepancy becomes particularly problematic when attempting to establish standardized performance benchmarks across different biosensor implementations.
Communication protocol diversity further complicates interoperability efforts. While Bluetooth Low Energy (BLE) has emerged as a dominant wireless standard for wearable devices, variations in implementation and profile support create connectivity challenges. Additionally, emerging protocols such as ANT+, Zigbee, and proprietary solutions fragment the communication landscape, requiring complex translation layers for cross-platform data exchange.
Data format standardization represents another critical interoperability consideration. The lack of universally accepted formats for biosensor data hampers seamless information exchange between platforms. While efforts like FHIR (Fast Healthcare Interoperability Resources) and IEEE 11073 standards aim to address these challenges, adoption remains inconsistent across the wearable technology industry, particularly among consumer-focused devices that prioritize proprietary ecosystems.
Security and privacy frameworks also vary significantly between platforms, creating additional interoperability hurdles. Different encryption standards, authentication mechanisms, and data access policies complicate the secure transfer of sensitive biometric information across platforms. These variations necessitate comprehensive security mapping and translation when implementing cross-platform solutions.
Recent industry initiatives have begun addressing these interoperability challenges through collaborative efforts like the Open mHealth initiative and the Continua Health Alliance. These consortia work toward establishing common data models, API specifications, and certification programs to facilitate seamless integration across different biosensor platforms. However, competitive market dynamics often counteract standardization efforts as manufacturers seek to maintain ecosystem lock-in through proprietary technologies.
For meaningful performance metric comparison across wearable biosensors, researchers and developers must implement robust normalization methodologies that account for platform-specific variations in data collection, processing, and reporting. This approach requires detailed understanding of each platform's technical specifications and algorithmic approaches to ensure valid cross-platform comparisons.
The fragmentation of standards across major wearable technology providers—including Apple Health, Google Fit, Samsung Health, and Fitbit—creates significant compatibility issues when comparing performance metrics. Each platform implements unique data processing algorithms and calibration methodologies, resulting in inconsistent measurements even when the underlying sensor technologies are similar. This discrepancy becomes particularly problematic when attempting to establish standardized performance benchmarks across different biosensor implementations.
Communication protocol diversity further complicates interoperability efforts. While Bluetooth Low Energy (BLE) has emerged as a dominant wireless standard for wearable devices, variations in implementation and profile support create connectivity challenges. Additionally, emerging protocols such as ANT+, Zigbee, and proprietary solutions fragment the communication landscape, requiring complex translation layers for cross-platform data exchange.
Data format standardization represents another critical interoperability consideration. The lack of universally accepted formats for biosensor data hampers seamless information exchange between platforms. While efforts like FHIR (Fast Healthcare Interoperability Resources) and IEEE 11073 standards aim to address these challenges, adoption remains inconsistent across the wearable technology industry, particularly among consumer-focused devices that prioritize proprietary ecosystems.
Security and privacy frameworks also vary significantly between platforms, creating additional interoperability hurdles. Different encryption standards, authentication mechanisms, and data access policies complicate the secure transfer of sensitive biometric information across platforms. These variations necessitate comprehensive security mapping and translation when implementing cross-platform solutions.
Recent industry initiatives have begun addressing these interoperability challenges through collaborative efforts like the Open mHealth initiative and the Continua Health Alliance. These consortia work toward establishing common data models, API specifications, and certification programs to facilitate seamless integration across different biosensor platforms. However, competitive market dynamics often counteract standardization efforts as manufacturers seek to maintain ecosystem lock-in through proprietary technologies.
For meaningful performance metric comparison across wearable biosensors, researchers and developers must implement robust normalization methodologies that account for platform-specific variations in data collection, processing, and reporting. This approach requires detailed understanding of each platform's technical specifications and algorithmic approaches to ensure valid cross-platform comparisons.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!