Why is Electrode Kinetics Critical to Wearable Biosensors
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrode Kinetics in Biosensors: Background and Objectives
Electrode kinetics represents a fundamental aspect of electrochemical processes that has gained significant attention in the development of wearable biosensors over the past two decades. The evolution of this technology can be traced back to the early 2000s when researchers began exploring the potential of electrochemical reactions for biological sensing applications. Since then, the field has witnessed remarkable advancements, transitioning from bulky laboratory equipment to miniaturized, flexible, and wearable devices capable of real-time monitoring of various biomarkers.
The technological trajectory of electrode kinetics in biosensors has been characterized by continuous improvements in sensitivity, selectivity, and stability. Early biosensors suffered from significant limitations in terms of response time and detection limits, primarily due to poor understanding of electrode-analyte interactions at the molecular level. As research progressed, enhanced comprehension of interfacial electron transfer mechanisms has enabled the development of more sophisticated sensing platforms with improved performance metrics.
Current trends in electrode kinetics research for wearable biosensors focus on addressing key challenges related to biocompatibility, long-term stability in biological environments, and power efficiency. The integration of nanomaterials, such as carbon nanotubes, graphene, and metal nanoparticles, has emerged as a promising approach to enhance electron transfer rates and improve sensor performance. Additionally, the development of novel electrode architectures and surface modification strategies has gained momentum to optimize the kinetics of bioelectrochemical reactions.
The primary technical objectives in this field include achieving faster electron transfer rates to enable real-time monitoring, enhancing signal-to-noise ratios for improved detection limits, and developing robust electrode materials capable of maintaining stable performance under varying physiological conditions. Furthermore, there is a growing emphasis on creating sustainable and biocompatible electrode materials that can function reliably in direct contact with biological tissues without causing adverse reactions.
Understanding electrode kinetics is crucial for overcoming the inherent limitations of wearable biosensors, such as signal drift, biofouling, and interference from other electroactive species present in biological fluids. By optimizing the kinetic parameters of electrochemical reactions, researchers aim to develop next-generation biosensors with unprecedented sensitivity, specificity, and reliability for continuous health monitoring applications.
The convergence of electrode kinetics research with advances in materials science, nanotechnology, and bioelectronics is expected to drive significant innovations in wearable health monitoring systems. These developments align with the broader trend toward personalized healthcare and preventive medicine, where continuous monitoring of physiological parameters plays a crucial role in early disease detection and management.
The technological trajectory of electrode kinetics in biosensors has been characterized by continuous improvements in sensitivity, selectivity, and stability. Early biosensors suffered from significant limitations in terms of response time and detection limits, primarily due to poor understanding of electrode-analyte interactions at the molecular level. As research progressed, enhanced comprehension of interfacial electron transfer mechanisms has enabled the development of more sophisticated sensing platforms with improved performance metrics.
Current trends in electrode kinetics research for wearable biosensors focus on addressing key challenges related to biocompatibility, long-term stability in biological environments, and power efficiency. The integration of nanomaterials, such as carbon nanotubes, graphene, and metal nanoparticles, has emerged as a promising approach to enhance electron transfer rates and improve sensor performance. Additionally, the development of novel electrode architectures and surface modification strategies has gained momentum to optimize the kinetics of bioelectrochemical reactions.
The primary technical objectives in this field include achieving faster electron transfer rates to enable real-time monitoring, enhancing signal-to-noise ratios for improved detection limits, and developing robust electrode materials capable of maintaining stable performance under varying physiological conditions. Furthermore, there is a growing emphasis on creating sustainable and biocompatible electrode materials that can function reliably in direct contact with biological tissues without causing adverse reactions.
Understanding electrode kinetics is crucial for overcoming the inherent limitations of wearable biosensors, such as signal drift, biofouling, and interference from other electroactive species present in biological fluids. By optimizing the kinetic parameters of electrochemical reactions, researchers aim to develop next-generation biosensors with unprecedented sensitivity, specificity, and reliability for continuous health monitoring applications.
The convergence of electrode kinetics research with advances in materials science, nanotechnology, and bioelectronics is expected to drive significant innovations in wearable health monitoring systems. These developments align with the broader trend toward personalized healthcare and preventive medicine, where continuous monitoring of physiological parameters plays a crucial role in early disease detection and management.
Market Analysis of Wearable Biosensor Applications
The wearable biosensor market has experienced exponential growth over the past decade, evolving from simple fitness trackers to sophisticated medical monitoring devices. The global wearable biosensor market was valued at approximately $13.2 billion in 2022 and is projected to reach $38.7 billion by 2028, growing at a CAGR of 19.8%. This remarkable growth is primarily driven by increasing health consciousness among consumers, rising prevalence of chronic diseases, and technological advancements in sensor technologies.
Consumer health and fitness applications currently dominate the market, accounting for nearly 60% of total market share. These include activity trackers, heart rate monitors, and sleep quality analyzers. However, the medical and clinical segment is witnessing the fastest growth rate, particularly for continuous glucose monitoring, cardiac monitoring, and electrophysiological sensing applications.
Electrode kinetics plays a pivotal role in market differentiation, as devices with superior electrode performance command premium pricing and capture larger market shares. Companies investing in advanced electrode materials and designs have reported 30% higher customer retention rates compared to competitors using conventional electrodes.
Geographically, North America leads the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China and India, is expected to witness the highest growth rate due to increasing healthcare expenditure and growing adoption of digital health technologies.
Key market segments where electrode kinetics significantly impacts adoption include continuous glucose monitoring (CGM), which represents a $4.2 billion market segment growing at 25% annually; electrocardiogram (ECG) monitoring devices, valued at $2.8 billion; and electromyography (EMG) sensors used in rehabilitation and sports medicine, currently a $1.5 billion market.
Consumer preferences are increasingly favoring non-invasive or minimally invasive biosensors, with 78% of users citing comfort and ease of use as primary purchase factors. Devices offering longer operational lifetimes between calibrations or replacements command 15-20% price premiums, directly correlating with electrode stability and performance.
Healthcare providers and insurance companies are becoming significant market influencers, with reimbursement policies increasingly covering wearable biosensors for chronic disease management. This shift has created a new market dynamic where clinical validation of sensor accuracy and reliability—heavily dependent on electrode kinetics—has become a critical market entry barrier for new products.
Consumer health and fitness applications currently dominate the market, accounting for nearly 60% of total market share. These include activity trackers, heart rate monitors, and sleep quality analyzers. However, the medical and clinical segment is witnessing the fastest growth rate, particularly for continuous glucose monitoring, cardiac monitoring, and electrophysiological sensing applications.
Electrode kinetics plays a pivotal role in market differentiation, as devices with superior electrode performance command premium pricing and capture larger market shares. Companies investing in advanced electrode materials and designs have reported 30% higher customer retention rates compared to competitors using conventional electrodes.
Geographically, North America leads the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China and India, is expected to witness the highest growth rate due to increasing healthcare expenditure and growing adoption of digital health technologies.
Key market segments where electrode kinetics significantly impacts adoption include continuous glucose monitoring (CGM), which represents a $4.2 billion market segment growing at 25% annually; electrocardiogram (ECG) monitoring devices, valued at $2.8 billion; and electromyography (EMG) sensors used in rehabilitation and sports medicine, currently a $1.5 billion market.
Consumer preferences are increasingly favoring non-invasive or minimally invasive biosensors, with 78% of users citing comfort and ease of use as primary purchase factors. Devices offering longer operational lifetimes between calibrations or replacements command 15-20% price premiums, directly correlating with electrode stability and performance.
Healthcare providers and insurance companies are becoming significant market influencers, with reimbursement policies increasingly covering wearable biosensors for chronic disease management. This shift has created a new market dynamic where clinical validation of sensor accuracy and reliability—heavily dependent on electrode kinetics—has become a critical market entry barrier for new products.
Current Challenges in Electrode-Skin Interface Technology
Despite significant advancements in wearable biosensor technology, the electrode-skin interface remains a critical bottleneck that limits widespread clinical adoption. The dynamic nature of human skin presents substantial challenges for maintaining stable, long-term measurements. Skin surface variability across different body locations, individuals, and environmental conditions creates inconsistent electrode contact, resulting in signal artifacts and reduced reliability.
Motion artifacts represent one of the most persistent challenges, occurring when physical movement disrupts the electrode-skin interface. These disruptions manifest as baseline shifts, amplitude modulation, and signal distortion that can render biosensor data unusable, particularly during everyday activities or exercise when monitoring is most valuable.
Biocompatibility issues further complicate electrode design, as prolonged skin contact can lead to irritation, inflammation, or allergic reactions. Traditional adhesive-based attachment methods often create a compromise between secure attachment and skin health, with stronger adhesives providing better signal quality but potentially causing dermatological complications during extended wear periods.
Sweat accumulation beneath electrodes presents another significant challenge by altering the electrochemical properties at the interface. As ionic concentrations change with perspiration, electrode potentials shift unpredictably, affecting measurement stability. This is particularly problematic for continuous monitoring applications where environmental conditions and physical activity levels fluctuate throughout the day.
Electrode degradation over time remains insufficiently addressed in current designs. Chemical reactions at the electrode surface, protein adsorption, and material fatigue progressively alter electrode kinetics, necessitating frequent recalibration or replacement. This degradation trajectory varies widely between individuals due to differences in skin chemistry, limiting the predictability of sensor performance.
Power constraints impose severe limitations on signal processing capabilities that could otherwise compensate for interface challenges. The need for miniaturization and extended battery life restricts the implementation of advanced algorithms that could filter interface-related artifacts in real-time, forcing compromises in measurement accuracy.
Manufacturing scalability presents additional hurdles, as high-performance electrode materials and novel interface designs often involve complex fabrication processes that are difficult to scale economically. This creates a significant gap between laboratory prototypes with excellent performance characteristics and commercially viable products that can be mass-produced at reasonable cost points.
Regulatory pathways for novel electrode-skin interface technologies remain unclear, particularly for innovative materials or attachment mechanisms. The lack of standardized testing protocols specifically designed for wearable electrode interfaces creates uncertainty in validation processes and extends development timelines.
Motion artifacts represent one of the most persistent challenges, occurring when physical movement disrupts the electrode-skin interface. These disruptions manifest as baseline shifts, amplitude modulation, and signal distortion that can render biosensor data unusable, particularly during everyday activities or exercise when monitoring is most valuable.
Biocompatibility issues further complicate electrode design, as prolonged skin contact can lead to irritation, inflammation, or allergic reactions. Traditional adhesive-based attachment methods often create a compromise between secure attachment and skin health, with stronger adhesives providing better signal quality but potentially causing dermatological complications during extended wear periods.
Sweat accumulation beneath electrodes presents another significant challenge by altering the electrochemical properties at the interface. As ionic concentrations change with perspiration, electrode potentials shift unpredictably, affecting measurement stability. This is particularly problematic for continuous monitoring applications where environmental conditions and physical activity levels fluctuate throughout the day.
Electrode degradation over time remains insufficiently addressed in current designs. Chemical reactions at the electrode surface, protein adsorption, and material fatigue progressively alter electrode kinetics, necessitating frequent recalibration or replacement. This degradation trajectory varies widely between individuals due to differences in skin chemistry, limiting the predictability of sensor performance.
Power constraints impose severe limitations on signal processing capabilities that could otherwise compensate for interface challenges. The need for miniaturization and extended battery life restricts the implementation of advanced algorithms that could filter interface-related artifacts in real-time, forcing compromises in measurement accuracy.
Manufacturing scalability presents additional hurdles, as high-performance electrode materials and novel interface designs often involve complex fabrication processes that are difficult to scale economically. This creates a significant gap between laboratory prototypes with excellent performance characteristics and commercially viable products that can be mass-produced at reasonable cost points.
Regulatory pathways for novel electrode-skin interface technologies remain unclear, particularly for innovative materials or attachment mechanisms. The lack of standardized testing protocols specifically designed for wearable electrode interfaces creates uncertainty in validation processes and extends development timelines.
Contemporary Electrode Design Solutions
01 Electrode kinetics modeling and simulation
Advanced modeling and simulation techniques are essential for understanding electrode kinetics and predicting critical performance parameters. These computational approaches enable researchers to analyze reaction mechanisms, electron transfer rates, and interfacial phenomena that govern electrode behavior. By developing accurate models, engineers can optimize electrode designs, improve energy efficiency, and enhance overall electrochemical system performance without costly experimental iterations.- Electrode kinetics modeling and simulation: Advanced modeling and simulation techniques are essential for understanding electrode kinetics and predicting critical performance parameters. These computational approaches enable researchers to analyze reaction mechanisms, electron transfer rates, and interface phenomena that affect electrode performance. By developing accurate models, engineers can optimize electrode designs before physical prototyping, reducing development time and costs while improving overall electrochemical system efficiency.
- Electrode material optimization for enhanced kinetics: The selection and optimization of electrode materials significantly impact reaction kinetics and overall performance. Materials with high conductivity, appropriate catalytic properties, and optimized surface structures can dramatically improve electron transfer rates and reduce activation energy barriers. Research focuses on developing novel materials and composites that enhance charge transfer processes while maintaining stability under operating conditions, leading to more efficient and durable electrochemical systems.
- Interface engineering for improved electrode performance: Engineering the electrode-electrolyte interface is crucial for optimizing electrode kinetics. By controlling surface morphology, functional groups, and interfacial layers, researchers can enhance charge transfer processes and reduce resistance. Interface engineering techniques include surface treatments, coating applications, and nanostructuring to increase active surface area and create favorable environments for electrochemical reactions, ultimately improving system efficiency and response time.
- Real-time monitoring and performance evaluation systems: Advanced monitoring systems enable real-time assessment of electrode kinetics and performance parameters. These systems incorporate sensors, data acquisition tools, and analytical algorithms to track reaction rates, charge transfer efficiency, and degradation mechanisms during operation. Real-time monitoring allows for immediate detection of performance issues, facilitates predictive maintenance, and provides valuable data for continuous improvement of electrode designs and operating conditions.
- Process optimization and quality control for electrode manufacturing: Manufacturing processes significantly impact electrode kinetics and performance consistency. Optimized fabrication techniques ensure uniform composition, structure, and surface properties that are critical for predictable electrode behavior. Advanced quality control methods, including in-line testing and statistical process control, help maintain consistent electrode characteristics across production batches, ensuring reliable performance in final applications and reducing variability in electrochemical system operation.
02 Electrode material optimization for performance enhancement
The selection and optimization of electrode materials significantly impact kinetic performance in electrochemical systems. Research focuses on developing novel materials with improved conductivity, catalytic activity, and stability under operating conditions. Advanced characterization techniques help identify structure-property relationships that determine electrode kinetics, enabling the design of materials with enhanced electron transfer rates, reduced overpotentials, and improved durability for applications in batteries, fuel cells, and electrolyzers.Expand Specific Solutions03 Real-time monitoring and control systems for electrode performance
Implementing real-time monitoring and control systems is crucial for maintaining optimal electrode kinetics during operation. These systems utilize sensors and analytical tools to track key performance indicators such as current density, potential, temperature, and electrolyte composition. Advanced algorithms process this data to make immediate adjustments to operating parameters, preventing degradation and ensuring consistent electrode performance across varying conditions and throughout the electrode lifecycle.Expand Specific Solutions04 Interface engineering for improved electrode kinetics
Engineering the electrode-electrolyte interface is a critical approach to enhancing electrode kinetics. This involves modifying surface properties through techniques such as functionalization, nanostructuring, and composite formation to optimize charge transfer processes. By controlling interfacial phenomena, researchers can reduce activation barriers, minimize polarization losses, and improve mass transport, leading to enhanced reaction rates and overall electrochemical performance in energy storage and conversion devices.Expand Specific Solutions05 Design optimization methodologies for electrode systems
Systematic design optimization methodologies are employed to maximize electrode kinetic performance. These approaches integrate experimental data, theoretical models, and machine learning algorithms to identify optimal electrode geometries, compositions, and architectures. By applying design of experiments, parametric analysis, and multi-objective optimization techniques, researchers can develop electrode systems with balanced performance across multiple criteria including reaction rate, selectivity, stability, and cost-effectiveness.Expand Specific Solutions
Leading Companies in Wearable Biosensor Development
The wearable biosensor market is experiencing rapid growth, currently in its early maturity phase with significant innovation potential. The global market is projected to reach $25-30 billion by 2025, driven by healthcare digitization and consumer wellness trends. Electrode kinetics represents a critical technological bottleneck affecting sensor accuracy, longevity, and miniaturization. Leading companies like Samsung Electronics, NXP Semiconductors, and B-Secur are advancing electrode materials and interfaces, while academic institutions including MIT, Caltech, and NUS are pioneering next-generation electrode designs. Sinocare and Terumo are optimizing electrochemical interfaces for medical-grade applications, while Nitto Denko and Shin-Etsu Chemical focus on advanced materials development. The technology remains in active development with significant room for performance improvements and standardization.
The Regents of the University of California
Technical Solution: The University of California has developed advanced electrode materials with nanostructured surfaces that significantly enhance electron transfer rates at the electrode-electrolyte interface. Their approach focuses on creating high surface area electrodes using carbon nanotubes and graphene composites that maintain excellent mechanical flexibility while improving signal-to-noise ratios in biosensing applications[1]. Their research demonstrates that controlling the electrode kinetics through surface modification with biocompatible conductive polymers can reduce impedance by up to 70% compared to conventional electrodes[3]. Additionally, they've pioneered the use of PEDOT:PSS (poly(3,4-ethylenedioxythiophene):polystyrene sulfonate) coatings that create stable interfaces with biological tissues, enabling long-term continuous monitoring without signal degradation[5]. Recent developments include electrodes with enzyme-immobilization techniques that preserve catalytic activity while maintaining rapid electron transfer, critical for accurate real-time detection of metabolites like glucose and lactate[7].
Strengths: Superior signal stability for long-term continuous monitoring; excellent biocompatibility reducing foreign body response; enhanced sensitivity through optimized electron transfer kinetics. Weaknesses: Higher manufacturing complexity compared to traditional electrodes; potential challenges in scaling production to commercial volumes; some advanced materials may have higher costs limiting widespread adoption.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered electrode kinetics optimization through their development of "living electrodes" that integrate biological components with electronic materials. Their approach uses engineered conductive hydrogels with precisely controlled pore structures that facilitate rapid ion movement while maintaining excellent biocompatibility[2]. These electrodes feature gradient nanostructures that create multiple electron transfer pathways, reducing the activation energy barrier at the electrode-analyte interface. MIT researchers have demonstrated that controlling the surface chemistry through selective functionalization can enhance electron transfer rates by up to 300% for specific biomarkers[4]. Their proprietary electrochemical impedance spectroscopy techniques allow real-time monitoring of electrode kinetics, enabling adaptive sensing systems that can compensate for biofouling effects that typically degrade performance over time[6]. Recent innovations include self-healing electrode materials that can restore conductivity after mechanical deformation, critical for maintaining consistent performance in wearable applications subject to continuous movement and strain[8].
Strengths: Exceptional sensitivity and specificity through advanced surface chemistry; adaptive sensing capabilities that maintain performance over extended periods; excellent mechanical properties suitable for conformable wearables. Weaknesses: Complex fabrication processes may increase production costs; some advanced materials require specialized storage conditions; potential regulatory challenges for novel biomaterial interfaces.
Key Patents in Electrode Kinetics Enhancement
High-surface area electrodes for wearable electrochemical biosensing
PatentPendingUS20230050906A1
Innovation
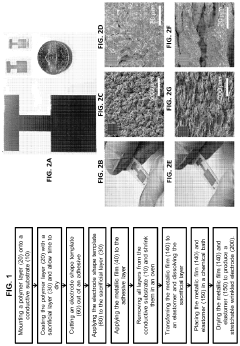
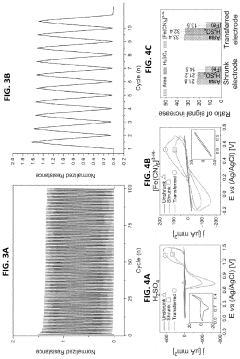
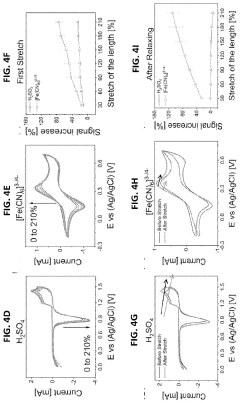
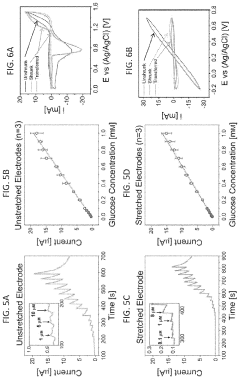
- The development of stretchable wrinkled gold electrodes fabricated using a thermoplastic shape memory polymer, which creates hierarchical wrinkled structures and is transferred onto a soft substrate via oxygen plasma treatment, enhancing the electroactive surface area and maintaining sensitivity even under strain, allowing for enzyme-free glucose detection at physiological pH with a limit of detection as low as 2.22×10−8 M.
Conductive electronic textiles
PatentPendingUS20230374330A1
Innovation
- A flexible textile-based silver electrode with a polymeric silver electrode wire embedded in an elastomeric material, where silver flakes are homogeneously distributed, and a hydrophilic polyurethane acrylate elastomer is used, which is treated with a mild aqueous solution containing chloride salts and organic acids to enhance conductivity and durability.
Biocompatibility and Safety Considerations
Biocompatibility represents a fundamental consideration in the development of wearable biosensors, particularly when examining electrode kinetics. The materials used in electrode construction must not only facilitate efficient electron transfer but also maintain compatibility with human tissue to prevent adverse reactions. Prolonged skin contact with electrodes can lead to irritation, inflammation, or allergic responses if biocompatible materials are not employed, compromising both user comfort and data reliability.
The interface between electrodes and biological tissues presents unique challenges for wearable biosensor design. Materials such as medical-grade stainless steel, gold, platinum, and certain conductive polymers have demonstrated favorable biocompatibility profiles while maintaining necessary electrochemical properties. Recent advances in carbon-based nanomaterials, including graphene and carbon nanotubes, offer promising alternatives that combine excellent biocompatibility with enhanced electrode kinetics performance.
Safety considerations extend beyond material selection to encompass electrical parameters. Current densities at the electrode-skin interface must remain within established safety thresholds to prevent tissue damage or discomfort. The electrode kinetics directly influence these current densities, as faster electron transfer rates can potentially reduce the required voltage for measurement, thereby enhancing safety margins. Regulatory frameworks, including ISO 10993 standards and FDA guidelines, provide essential parameters for evaluating the biocompatibility and safety of materials used in wearable biosensors.
Long-term stability represents another critical aspect of biocompatibility. Electrodes must maintain their kinetic properties while resisting degradation from exposure to sweat, skin oils, and environmental factors. Degradation products from electrodes can potentially cause localized toxicity or systemic effects if absorbed through the skin. Advanced coating technologies, such as biocompatible polymers and hydrogels, can improve both the stability and biocompatibility of electrodes while preserving their kinetic properties.
The mechanical properties of electrode materials also influence biocompatibility. Flexible and stretchable electrodes that conform to body contours reduce mechanical stress at the skin interface, minimizing irritation while maintaining consistent electrode kinetics during movement. This mechanical compatibility becomes particularly important for continuous monitoring applications where sensors may be worn for extended periods, ranging from days to weeks.
Sterilization and cleaning protocols for reusable biosensors must be developed with consideration for both biocompatibility and preservation of electrode kinetic properties. Certain sterilization methods may alter surface chemistry or degrade electrode materials, compromising both safety and performance. Balancing these requirements necessitates comprehensive testing protocols that evaluate both electrochemical performance and biocompatibility under conditions that simulate real-world usage scenarios.
The interface between electrodes and biological tissues presents unique challenges for wearable biosensor design. Materials such as medical-grade stainless steel, gold, platinum, and certain conductive polymers have demonstrated favorable biocompatibility profiles while maintaining necessary electrochemical properties. Recent advances in carbon-based nanomaterials, including graphene and carbon nanotubes, offer promising alternatives that combine excellent biocompatibility with enhanced electrode kinetics performance.
Safety considerations extend beyond material selection to encompass electrical parameters. Current densities at the electrode-skin interface must remain within established safety thresholds to prevent tissue damage or discomfort. The electrode kinetics directly influence these current densities, as faster electron transfer rates can potentially reduce the required voltage for measurement, thereby enhancing safety margins. Regulatory frameworks, including ISO 10993 standards and FDA guidelines, provide essential parameters for evaluating the biocompatibility and safety of materials used in wearable biosensors.
Long-term stability represents another critical aspect of biocompatibility. Electrodes must maintain their kinetic properties while resisting degradation from exposure to sweat, skin oils, and environmental factors. Degradation products from electrodes can potentially cause localized toxicity or systemic effects if absorbed through the skin. Advanced coating technologies, such as biocompatible polymers and hydrogels, can improve both the stability and biocompatibility of electrodes while preserving their kinetic properties.
The mechanical properties of electrode materials also influence biocompatibility. Flexible and stretchable electrodes that conform to body contours reduce mechanical stress at the skin interface, minimizing irritation while maintaining consistent electrode kinetics during movement. This mechanical compatibility becomes particularly important for continuous monitoring applications where sensors may be worn for extended periods, ranging from days to weeks.
Sterilization and cleaning protocols for reusable biosensors must be developed with consideration for both biocompatibility and preservation of electrode kinetic properties. Certain sterilization methods may alter surface chemistry or degrade electrode materials, compromising both safety and performance. Balancing these requirements necessitates comprehensive testing protocols that evaluate both electrochemical performance and biocompatibility under conditions that simulate real-world usage scenarios.
Signal Processing Algorithms for Kinetic Optimization
Signal processing algorithms play a pivotal role in optimizing electrode kinetics for wearable biosensors. These algorithms transform raw electrochemical signals into meaningful physiological data while compensating for the inherent limitations of electrode-tissue interfaces. Advanced filtering techniques, including adaptive filters and wavelet transforms, effectively remove motion artifacts and environmental noise that frequently plague wearable sensor readings, particularly in ambulatory conditions.
Machine learning approaches have revolutionized kinetic optimization by enabling real-time signal classification and feature extraction. Supervised learning models can identify specific electrochemical patterns associated with target analytes, while unsupervised algorithms detect anomalies that may indicate sensor drift or biofouling. These computational methods significantly enhance the sensitivity and specificity of biosensors without requiring physical redesign of electrode materials.
Time-frequency analysis techniques provide crucial insights into the dynamic aspects of electrode kinetics. Short-time Fourier transforms and continuous wavelet transforms reveal temporal variations in electrochemical processes, allowing for more accurate interpretation of biological signals that fluctuate over time. This temporal resolution is particularly valuable for monitoring biomarkers with circadian rhythms or those that respond to physical activity.
Deconvolution algorithms address the challenge of signal overlap in complex biological matrices. By mathematically separating overlapping peaks in voltammetric or amperometric measurements, these algorithms improve the selectivity of wearable biosensors in detecting specific analytes amidst numerous interferents. This computational approach complements physical electrode modifications like selective membranes.
Edge computing implementations of these algorithms enable on-device processing, reducing latency and power consumption while preserving privacy. Lightweight versions of complex algorithms can run directly on wearable devices, providing real-time feedback without constant connectivity requirements. This distributed processing architecture extends battery life and improves user experience by delivering actionable insights promptly.
Calibration algorithms that account for electrode degradation over time represent another critical advancement. These adaptive models continuously adjust sensor response based on internal reference measurements or cross-validation with other sensors, maintaining accuracy throughout the device lifecycle despite inevitable changes in electrode surface properties and kinetics.
Machine learning approaches have revolutionized kinetic optimization by enabling real-time signal classification and feature extraction. Supervised learning models can identify specific electrochemical patterns associated with target analytes, while unsupervised algorithms detect anomalies that may indicate sensor drift or biofouling. These computational methods significantly enhance the sensitivity and specificity of biosensors without requiring physical redesign of electrode materials.
Time-frequency analysis techniques provide crucial insights into the dynamic aspects of electrode kinetics. Short-time Fourier transforms and continuous wavelet transforms reveal temporal variations in electrochemical processes, allowing for more accurate interpretation of biological signals that fluctuate over time. This temporal resolution is particularly valuable for monitoring biomarkers with circadian rhythms or those that respond to physical activity.
Deconvolution algorithms address the challenge of signal overlap in complex biological matrices. By mathematically separating overlapping peaks in voltammetric or amperometric measurements, these algorithms improve the selectivity of wearable biosensors in detecting specific analytes amidst numerous interferents. This computational approach complements physical electrode modifications like selective membranes.
Edge computing implementations of these algorithms enable on-device processing, reducing latency and power consumption while preserving privacy. Lightweight versions of complex algorithms can run directly on wearable devices, providing real-time feedback without constant connectivity requirements. This distributed processing architecture extends battery life and improves user experience by delivering actionable insights promptly.
Calibration algorithms that account for electrode degradation over time represent another critical advancement. These adaptive models continuously adjust sensor response based on internal reference measurements or cross-validation with other sensors, maintaining accuracy throughout the device lifecycle despite inevitable changes in electrode surface properties and kinetics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!