How Wearable Biosensors Meet Medical Device Regulations
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Wearable Biosensor Regulatory Landscape and Objectives
Wearable biosensors represent a significant technological advancement in healthcare monitoring, evolving from simple fitness trackers to sophisticated medical devices capable of continuous physiological monitoring. This evolution has created a complex regulatory landscape that manufacturers must navigate to bring products to market successfully. The regulatory framework for wearable biosensors varies significantly across global markets, with the FDA in the United States, the MDR in Europe, and the NMPA in China establishing distinct requirements for medical device classification and approval.
The primary objective of regulatory compliance for wearable biosensors is to ensure patient safety while facilitating innovation. These devices occupy a unique position at the intersection of consumer electronics and medical devices, creating regulatory challenges that traditional frameworks were not designed to address. Regulatory bodies worldwide are actively adapting their approaches to accommodate the rapid technological advancement in this sector while maintaining rigorous safety standards.
Historical development of wearable biosensor regulations shows a gradual shift from treating all health-monitoring devices as medical devices toward more nuanced approaches that consider intended use, risk profile, and clinical claims. The FDA's Digital Health Innovation Action Plan and Software Pre-Certification Program exemplify efforts to streamline regulatory pathways for digital health technologies, including certain wearable biosensors.
Current regulatory classifications typically depend on the device's intended use, risk level, and claimed medical benefits. Low-risk wellness devices that make no medical claims often face minimal regulatory scrutiny, while devices intended for diagnosis, treatment, or monitoring of specific medical conditions must undergo rigorous premarket review processes. This distinction creates a critical decision point for manufacturers in product positioning and marketing strategy.
The technical objectives for regulatory compliance include developing validation protocols that demonstrate measurement accuracy, precision, and clinical relevance. Manufacturers must establish robust quality management systems, conduct appropriate clinical evaluations, and implement comprehensive risk management processes throughout the product lifecycle. Additionally, cybersecurity and data privacy considerations have become increasingly important regulatory concerns as these devices collect and transmit sensitive health information.
Looking forward, regulatory harmonization efforts aim to reduce the burden of multiple submissions across different markets while maintaining high safety standards. The International Medical Device Regulators Forum (IMDRF) is working toward consensus on common principles for regulating Software as a Medical Device (SaMD), which affects many advanced wearable biosensors. These efforts seek to balance innovation with patient protection in this rapidly evolving technological landscape.
The primary objective of regulatory compliance for wearable biosensors is to ensure patient safety while facilitating innovation. These devices occupy a unique position at the intersection of consumer electronics and medical devices, creating regulatory challenges that traditional frameworks were not designed to address. Regulatory bodies worldwide are actively adapting their approaches to accommodate the rapid technological advancement in this sector while maintaining rigorous safety standards.
Historical development of wearable biosensor regulations shows a gradual shift from treating all health-monitoring devices as medical devices toward more nuanced approaches that consider intended use, risk profile, and clinical claims. The FDA's Digital Health Innovation Action Plan and Software Pre-Certification Program exemplify efforts to streamline regulatory pathways for digital health technologies, including certain wearable biosensors.
Current regulatory classifications typically depend on the device's intended use, risk level, and claimed medical benefits. Low-risk wellness devices that make no medical claims often face minimal regulatory scrutiny, while devices intended for diagnosis, treatment, or monitoring of specific medical conditions must undergo rigorous premarket review processes. This distinction creates a critical decision point for manufacturers in product positioning and marketing strategy.
The technical objectives for regulatory compliance include developing validation protocols that demonstrate measurement accuracy, precision, and clinical relevance. Manufacturers must establish robust quality management systems, conduct appropriate clinical evaluations, and implement comprehensive risk management processes throughout the product lifecycle. Additionally, cybersecurity and data privacy considerations have become increasingly important regulatory concerns as these devices collect and transmit sensitive health information.
Looking forward, regulatory harmonization efforts aim to reduce the burden of multiple submissions across different markets while maintaining high safety standards. The International Medical Device Regulators Forum (IMDRF) is working toward consensus on common principles for regulating Software as a Medical Device (SaMD), which affects many advanced wearable biosensors. These efforts seek to balance innovation with patient protection in this rapidly evolving technological landscape.
Market Demand Analysis for Medically Compliant Wearables
The global market for medically compliant wearable biosensors is experiencing unprecedented growth, driven by increasing healthcare costs, aging populations, and the rising prevalence of chronic diseases. Current market projections indicate that the medical wearables sector is expected to reach $27.4 billion by 2026, growing at a compound annual growth rate (CAGR) of 17.8% from 2021. This remarkable expansion reflects the substantial demand for devices that can effectively monitor health parameters while adhering to stringent medical regulations.
Consumer demand for these devices stems primarily from three key demographics: aging populations requiring continuous health monitoring, chronic disease patients needing regular vital sign tracking, and health-conscious individuals seeking preventive care solutions. The COVID-19 pandemic has further accelerated this demand, as remote patient monitoring became essential during periods of limited healthcare access, creating a 103% increase in telehealth utilization compared to pre-pandemic levels.
Healthcare providers represent another significant market segment, increasingly adopting regulated wearable technologies to reduce hospital readmissions, decrease healthcare delivery costs, and improve patient outcomes. Insurance companies are also emerging as key stakeholders, with many now offering premium discounts or incentives for patients who utilize approved medical wearables for condition management.
Market research indicates that consumers and healthcare professionals prioritize several features in medically compliant wearables: clinical-grade accuracy (cited by 87% of healthcare providers as essential), regulatory compliance with standards such as FDA clearance or CE marking, seamless data integration with electronic health records, and enhanced data security protocols that meet HIPAA requirements.
Regional analysis reveals varying adoption rates and regulatory landscapes. North America currently dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is projected to witness the fastest growth rate of 19.6% through 2026, driven by improving healthcare infrastructure and increasing digital health initiatives in countries like China, Japan, and India.
The market is experiencing a notable shift from consumer-grade fitness trackers toward clinically validated devices that can receive regulatory approval. This transition is evidenced by the 34% increase in FDA submissions for wearable medical devices over the past three years. Devices capable of monitoring multiple parameters simultaneously (such as ECG, blood oxygen, and temperature) are showing particularly strong demand growth, with sales increasing by 47% year-over-year.
Industry forecasts suggest that future market expansion will be fueled by innovations in non-invasive continuous glucose monitoring, wearable dialysis devices, and advanced neurological monitoring systems, all of which require rigorous regulatory compliance to achieve market acceptance and clinical adoption.
Consumer demand for these devices stems primarily from three key demographics: aging populations requiring continuous health monitoring, chronic disease patients needing regular vital sign tracking, and health-conscious individuals seeking preventive care solutions. The COVID-19 pandemic has further accelerated this demand, as remote patient monitoring became essential during periods of limited healthcare access, creating a 103% increase in telehealth utilization compared to pre-pandemic levels.
Healthcare providers represent another significant market segment, increasingly adopting regulated wearable technologies to reduce hospital readmissions, decrease healthcare delivery costs, and improve patient outcomes. Insurance companies are also emerging as key stakeholders, with many now offering premium discounts or incentives for patients who utilize approved medical wearables for condition management.
Market research indicates that consumers and healthcare professionals prioritize several features in medically compliant wearables: clinical-grade accuracy (cited by 87% of healthcare providers as essential), regulatory compliance with standards such as FDA clearance or CE marking, seamless data integration with electronic health records, and enhanced data security protocols that meet HIPAA requirements.
Regional analysis reveals varying adoption rates and regulatory landscapes. North America currently dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is projected to witness the fastest growth rate of 19.6% through 2026, driven by improving healthcare infrastructure and increasing digital health initiatives in countries like China, Japan, and India.
The market is experiencing a notable shift from consumer-grade fitness trackers toward clinically validated devices that can receive regulatory approval. This transition is evidenced by the 34% increase in FDA submissions for wearable medical devices over the past three years. Devices capable of monitoring multiple parameters simultaneously (such as ECG, blood oxygen, and temperature) are showing particularly strong demand growth, with sales increasing by 47% year-over-year.
Industry forecasts suggest that future market expansion will be fueled by innovations in non-invasive continuous glucose monitoring, wearable dialysis devices, and advanced neurological monitoring systems, all of which require rigorous regulatory compliance to achieve market acceptance and clinical adoption.
Current Regulatory Challenges for Wearable Biosensors
Wearable biosensors face a complex regulatory landscape that varies significantly across global markets. In the United States, the FDA categorizes these devices based on risk levels, with Class I devices requiring minimal oversight while Class II and III devices face progressively stringent requirements. The 510(k) clearance pathway remains the most common route for wearable biosensors, though the De Novo classification process has gained relevance for novel technologies without predicates.
The European Union's transition from the Medical Device Directive (MDD) to the Medical Device Regulation (MDR) has created substantial challenges for manufacturers. The MDR imposes more rigorous clinical evidence requirements, post-market surveillance obligations, and unique device identification systems. Many wearable biosensors previously classified as general wellness products now fall under medical device regulations, necessitating significant documentation and testing investments.
A fundamental regulatory challenge stems from the hybrid nature of wearable biosensors, which often combine hardware, software, and data analytics components. Regulatory frameworks traditionally designed for conventional medical devices struggle to address the rapid iteration cycles and continuous software updates characteristic of wearable technology. The FDA's Digital Health Software Precertification Program represents an attempt to adapt regulatory approaches to this reality, but remains in pilot stages.
Data privacy and security regulations present another layer of complexity. HIPAA in the US, GDPR in Europe, and similar frameworks worldwide impose strict requirements on health data collection, storage, and transmission. Manufacturers must implement robust cybersecurity measures and transparent data governance policies, particularly challenging for devices that continuously collect sensitive biometric information.
The distinction between consumer wellness devices and regulated medical devices creates a significant gray area. Many manufacturers deliberately position their products as wellness tools to avoid medical device regulations, even when the technology could support clinical applications. This regulatory arbitrage creates market confusion and potentially undermines patient safety.
Interoperability standards represent another regulatory hurdle. As healthcare ecosystems increasingly rely on connected devices, regulators are developing frameworks for ensuring safe, secure data exchange between wearable biosensors and electronic health records or clinical decision support systems. The FDA's guidance on interoperability and the International Medical Device Regulators Forum's standards are evolving to address these concerns.
Global regulatory harmonization remains elusive despite efforts through organizations like the International Medical Device Regulators Forum. Manufacturers face the burden of navigating different requirements across markets, often necessitating multiple clinical studies and documentation packages, significantly increasing time-to-market and development costs.
The European Union's transition from the Medical Device Directive (MDD) to the Medical Device Regulation (MDR) has created substantial challenges for manufacturers. The MDR imposes more rigorous clinical evidence requirements, post-market surveillance obligations, and unique device identification systems. Many wearable biosensors previously classified as general wellness products now fall under medical device regulations, necessitating significant documentation and testing investments.
A fundamental regulatory challenge stems from the hybrid nature of wearable biosensors, which often combine hardware, software, and data analytics components. Regulatory frameworks traditionally designed for conventional medical devices struggle to address the rapid iteration cycles and continuous software updates characteristic of wearable technology. The FDA's Digital Health Software Precertification Program represents an attempt to adapt regulatory approaches to this reality, but remains in pilot stages.
Data privacy and security regulations present another layer of complexity. HIPAA in the US, GDPR in Europe, and similar frameworks worldwide impose strict requirements on health data collection, storage, and transmission. Manufacturers must implement robust cybersecurity measures and transparent data governance policies, particularly challenging for devices that continuously collect sensitive biometric information.
The distinction between consumer wellness devices and regulated medical devices creates a significant gray area. Many manufacturers deliberately position their products as wellness tools to avoid medical device regulations, even when the technology could support clinical applications. This regulatory arbitrage creates market confusion and potentially undermines patient safety.
Interoperability standards represent another regulatory hurdle. As healthcare ecosystems increasingly rely on connected devices, regulators are developing frameworks for ensuring safe, secure data exchange between wearable biosensors and electronic health records or clinical decision support systems. The FDA's guidance on interoperability and the International Medical Device Regulators Forum's standards are evolving to address these concerns.
Global regulatory harmonization remains elusive despite efforts through organizations like the International Medical Device Regulators Forum. Manufacturers face the burden of navigating different requirements across markets, often necessitating multiple clinical studies and documentation packages, significantly increasing time-to-market and development costs.
Current Compliance Strategies and Frameworks
01 Wearable biosensors for health monitoring
Wearable biosensors can be designed to continuously monitor various health parameters such as heart rate, blood pressure, body temperature, and blood glucose levels. These devices typically use non-invasive or minimally invasive methods to collect physiological data from the user. The sensors can be integrated into everyday wearable items like watches, patches, or clothing, allowing for real-time health monitoring without disrupting daily activities. The collected data can be transmitted wirelessly to smartphones or healthcare providers for analysis and early detection of health issues.- Wearable biosensors for health monitoring: Wearable biosensors designed for continuous health monitoring can track various physiological parameters such as heart rate, blood pressure, body temperature, and glucose levels. These devices enable real-time health tracking and early detection of abnormalities, allowing for timely medical intervention. The sensors are typically integrated into comfortable, non-invasive wearable formats that can be used for extended periods in daily life.
- Sweat-based biosensing technologies: Biosensors that analyze sweat composition can provide valuable insights into a person's health status without invasive procedures. These sensors detect various biomarkers in sweat, including electrolytes, metabolites, and hormones, which can indicate hydration levels, stress, and potential health issues. The technology typically involves flexible materials that conform to the skin and microfluidic channels that collect and analyze sweat in real-time.
- Implantable and minimally invasive biosensors: Advanced biosensing technologies that can be implanted under the skin or inserted into the body with minimal invasion. These sensors provide continuous monitoring of internal biomarkers such as glucose, lactate, or specific proteins that indicate disease progression. The devices often incorporate biocompatible materials to reduce rejection and inflammation, and wireless communication capabilities to transmit data to external devices for analysis.
- Smart textile integrated biosensors: Biosensors integrated directly into textiles and clothing items create unobtrusive health monitoring solutions. These smart textiles incorporate conductive fibers, flexible electronics, and sensing elements that can detect physiological signals, body movements, and environmental conditions. The technology enables comfortable, everyday wearables that continuously monitor health parameters without disrupting normal activities.
- Data processing and AI for wearable biosensors: Advanced algorithms and artificial intelligence systems designed to process and analyze the large volumes of data generated by wearable biosensors. These computational approaches enable pattern recognition, anomaly detection, and predictive analytics that transform raw sensor data into actionable health insights. The technology includes edge computing capabilities for real-time processing and secure data transmission protocols to protect sensitive health information.
02 Biosensor materials and fabrication techniques
Advanced materials and fabrication techniques are crucial for developing effective wearable biosensors. These may include flexible electronics, conductive polymers, and nanomaterials that can conform to the body's contours while maintaining sensing accuracy. Techniques such as screen printing, inkjet printing, and microfabrication are employed to create sensors that are both sensitive and durable. The materials must be biocompatible to prevent skin irritation during prolonged wear and should maintain functionality under various environmental conditions including moisture and temperature fluctuations.Expand Specific Solutions03 Data processing and analysis systems for biosensors
Wearable biosensors generate large volumes of data that require sophisticated processing and analysis systems. These systems often employ artificial intelligence and machine learning algorithms to interpret the collected physiological data, identify patterns, and provide meaningful insights. Edge computing capabilities may be integrated into the wearable devices to perform preliminary data processing before transmission, reducing bandwidth requirements and enabling faster response times. The analysis systems can also include alert mechanisms for abnormal readings that require immediate attention from healthcare providers.Expand Specific Solutions04 Integration of biosensors with IoT and healthcare systems
Wearable biosensors are increasingly being integrated with Internet of Things (IoT) platforms and healthcare information systems to create comprehensive health monitoring ecosystems. This integration enables seamless data flow from the wearable device to electronic health records, telemedicine platforms, and clinical decision support systems. The connected nature of these biosensors allows for remote patient monitoring, reducing the need for in-person clinical visits and enabling continuous care for chronic conditions. Security protocols and data encryption methods are implemented to protect sensitive health information during transmission and storage.Expand Specific Solutions05 Specialized biosensors for specific biomarkers and conditions
Specialized wearable biosensors are being developed to detect specific biomarkers associated with various health conditions. These include sensors for monitoring stress hormones, inflammatory markers, electrolytes, and metabolites in bodily fluids such as sweat, tears, or interstitial fluid. Some biosensors are designed to track particular conditions like diabetes, cardiovascular diseases, or respiratory disorders with high specificity and sensitivity. These specialized sensors often employ unique detection mechanisms such as electrochemical, optical, or piezoelectric sensing to accurately measure the target biomarkers, providing valuable information for disease management and prevention.Expand Specific Solutions
Key Regulatory Bodies and Industry Stakeholders
The wearable biosensor market is in a growth phase, with increasing regulatory scrutiny as these devices transition from consumer gadgets to medical tools. The global market is projected to reach significant scale, driven by healthcare digitization and remote monitoring demands. From a technical maturity perspective, established medical device manufacturers like Medtronic and Samsung Electronics lead with regulatory expertise, while technology companies such as Google and Huawei are rapidly advancing sensing capabilities. Academic institutions including MIT and Caltech contribute fundamental research, while specialized firms like Affectiva and Polar Electro focus on niche applications. The competitive landscape reflects a convergence of traditional medical device regulation with consumer electronics innovation, creating both challenges and opportunities for market participants.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has implemented a dual-track regulatory strategy for their wearable biosensors, distinguishing between consumer wellness devices and medical-grade products. Their Samsung Health platform serves as the foundation for their consumer health wearables, while their Samsung Health Monitor application undergoes more rigorous medical device regulatory scrutiny. For medical-grade features, Samsung has successfully navigated FDA clearance and CE marking processes for specific functionalities like ECG and blood pressure monitoring on their Galaxy Watch series[2]. Their approach includes a modular software architecture that allows for separate regulatory submissions for different health monitoring features, enabling faster market entry for non-regulated functions while pursuing clearance for medical applications. Samsung's regulatory compliance strategy leverages their experience in both consumer electronics and healthcare sectors, implementing design controls that satisfy IEC 62304 for software lifecycle processes and ISO 14971 for risk management[4]. They've established dedicated regulatory affairs teams that collaborate with regional authorities to address market-specific requirements, particularly focusing on data privacy frameworks that comply with both GDPR and HIPAA standards.
Strengths: Strong integration between consumer electronics expertise and healthcare regulatory knowledge; established global supply chain with quality management systems that meet medical device standards; significant R&D resources to address evolving regulatory requirements. Weaknesses: Balancing consumer expectations for rapid innovation with medical device regulatory timelines creates market positioning challenges; their dual-track approach may create consumer confusion about which features have regulatory clearance versus wellness applications.
Medtronic, Inc.
Technical Solution: Medtronic has developed a comprehensive regulatory compliance framework for their wearable biosensors that integrates with their established medical device ecosystem. Their approach includes a modular design philosophy that separates sensing components from data processing units, allowing for streamlined regulatory pathways. Medtronic's Guardian™ Connect continuous glucose monitoring system exemplifies their regulatory strategy, utilizing a risk-based classification approach that addresses both hardware and software components separately[1]. The company employs a "pre-submission" consultation process with regulatory bodies like FDA and EU MDR authorities to establish clear pathways before formal submissions. Their quality management system specifically addresses the unique challenges of wearable biosensors, including biocompatibility testing protocols, long-term skin contact considerations, and data security frameworks that comply with HIPAA and GDPR requirements[3]. Medtronic has pioneered the concept of "regulatory co-development," where regulatory specialists are embedded within R&D teams from initial concept stages.
Strengths: Extensive experience navigating complex regulatory pathways across global markets; established relationships with regulatory bodies; comprehensive quality management systems specifically tailored for medical wearables. Weaknesses: Their rigorous compliance approach may extend development timelines compared to consumer-focused competitors; higher associated costs for regulatory compliance may impact pricing strategy and market accessibility.
Critical Standards and Certification Pathways
Wearable biosensors and applications thereof
PatentActiveUS11813057B2
Innovation
- Development of highly sensitive In2O3 nanoribbon transistor biosensors with integrated on-chip gold gate electrodes, deposited on flexible polyethylene terephthalate substrates, functionalized with glucose oxidase, chitosan, and single-walled carbon nanotubes, capable of detecting glucose concentrations between 10 nM to 1 mM in external body fluids without breaking the skin.
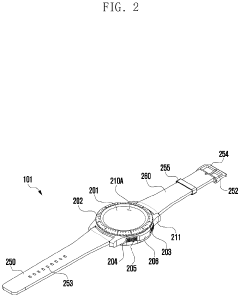
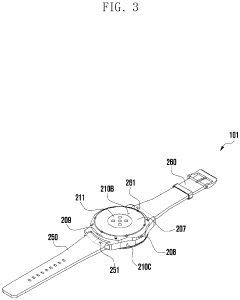
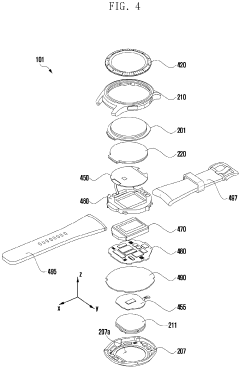
Wearable electronic device including plurality of biometric sensors
PatentPendingEP4275599A1
Innovation
- The wearable electronic device employs a configuration where a first biosensor with a light transmitter on one substrate and a light receiver on an overlapping second substrate calculates bio signals, allowing for a miniaturized design by separating the components while maintaining effective signal transmission and reception.
Risk Management and Patient Safety Considerations
Risk management in wearable biosensors represents a critical component of regulatory compliance and patient safety assurance. The integration of these devices into healthcare delivery systems introduces unique risk profiles that must be systematically identified, analyzed, and mitigated. Manufacturers must implement comprehensive risk management processes aligned with ISO 14971, which provides a framework for identifying hazards, estimating and evaluating associated risks, and implementing control measures throughout the product lifecycle.
Patient safety considerations begin with the physical design of wearable biosensors, addressing biocompatibility of materials in direct contact with skin, potential allergic reactions, and electrical safety parameters. The risk of skin irritation or burns from prolonged wear requires careful material selection and design validation through extensive biocompatibility testing according to ISO 10993 standards.
Data security vulnerabilities present another significant risk dimension, as these devices collect, process, and transmit sensitive health information. Unauthorized access to this data could compromise patient privacy and potentially lead to incorrect clinical decisions if data integrity is compromised. Manufacturers must implement robust encryption protocols, secure authentication mechanisms, and regular security updates to mitigate these risks.
Measurement accuracy and reliability constitute fundamental safety considerations, as healthcare decisions may be based on data from these devices. Factors affecting accuracy include sensor drift, calibration stability, and environmental interferences. Rigorous validation protocols must verify performance across the intended operating conditions and user demographics, with clear documentation of limitations and potential sources of error.
The risk of misinterpretation of data by both healthcare providers and patients necessitates clear labeling, comprehensive user instructions, and appropriate training materials. Manufacturers must consider the varying levels of technical literacy among users and implement design features that minimize the possibility of misuse or misunderstanding of device outputs.
Post-market surveillance systems play a crucial role in ongoing risk management, enabling manufacturers to identify emerging safety issues not detected during pre-market evaluation. These systems should include adverse event reporting mechanisms, periodic safety update reviews, and processes for implementing corrective actions when necessary. The feedback loop between real-world use and product improvement represents a cornerstone of effective risk management for wearable biosensors.
Patient safety considerations begin with the physical design of wearable biosensors, addressing biocompatibility of materials in direct contact with skin, potential allergic reactions, and electrical safety parameters. The risk of skin irritation or burns from prolonged wear requires careful material selection and design validation through extensive biocompatibility testing according to ISO 10993 standards.
Data security vulnerabilities present another significant risk dimension, as these devices collect, process, and transmit sensitive health information. Unauthorized access to this data could compromise patient privacy and potentially lead to incorrect clinical decisions if data integrity is compromised. Manufacturers must implement robust encryption protocols, secure authentication mechanisms, and regular security updates to mitigate these risks.
Measurement accuracy and reliability constitute fundamental safety considerations, as healthcare decisions may be based on data from these devices. Factors affecting accuracy include sensor drift, calibration stability, and environmental interferences. Rigorous validation protocols must verify performance across the intended operating conditions and user demographics, with clear documentation of limitations and potential sources of error.
The risk of misinterpretation of data by both healthcare providers and patients necessitates clear labeling, comprehensive user instructions, and appropriate training materials. Manufacturers must consider the varying levels of technical literacy among users and implement design features that minimize the possibility of misuse or misunderstanding of device outputs.
Post-market surveillance systems play a crucial role in ongoing risk management, enabling manufacturers to identify emerging safety issues not detected during pre-market evaluation. These systems should include adverse event reporting mechanisms, periodic safety update reviews, and processes for implementing corrective actions when necessary. The feedback loop between real-world use and product improvement represents a cornerstone of effective risk management for wearable biosensors.
Global Harmonization Efforts for Wearable Regulations
The global landscape of wearable biosensor regulations presents a complex and fragmented picture, with different countries and regions implementing varying standards and approval processes. This fragmentation creates significant challenges for manufacturers seeking to distribute their products internationally, often requiring multiple submissions and compliance with diverse regulatory frameworks. Recognizing these inefficiencies, several international initiatives have emerged to harmonize regulatory approaches for wearable medical technologies.
The International Medical Device Regulators Forum (IMDRF) has taken a leading role in promoting regulatory convergence. Through its working groups focused specifically on Software as a Medical Device (SaMD) and cybersecurity, the IMDRF has developed guidance documents that address many aspects relevant to wearable biosensors. These efforts aim to establish common principles and terminology that can be adopted across jurisdictions, reducing redundancy in regulatory submissions.
The Medical Device Single Audit Program (MDSAP) represents another significant harmonization effort, allowing manufacturers to undergo a single audit that satisfies the requirements of multiple regulatory authorities. Currently, regulatory bodies from Australia, Brazil, Canada, Japan, and the United States participate in this program, with the European Union serving as an observer. For wearable biosensor manufacturers, MDSAP offers a streamlined pathway to multi-market compliance.
Regional harmonization initiatives have also gained momentum. The Asia-Pacific Economic Cooperation (APEC) Life Sciences Innovation Forum has established the Regulatory Harmonization Steering Committee, which works to align medical device regulations across its 21 member economies. Similarly, the Association of Southeast Asian Nations (ASEAN) has developed the ASEAN Medical Device Directive to standardize requirements across its member states.
Standardization bodies like the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC) contribute significantly to harmonization through the development of international standards. The ISO 13485 for quality management systems and IEC 60601 series for medical electrical equipment safety provide globally recognized benchmarks that many regulatory frameworks reference or incorporate.
Despite these promising developments, challenges remain in achieving true global harmonization. Cultural differences in risk perception, varying healthcare system structures, and differing political priorities continue to impede complete alignment. Additionally, the rapid pace of technological innovation in wearable biosensors often outstrips the development of harmonized regulatory approaches, creating temporary regulatory gaps that countries may fill with divergent interim measures.
The International Medical Device Regulators Forum (IMDRF) has taken a leading role in promoting regulatory convergence. Through its working groups focused specifically on Software as a Medical Device (SaMD) and cybersecurity, the IMDRF has developed guidance documents that address many aspects relevant to wearable biosensors. These efforts aim to establish common principles and terminology that can be adopted across jurisdictions, reducing redundancy in regulatory submissions.
The Medical Device Single Audit Program (MDSAP) represents another significant harmonization effort, allowing manufacturers to undergo a single audit that satisfies the requirements of multiple regulatory authorities. Currently, regulatory bodies from Australia, Brazil, Canada, Japan, and the United States participate in this program, with the European Union serving as an observer. For wearable biosensor manufacturers, MDSAP offers a streamlined pathway to multi-market compliance.
Regional harmonization initiatives have also gained momentum. The Asia-Pacific Economic Cooperation (APEC) Life Sciences Innovation Forum has established the Regulatory Harmonization Steering Committee, which works to align medical device regulations across its 21 member economies. Similarly, the Association of Southeast Asian Nations (ASEAN) has developed the ASEAN Medical Device Directive to standardize requirements across its member states.
Standardization bodies like the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC) contribute significantly to harmonization through the development of international standards. The ISO 13485 for quality management systems and IEC 60601 series for medical electrical equipment safety provide globally recognized benchmarks that many regulatory frameworks reference or incorporate.
Despite these promising developments, challenges remain in achieving true global harmonization. Cultural differences in risk perception, varying healthcare system structures, and differing political priorities continue to impede complete alignment. Additionally, the rapid pace of technological innovation in wearable biosensors often outstrips the development of harmonized regulatory approaches, creating temporary regulatory gaps that countries may fill with divergent interim measures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!