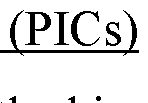Wearable Biosensors: Catalysts for Next-Gen Medical Devices
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Wearable Biosensor Evolution and Objectives
Wearable biosensors have undergone a remarkable evolution over the past two decades, transforming from rudimentary step counters to sophisticated multi-parameter monitoring systems capable of continuous health assessment. The journey began in the early 2000s with simple pedometers and heart rate monitors, primarily targeting fitness enthusiasts. These early devices laid the foundation for what would become a revolutionary approach to healthcare monitoring and management.
By the mid-2010s, the integration of advanced microelectronics and improved sensor technologies enabled the development of more sophisticated wearable biosensors with enhanced capabilities for monitoring various physiological parameters. This period witnessed the emergence of smartwatches and fitness bands equipped with photoplethysmography (PPG) sensors for heart rate monitoring, accelerometers for activity tracking, and basic electrodermal activity sensors.
The technological trajectory has been characterized by miniaturization, increased sensor accuracy, improved power efficiency, and enhanced data processing capabilities. Recent advancements have introduced non-invasive glucose monitoring, continuous blood pressure assessment, and electrochemical sensors capable of analyzing sweat composition for biomarkers. These developments represent significant milestones in the evolution of wearable biosensor technology.
Current research is focused on addressing key challenges such as sensor drift, biocompatibility, power consumption, and data accuracy. The integration of artificial intelligence and machine learning algorithms has further enhanced the analytical capabilities of these devices, enabling predictive health insights and personalized recommendations based on individual health patterns.
The primary objectives of wearable biosensor technology development are multifaceted. First, to achieve clinical-grade accuracy and reliability comparable to traditional medical devices, ensuring that data collected can be used for diagnostic and treatment decisions. Second, to expand the range of biomarkers that can be monitored non-invasively, particularly focusing on indicators relevant to chronic disease management.
Additionally, there is a strong emphasis on improving user experience through enhanced comfort, extended battery life, and seamless integration with healthcare systems. The ultimate goal is to transition from reactive to preventive healthcare by enabling continuous monitoring that can detect subtle physiological changes before they manifest as clinical symptoms.
Looking forward, the technological roadmap aims to develop fully integrated biosensing platforms capable of simultaneous multi-parameter monitoring with minimal user intervention. This includes the development of self-powered sensors, biodegradable components, and advanced materials that can conform to the human body while maintaining optimal sensing performance.
By the mid-2010s, the integration of advanced microelectronics and improved sensor technologies enabled the development of more sophisticated wearable biosensors with enhanced capabilities for monitoring various physiological parameters. This period witnessed the emergence of smartwatches and fitness bands equipped with photoplethysmography (PPG) sensors for heart rate monitoring, accelerometers for activity tracking, and basic electrodermal activity sensors.
The technological trajectory has been characterized by miniaturization, increased sensor accuracy, improved power efficiency, and enhanced data processing capabilities. Recent advancements have introduced non-invasive glucose monitoring, continuous blood pressure assessment, and electrochemical sensors capable of analyzing sweat composition for biomarkers. These developments represent significant milestones in the evolution of wearable biosensor technology.
Current research is focused on addressing key challenges such as sensor drift, biocompatibility, power consumption, and data accuracy. The integration of artificial intelligence and machine learning algorithms has further enhanced the analytical capabilities of these devices, enabling predictive health insights and personalized recommendations based on individual health patterns.
The primary objectives of wearable biosensor technology development are multifaceted. First, to achieve clinical-grade accuracy and reliability comparable to traditional medical devices, ensuring that data collected can be used for diagnostic and treatment decisions. Second, to expand the range of biomarkers that can be monitored non-invasively, particularly focusing on indicators relevant to chronic disease management.
Additionally, there is a strong emphasis on improving user experience through enhanced comfort, extended battery life, and seamless integration with healthcare systems. The ultimate goal is to transition from reactive to preventive healthcare by enabling continuous monitoring that can detect subtle physiological changes before they manifest as clinical symptoms.
Looking forward, the technological roadmap aims to develop fully integrated biosensing platforms capable of simultaneous multi-parameter monitoring with minimal user intervention. This includes the development of self-powered sensors, biodegradable components, and advanced materials that can conform to the human body while maintaining optimal sensing performance.
Healthcare Market Demand Analysis
The global wearable biosensor market is experiencing unprecedented growth, driven by increasing healthcare costs, aging populations, and the rising prevalence of chronic diseases. Current market valuations place the wearable biosensor sector at approximately 12 billion USD in 2023, with projections indicating a compound annual growth rate of 18-20% through 2030. This remarkable expansion reflects the growing demand for continuous health monitoring solutions that can reduce hospital readmissions and enable preventive healthcare approaches.
Consumer demand for personalized healthcare is reshaping the medical device landscape. Patients increasingly seek active participation in their health management, creating a robust market for devices that provide real-time physiological data. This shift toward patient-centered care has accelerated the adoption of wearable biosensors across various healthcare settings, from hospitals to home care environments.
The remote patient monitoring segment represents the fastest-growing application area, with healthcare providers increasingly implementing telehealth solutions to manage patients with chronic conditions. Diabetes management leads this category, with continuous glucose monitoring systems witnessing adoption rates increasing by nearly 40% annually in developed markets. Cardiovascular monitoring follows closely, driven by the prevalence of heart disease and the clinical value of continuous ECG and blood pressure data.
Healthcare systems worldwide are recognizing the economic benefits of wearable biosensors. Studies indicate that remote monitoring can reduce hospital readmissions by 25-30% for certain chronic conditions, translating to significant cost savings. Insurance companies have begun offering premium reductions for patients utilizing approved monitoring devices, further stimulating market demand.
Regional analysis reveals North America currently dominates the market share at approximately 40%, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next five years due to improving healthcare infrastructure, increasing disposable income, and greater smartphone penetration enabling connected health solutions.
The COVID-19 pandemic has served as a market accelerator, with healthcare providers rapidly adopting remote monitoring solutions to minimize in-person visits. This has created lasting changes in healthcare delivery models, with 76% of hospitals now implementing some form of remote patient monitoring program compared to just 24% pre-pandemic.
Consumer surveys indicate growing acceptance of wearable health technology, with over 60% of adults in developed markets expressing willingness to use wearable devices for health monitoring if recommended by healthcare providers. This represents a significant shift from just five years ago when adoption intention rates were below 40%.
Consumer demand for personalized healthcare is reshaping the medical device landscape. Patients increasingly seek active participation in their health management, creating a robust market for devices that provide real-time physiological data. This shift toward patient-centered care has accelerated the adoption of wearable biosensors across various healthcare settings, from hospitals to home care environments.
The remote patient monitoring segment represents the fastest-growing application area, with healthcare providers increasingly implementing telehealth solutions to manage patients with chronic conditions. Diabetes management leads this category, with continuous glucose monitoring systems witnessing adoption rates increasing by nearly 40% annually in developed markets. Cardiovascular monitoring follows closely, driven by the prevalence of heart disease and the clinical value of continuous ECG and blood pressure data.
Healthcare systems worldwide are recognizing the economic benefits of wearable biosensors. Studies indicate that remote monitoring can reduce hospital readmissions by 25-30% for certain chronic conditions, translating to significant cost savings. Insurance companies have begun offering premium reductions for patients utilizing approved monitoring devices, further stimulating market demand.
Regional analysis reveals North America currently dominates the market share at approximately 40%, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next five years due to improving healthcare infrastructure, increasing disposable income, and greater smartphone penetration enabling connected health solutions.
The COVID-19 pandemic has served as a market accelerator, with healthcare providers rapidly adopting remote monitoring solutions to minimize in-person visits. This has created lasting changes in healthcare delivery models, with 76% of hospitals now implementing some form of remote patient monitoring program compared to just 24% pre-pandemic.
Consumer surveys indicate growing acceptance of wearable health technology, with over 60% of adults in developed markets expressing willingness to use wearable devices for health monitoring if recommended by healthcare providers. This represents a significant shift from just five years ago when adoption intention rates were below 40%.
Current Biosensor Technologies and Limitations
Wearable biosensor technologies have evolved significantly over the past decade, transitioning from simple activity trackers to sophisticated medical monitoring devices. Current biosensor technologies encompass a diverse range of sensing modalities including electrochemical, optical, piezoelectric, and thermal sensors. Electrochemical sensors dominate the market due to their ability to detect specific biomarkers through redox reactions, offering high sensitivity for monitoring glucose levels, electrolytes, and metabolites in bodily fluids.
Optical biosensors utilize spectroscopic techniques such as fluorescence, surface plasmon resonance, and colorimetry to detect biomolecular interactions without requiring direct electrical connections. These sensors excel in applications requiring non-invasive monitoring, such as pulse oximetry and continuous heart rate measurement, though they often struggle with signal interference from ambient light and skin pigmentation.
Despite significant advancements, current wearable biosensor technologies face substantial limitations. Power consumption remains a critical constraint, with most advanced sensors requiring frequent recharging or battery replacement, limiting their practical continuous monitoring capabilities. This power limitation often forces compromises between measurement frequency, accuracy, and device longevity.
Biocompatibility presents another significant challenge, as prolonged skin contact can cause irritation, allergic reactions, or discomfort. Materials that are simultaneously biocompatible, flexible, and durable enough for extended wear remain elusive, particularly for sensors requiring direct contact with bodily fluids or implantation.
Data accuracy and reliability constitute persistent limitations, especially in real-world conditions involving motion artifacts, environmental interference, and physiological variations between users. Most current sensors require frequent calibration and struggle to maintain accuracy during physical activity or environmental changes, limiting their clinical utility.
Miniaturization challenges continue to impede progress, as integrating multiple sensing modalities, power sources, and data transmission capabilities into comfortable, unobtrusive form factors remains technically challenging. The trade-off between sensor size and performance often results in compromises that affect user adoption and clinical value.
Regulatory hurdles further complicate advancement, with medical-grade wearable biosensors facing stringent approval processes that significantly extend development timelines and increase costs. Many promising technologies remain in research phases due to difficulties in meeting regulatory requirements for accuracy, reliability, and safety in diverse patient populations.
Optical biosensors utilize spectroscopic techniques such as fluorescence, surface plasmon resonance, and colorimetry to detect biomolecular interactions without requiring direct electrical connections. These sensors excel in applications requiring non-invasive monitoring, such as pulse oximetry and continuous heart rate measurement, though they often struggle with signal interference from ambient light and skin pigmentation.
Despite significant advancements, current wearable biosensor technologies face substantial limitations. Power consumption remains a critical constraint, with most advanced sensors requiring frequent recharging or battery replacement, limiting their practical continuous monitoring capabilities. This power limitation often forces compromises between measurement frequency, accuracy, and device longevity.
Biocompatibility presents another significant challenge, as prolonged skin contact can cause irritation, allergic reactions, or discomfort. Materials that are simultaneously biocompatible, flexible, and durable enough for extended wear remain elusive, particularly for sensors requiring direct contact with bodily fluids or implantation.
Data accuracy and reliability constitute persistent limitations, especially in real-world conditions involving motion artifacts, environmental interference, and physiological variations between users. Most current sensors require frequent calibration and struggle to maintain accuracy during physical activity or environmental changes, limiting their clinical utility.
Miniaturization challenges continue to impede progress, as integrating multiple sensing modalities, power sources, and data transmission capabilities into comfortable, unobtrusive form factors remains technically challenging. The trade-off between sensor size and performance often results in compromises that affect user adoption and clinical value.
Regulatory hurdles further complicate advancement, with medical-grade wearable biosensors facing stringent approval processes that significantly extend development timelines and increase costs. Many promising technologies remain in research phases due to difficulties in meeting regulatory requirements for accuracy, reliability, and safety in diverse patient populations.
Current Biosensor Integration Solutions
01 Wearable biosensors for health monitoring
Wearable biosensors can be designed to continuously monitor various health parameters such as heart rate, blood pressure, body temperature, and blood glucose levels. These devices typically use non-invasive or minimally invasive methods to collect physiological data from the user. The sensors can be integrated into everyday wearable items like watches, bands, or patches, allowing for real-time health monitoring without disrupting daily activities. The collected data can be transmitted wirelessly to smartphones or healthcare systems for analysis and early detection of health issues.- Wearable biosensors for health monitoring: Wearable biosensors can be designed to continuously monitor various health parameters such as heart rate, blood pressure, body temperature, and glucose levels. These devices typically use non-invasive or minimally invasive methods to collect physiological data from the user. The sensors can be integrated into everyday wearable items like watches, patches, or clothing, allowing for real-time health monitoring without disrupting daily activities. The collected data can be transmitted wirelessly to smartphones or healthcare providers for analysis and early detection of health issues.
- Flexible and stretchable biosensor technologies: Advanced materials and manufacturing techniques enable the development of flexible and stretchable biosensors that conform to the body's contours. These sensors incorporate conductive polymers, nanomaterials, or thin-film electronics that maintain functionality while being bent, twisted, or stretched. The flexibility improves user comfort and ensures consistent contact with the skin for accurate measurements. These technologies allow for long-term wear without causing irritation or discomfort, making them suitable for continuous monitoring applications.
- Biochemical sensing mechanisms: Wearable biosensors employ various biochemical sensing mechanisms to detect and measure biomarkers in bodily fluids such as sweat, tears, or interstitial fluid. These mechanisms include electrochemical sensors, optical sensors, and enzymatic reactions that can detect specific molecules like glucose, lactate, electrolytes, or stress hormones. The sensors may incorporate selective membranes or recognition elements like antibodies or aptamers to enhance specificity. Some advanced designs include microfluidic channels to collect and direct small volumes of fluid to the sensing elements.
- Data processing and wireless communication systems: Wearable biosensors integrate sophisticated data processing capabilities and wireless communication systems to handle the collected biological information. These systems include microcontrollers for signal processing, algorithms for data analysis, and wireless protocols like Bluetooth, NFC, or cellular connectivity for transmitting data to external devices. Power management systems optimize battery life while maintaining continuous monitoring capabilities. Some advanced systems incorporate edge computing to process data locally before transmission, reducing bandwidth requirements and enhancing privacy.
- Application-specific biosensor designs: Wearable biosensors are designed for specific applications across healthcare, fitness, workplace safety, and environmental monitoring. These specialized designs may focus on particular user groups like athletes, elderly patients, or industrial workers. For example, biosensors for diabetes management continuously monitor glucose levels, while athletic performance sensors track multiple physiological parameters during exercise. Other specialized designs include sensors for detecting exposure to environmental toxins, monitoring medication adherence, or assessing cognitive states through physiological markers.
02 Flexible and stretchable biosensor technologies
Advanced materials and manufacturing techniques enable the development of flexible and stretchable biosensors that conform to the body's contours. These sensors incorporate conductive polymers, nanomaterials, or thin-film electronics that maintain functionality while bending or stretching. The flexibility improves user comfort and ensures consistent contact with the skin for accurate measurements. These technologies allow biosensors to be integrated into clothing, adhesive patches, or directly attached to the skin, expanding the potential applications and improving user compliance.Expand Specific Solutions03 Electrochemical and optical sensing mechanisms
Wearable biosensors employ various sensing mechanisms, with electrochemical and optical methods being particularly prominent. Electrochemical sensors detect biological analytes through chemical reactions that generate measurable electrical signals. These sensors can monitor glucose, lactate, electrolytes, and other biomarkers in bodily fluids like sweat or interstitial fluid. Optical sensors use light-based detection methods such as photoplethysmography, spectroscopy, or fluorescence to measure parameters like blood oxygen saturation, heart rate, or specific biomarkers. The choice of sensing mechanism depends on the target analyte and the required sensitivity and specificity.Expand Specific Solutions04 Data processing and artificial intelligence integration
Modern wearable biosensors incorporate sophisticated data processing capabilities and artificial intelligence algorithms to enhance their functionality. These systems can filter noise, compensate for motion artifacts, and apply calibration algorithms to improve measurement accuracy. Machine learning algorithms analyze patterns in the collected data to provide personalized insights, detect anomalies, or predict potential health issues before they become severe. Cloud connectivity enables secure storage of historical data and facilitates remote monitoring by healthcare providers, creating opportunities for telemedicine and personalized healthcare.Expand Specific Solutions05 Energy harvesting and power management solutions
Innovative energy solutions address the power requirements of wearable biosensors, which need to operate continuously for extended periods. Energy harvesting technologies capture power from body heat, movement, or ambient light to supplement or replace traditional batteries. Advanced power management systems optimize energy consumption by adjusting sampling rates, processing loads, and transmission frequencies based on usage patterns or detected events. Low-power electronic components and efficient communication protocols further extend operating time between charges, enhancing the practicality of continuous monitoring applications.Expand Specific Solutions
Leading Companies in Wearable Medical Technology
The wearable biosensors market is experiencing rapid growth in the early maturity phase, with an estimated market size exceeding $12 billion and projected to grow at a CAGR of 25-30% through 2028. Major technology players like Google (through Verily Life Sciences), Samsung Electronics, and Philips are competing alongside specialized medical device companies such as Biosense Webster and Cardiac Pacemakers. Academic institutions including MIT, Caltech, and Northwestern University are driving innovation through research partnerships with industry leaders. The technology is approaching mainstream adoption with continuous glucose monitors and cardiac monitoring devices already commercialized, while next-generation sensors utilizing advanced materials and AI integration are being developed by companies like Sinocare and Affectiva to enable real-time health monitoring and predictive analytics capabilities.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed a comprehensive wearable biosensor ecosystem centered around their Galaxy Watch series and Samsung Health platform. Their advanced biosensors utilize photoplethysmography (PPG) technology for continuous heart rate monitoring, electrocardiogram (ECG) capabilities for detecting irregular heart rhythms, and bioelectrical impedance analysis (BIA) for body composition measurements. Samsung's latest sensors incorporate reflective spectroscopy techniques that enable blood pressure monitoring and blood oxygen saturation (SpO2) tracking without invasive procedures[1]. The company has also pioneered integration of these sensors with their semiconductor expertise, creating ultra-low-power biosensing chips that extend battery life while maintaining high sensitivity. Their recent developments include stretchable, skin-adherent patches that can monitor multiple physiological parameters simultaneously while transmitting data wirelessly to medical providers[3].
Strengths: Samsung leverages vertical integration of hardware and software, controlling the entire ecosystem from sensor manufacturing to data analysis platforms. Their massive consumer electronics reach provides immediate commercialization pathways. Weaknesses: Their consumer-focused approach sometimes prioritizes user experience over clinical-grade accuracy, and regulatory approval processes for medical-grade features can delay implementation of cutting-edge technologies.
Cardiac Pacemakers, Inc.
Technical Solution: Cardiac Pacemakers, Inc. (a subsidiary of Boston Scientific) has developed advanced implantable and wearable biosensor technologies focused on cardiac monitoring and therapy. Their latest wearable solutions include the HeartLogic Heart Failure Diagnostic Service, which combines multiple physiological sensors to detect early signs of worsening heart failure up to weeks before symptom onset[7]. The company's biosensors measure thoracic impedance, heart sounds, respiratory rate and volume, heart rate, and patient activity levels, integrating these data streams through proprietary algorithms that have demonstrated 70% sensitivity in predicting heart failure events. Their technology incorporates miniaturized accelerometers and acoustic sensors that can detect subtle changes in cardiac function through the chest wall. Recent innovations include subcutaneous sensors that can be implanted with minimally invasive procedures while providing continuous monitoring for arrhythmias and hemodynamic parameters[8]. The company has also pioneered communication protocols that enable their biosensors to transmit data securely to healthcare providers while minimizing power consumption.
Strengths: Cardiac Pacemakers brings unparalleled expertise in cardiac monitoring technology and established clinical validation pathways. Their solutions are designed with direct clinical applications in mind, addressing specific cardiac conditions with proven outcomes. Weaknesses: Their highly specialized focus on cardiac applications may limit broader application across other medical domains, and their technologies often require physician involvement for implementation, potentially limiting direct consumer access.
Key Patents and Research in Biosensor Technology
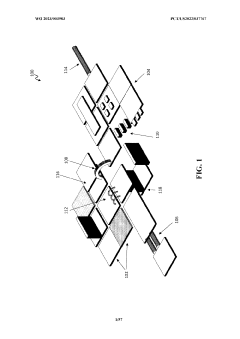
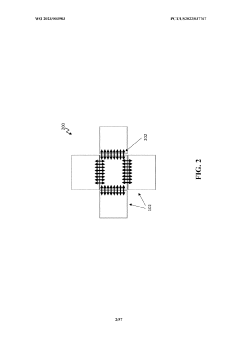
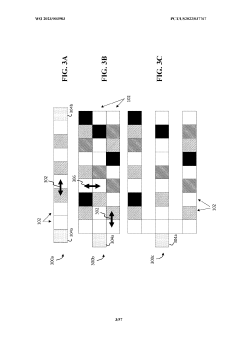
Wearable biosensors for semi-invasive, real-time monitoring of analytes, and related methods and apparatus
PatentWO2023003983A1
Innovation
- Development of integrated photonic systems with sensor photonic integrated circuits (PICs) functionalized with binding ligands, capable of real-time monitoring using refractive index-based biosensing, absorption spectroscopy, and fluorescence spectroscopy, integrated into wearable devices with microneedles for semi-invasive sampling and optical analyte sensors configured to detect multiple analytes simultaneously.
Wearable biosensors and applications thereof
PatentActiveUS11813057B2
Innovation
- Development of highly sensitive In2O3 nanoribbon transistor biosensors with integrated on-chip gold gate electrodes, deposited on flexible polyethylene terephthalate substrates, functionalized with glucose oxidase, chitosan, and single-walled carbon nanotubes, capable of detecting glucose concentrations between 10 nM to 1 mM in external body fluids without breaking the skin.
Regulatory Framework for Medical Wearables
The regulatory landscape for wearable medical devices represents a complex and evolving framework that significantly impacts product development, market entry, and commercial success. In the United States, the FDA classifies wearable biosensors based on their intended use and risk profile, with most falling under Class I (low risk) or Class II (moderate risk) categories requiring 510(k) clearance. The regulatory pathway depends critically on whether the device is marketed for general wellness or for specific medical purposes, with the latter facing more stringent requirements.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced more comprehensive requirements for wearable medical technologies, including stricter clinical evidence standards and post-market surveillance. Notably, the EU's GDPR adds another layer of compliance requirements regarding the handling of personal health data collected by these devices.
Asian markets present varying regulatory approaches. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific pathways for software as a medical device (SaMD), while China's National Medical Products Administration (NMPA) has recently updated its framework to accommodate innovative medical wearables, though with significant requirements for local testing.
A critical challenge across all jurisdictions is the rapid pace of technological innovation outstripping regulatory frameworks. Many regulatory bodies are implementing special programs to address this gap, such as the FDA's Digital Health Software Precertification Program and the UK MHRA's Innovation Office, designed to provide more agile regulatory pathways for digital health technologies.
Data privacy and security regulations represent another crucial dimension, with requirements varying significantly across regions. HIPAA compliance in the US, GDPR in Europe, and the Personal Information Protection Law in China all impose specific obligations on manufacturers regarding data protection, consent management, and breach notification.
Interoperability standards are increasingly emphasized by regulators, with the FDA and other agencies promoting adherence to frameworks like FHIR (Fast Healthcare Interoperability Resources) to ensure seamless integration with healthcare systems. This focus on interoperability reflects the growing recognition that the value of wearable biosensors is maximized when their data can be effectively incorporated into broader healthcare ecosystems.
Regulatory harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), are working to establish more consistent global approaches to novel medical technologies, potentially reducing the compliance burden for manufacturers targeting multiple markets.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced more comprehensive requirements for wearable medical technologies, including stricter clinical evidence standards and post-market surveillance. Notably, the EU's GDPR adds another layer of compliance requirements regarding the handling of personal health data collected by these devices.
Asian markets present varying regulatory approaches. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific pathways for software as a medical device (SaMD), while China's National Medical Products Administration (NMPA) has recently updated its framework to accommodate innovative medical wearables, though with significant requirements for local testing.
A critical challenge across all jurisdictions is the rapid pace of technological innovation outstripping regulatory frameworks. Many regulatory bodies are implementing special programs to address this gap, such as the FDA's Digital Health Software Precertification Program and the UK MHRA's Innovation Office, designed to provide more agile regulatory pathways for digital health technologies.
Data privacy and security regulations represent another crucial dimension, with requirements varying significantly across regions. HIPAA compliance in the US, GDPR in Europe, and the Personal Information Protection Law in China all impose specific obligations on manufacturers regarding data protection, consent management, and breach notification.
Interoperability standards are increasingly emphasized by regulators, with the FDA and other agencies promoting adherence to frameworks like FHIR (Fast Healthcare Interoperability Resources) to ensure seamless integration with healthcare systems. This focus on interoperability reflects the growing recognition that the value of wearable biosensors is maximized when their data can be effectively incorporated into broader healthcare ecosystems.
Regulatory harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), are working to establish more consistent global approaches to novel medical technologies, potentially reducing the compliance burden for manufacturers targeting multiple markets.
Data Security and Patient Privacy Considerations
The integration of wearable biosensors into medical devices introduces significant data security and patient privacy challenges that must be addressed comprehensively. As these devices continuously collect sensitive biometric data, they create unprecedented vulnerabilities requiring robust protection frameworks.
Healthcare data breaches have increased by 55% since 2019, with wearable device vulnerabilities contributing significantly to this trend. The sensitive nature of biosensor-collected data—including heart rate patterns, glucose levels, and neurological signals—makes it particularly valuable to malicious actors, commanding premium prices on illicit markets.
Current regulatory frameworks like HIPAA in the United States and GDPR in Europe provide baseline protection requirements, but their application to continuously evolving wearable biosensor technology remains challenging. The boundary between medical and consumer-grade wearables further complicates compliance efforts, creating regulatory gray areas that manufacturers must navigate carefully.
Encryption technologies represent the first line of defense for wearable biosensors. Advanced encryption standards (AES-256) and secure element architecture are becoming industry standards, though they introduce power consumption challenges for small form-factor devices. Emerging lightweight cryptographic protocols specifically designed for resource-constrained IoT devices show promise in balancing security with power efficiency.
Authentication mechanisms present another critical security dimension. Multi-factor authentication, biometric verification, and zero-knowledge proof systems are being implemented to ensure only authorized access to sensitive biosensor data. However, these systems must balance security with usability to avoid compromising patient experience or emergency access.
Data minimization principles are increasingly important in biosensor design. By processing data at the edge and transmitting only essential information, manufacturers can reduce attack surfaces and privacy risks. This approach requires sophisticated on-device algorithms that can extract clinically relevant insights while discarding raw data that could compromise patient privacy.
Consent management frameworks represent another evolving area. Dynamic consent models allow patients to maintain granular control over their biosensor data, specifying which data points can be collected, how long they can be stored, and with whom they can be shared. These systems must be intuitive enough for patients with varying technical literacy while remaining comprehensive enough to satisfy regulatory requirements.
As wearable biosensors become more integrated into clinical decision-making, establishing clear liability frameworks for data breaches becomes increasingly important. The distributed nature of these systems—spanning device manufacturers, software developers, healthcare providers, and cloud services—creates complex questions of responsibility that remain largely unresolved in current legal frameworks.
Healthcare data breaches have increased by 55% since 2019, with wearable device vulnerabilities contributing significantly to this trend. The sensitive nature of biosensor-collected data—including heart rate patterns, glucose levels, and neurological signals—makes it particularly valuable to malicious actors, commanding premium prices on illicit markets.
Current regulatory frameworks like HIPAA in the United States and GDPR in Europe provide baseline protection requirements, but their application to continuously evolving wearable biosensor technology remains challenging. The boundary between medical and consumer-grade wearables further complicates compliance efforts, creating regulatory gray areas that manufacturers must navigate carefully.
Encryption technologies represent the first line of defense for wearable biosensors. Advanced encryption standards (AES-256) and secure element architecture are becoming industry standards, though they introduce power consumption challenges for small form-factor devices. Emerging lightweight cryptographic protocols specifically designed for resource-constrained IoT devices show promise in balancing security with power efficiency.
Authentication mechanisms present another critical security dimension. Multi-factor authentication, biometric verification, and zero-knowledge proof systems are being implemented to ensure only authorized access to sensitive biosensor data. However, these systems must balance security with usability to avoid compromising patient experience or emergency access.
Data minimization principles are increasingly important in biosensor design. By processing data at the edge and transmitting only essential information, manufacturers can reduce attack surfaces and privacy risks. This approach requires sophisticated on-device algorithms that can extract clinically relevant insights while discarding raw data that could compromise patient privacy.
Consent management frameworks represent another evolving area. Dynamic consent models allow patients to maintain granular control over their biosensor data, specifying which data points can be collected, how long they can be stored, and with whom they can be shared. These systems must be intuitive enough for patients with varying technical literacy while remaining comprehensive enough to satisfy regulatory requirements.
As wearable biosensors become more integrated into clinical decision-making, establishing clear liability frameworks for data breaches becomes increasingly important. The distributed nature of these systems—spanning device manufacturers, software developers, healthcare providers, and cloud services—creates complex questions of responsibility that remain largely unresolved in current legal frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!