Regulatory Compliance for Wearable Biosensors in Europe
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
European Wearable Biosensor Regulatory Landscape
The European regulatory landscape for wearable biosensors is characterized by a complex framework of directives, regulations, and standards that manufacturers must navigate to ensure market access. At the forefront is the Medical Device Regulation (MDR 2017/745), which replaced the Medical Device Directive in May 2021, introducing more stringent requirements for clinical evaluation, post-market surveillance, and technical documentation for medical devices, including many wearable biosensors.
The General Data Protection Regulation (GDPR) constitutes another critical component of this landscape, governing the collection, processing, and storage of personal health data generated by biosensors. GDPR mandates privacy by design, data minimization principles, and explicit consent mechanisms, significantly impacting how biosensor manufacturers design their data handling architectures.
For wearable biosensors that utilize wireless communication technologies, compliance with the Radio Equipment Directive (RED 2014/53/EU) is mandatory, ensuring that devices meet essential requirements for radio spectrum usage, electromagnetic compatibility, and safety.
The European regulatory framework also includes the In Vitro Diagnostic Regulation (IVDR 2017/746) for biosensors that perform diagnostic functions, such as glucose monitoring or blood analysis. This regulation introduces a new risk classification system and enhanced requirements for clinical evidence and performance evaluation.
Harmonized standards play a crucial role in demonstrating compliance with these regulations. Standards such as EN ISO 13485 for quality management systems, IEC 60601 for electrical safety, and ISO 14971 for risk management provide manufacturers with presumption of conformity to regulatory requirements when properly implemented.
The Conformité Européenne (CE) marking process represents the culmination of regulatory compliance efforts, signifying that a product meets all applicable EU requirements. For higher-risk biosensors, this process typically involves assessment by a Notified Body, an independent organization designated by EU member states to assess conformity.
Recent regulatory developments include the European Commission's focus on artificial intelligence regulation, which will impact biosensors utilizing AI for data analysis, and the European Health Data Space initiative, aimed at facilitating secure access to health data across borders while maintaining privacy protections.
National competent authorities in each EU member state oversee market surveillance and enforcement of these regulations, with coordination at the EU level through bodies such as the Medical Device Coordination Group (MDCG) and the European Medicines Agency (EMA) for combination products.
Understanding this multifaceted regulatory landscape is essential for manufacturers seeking to develop and market wearable biosensors in Europe, as compliance requirements significantly influence product design, development timelines, and go-to-market strategies.
The General Data Protection Regulation (GDPR) constitutes another critical component of this landscape, governing the collection, processing, and storage of personal health data generated by biosensors. GDPR mandates privacy by design, data minimization principles, and explicit consent mechanisms, significantly impacting how biosensor manufacturers design their data handling architectures.
For wearable biosensors that utilize wireless communication technologies, compliance with the Radio Equipment Directive (RED 2014/53/EU) is mandatory, ensuring that devices meet essential requirements for radio spectrum usage, electromagnetic compatibility, and safety.
The European regulatory framework also includes the In Vitro Diagnostic Regulation (IVDR 2017/746) for biosensors that perform diagnostic functions, such as glucose monitoring or blood analysis. This regulation introduces a new risk classification system and enhanced requirements for clinical evidence and performance evaluation.
Harmonized standards play a crucial role in demonstrating compliance with these regulations. Standards such as EN ISO 13485 for quality management systems, IEC 60601 for electrical safety, and ISO 14971 for risk management provide manufacturers with presumption of conformity to regulatory requirements when properly implemented.
The Conformité Européenne (CE) marking process represents the culmination of regulatory compliance efforts, signifying that a product meets all applicable EU requirements. For higher-risk biosensors, this process typically involves assessment by a Notified Body, an independent organization designated by EU member states to assess conformity.
Recent regulatory developments include the European Commission's focus on artificial intelligence regulation, which will impact biosensors utilizing AI for data analysis, and the European Health Data Space initiative, aimed at facilitating secure access to health data across borders while maintaining privacy protections.
National competent authorities in each EU member state oversee market surveillance and enforcement of these regulations, with coordination at the EU level through bodies such as the Medical Device Coordination Group (MDCG) and the European Medicines Agency (EMA) for combination products.
Understanding this multifaceted regulatory landscape is essential for manufacturers seeking to develop and market wearable biosensors in Europe, as compliance requirements significantly influence product design, development timelines, and go-to-market strategies.
Market Demand Analysis for Compliant Biosensor Wearables
The wearable biosensor market in Europe is experiencing significant growth, driven by increasing health consciousness, aging populations, and the rising prevalence of chronic diseases. Current market research indicates that the European wearable medical device market is projected to grow at a compound annual growth rate of approximately 18% through 2027, with biosensors representing a substantial segment of this expansion.
Consumer demand for health monitoring solutions has accelerated dramatically following the COVID-19 pandemic, as individuals have become more proactive about tracking their vital signs and overall wellness. This shift has created a robust market for compliant biosensor wearables that can monitor parameters such as heart rate, blood oxygen levels, glucose, and stress indicators in real-time.
Healthcare systems across Europe are increasingly adopting remote patient monitoring solutions to reduce hospitalization costs and improve patient outcomes. This institutional demand complements consumer interest, creating a dual market dynamic that favors compliant biosensor technologies. Countries like Germany, the UK, and France are leading this adoption, with healthcare providers allocating growing portions of their budgets to digital health solutions.
The regulatory landscape, particularly the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), has created distinct market segments for consumer wellness devices versus medical-grade biosensors. This bifurcation has resulted in differentiated demand patterns, with medical-grade devices commanding premium prices but facing longer development cycles due to stringent compliance requirements.
Market research reveals that European consumers are increasingly willing to pay premium prices for biosensor wearables that offer clinical-grade accuracy and regulatory compliance. This trend is particularly evident in markets like Switzerland, Sweden, and Denmark, where health expenditure per capita is among the highest globally.
Corporate wellness programs represent another significant market driver, with European businesses increasingly investing in employee health monitoring solutions. These programs typically require devices with strong data protection features to comply with GDPR, creating demand for biosensors with enhanced privacy and security capabilities.
The aging European population presents a substantial market opportunity, with seniors and their caregivers seeking remote monitoring solutions that can detect falls, medication adherence, and vital sign abnormalities. This demographic shift is expected to sustain long-term demand growth for compliant biosensor wearables, particularly those designed for elder care applications.
Insurance companies across Europe are gradually implementing incentive programs that reward policyholders for using health monitoring devices, creating additional market pull for compliant biosensor technologies. This trend is expected to accelerate as more insurers recognize the preventive health benefits and cost savings associated with continuous biometric monitoring.
Consumer demand for health monitoring solutions has accelerated dramatically following the COVID-19 pandemic, as individuals have become more proactive about tracking their vital signs and overall wellness. This shift has created a robust market for compliant biosensor wearables that can monitor parameters such as heart rate, blood oxygen levels, glucose, and stress indicators in real-time.
Healthcare systems across Europe are increasingly adopting remote patient monitoring solutions to reduce hospitalization costs and improve patient outcomes. This institutional demand complements consumer interest, creating a dual market dynamic that favors compliant biosensor technologies. Countries like Germany, the UK, and France are leading this adoption, with healthcare providers allocating growing portions of their budgets to digital health solutions.
The regulatory landscape, particularly the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), has created distinct market segments for consumer wellness devices versus medical-grade biosensors. This bifurcation has resulted in differentiated demand patterns, with medical-grade devices commanding premium prices but facing longer development cycles due to stringent compliance requirements.
Market research reveals that European consumers are increasingly willing to pay premium prices for biosensor wearables that offer clinical-grade accuracy and regulatory compliance. This trend is particularly evident in markets like Switzerland, Sweden, and Denmark, where health expenditure per capita is among the highest globally.
Corporate wellness programs represent another significant market driver, with European businesses increasingly investing in employee health monitoring solutions. These programs typically require devices with strong data protection features to comply with GDPR, creating demand for biosensors with enhanced privacy and security capabilities.
The aging European population presents a substantial market opportunity, with seniors and their caregivers seeking remote monitoring solutions that can detect falls, medication adherence, and vital sign abnormalities. This demographic shift is expected to sustain long-term demand growth for compliant biosensor wearables, particularly those designed for elder care applications.
Insurance companies across Europe are gradually implementing incentive programs that reward policyholders for using health monitoring devices, creating additional market pull for compliant biosensor technologies. This trend is expected to accelerate as more insurers recognize the preventive health benefits and cost savings associated with continuous biometric monitoring.
Current Regulatory Challenges for Wearable Biosensors
The regulatory landscape for wearable biosensors in Europe presents significant challenges for manufacturers, developers, and healthcare providers. The primary regulatory framework governing these devices is the European Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Devices Directive (MDD) with full implementation in May 2021. This transition has created substantial compliance hurdles as the MDR introduces more stringent requirements for clinical evaluation, post-market surveillance, and technical documentation.
One of the most pressing challenges is the classification ambiguity of wearable biosensors. Depending on their intended use and functionality, these devices may be classified anywhere from Class I (low risk) to Class III (high risk) medical devices, or potentially as wellness products outside medical device regulation altogether. The boundary between wellness monitoring and medical diagnosis remains particularly problematic, with many manufacturers struggling to determine the appropriate regulatory pathway.
Data protection compliance represents another significant obstacle, as wearable biosensors typically collect vast amounts of sensitive health data. The General Data Protection Regulation (GDPR) imposes strict requirements on data processing, storage, and transfer, necessitating robust data management systems and explicit user consent mechanisms. The intersection of MDR and GDPR creates a complex compliance matrix that many companies find difficult to navigate.
The conformity assessment procedures have become more rigorous under the MDR, requiring involvement of Notified Bodies for a wider range of devices. However, the limited number of designated Notified Bodies has created bottlenecks in the certification process, leading to delays in bringing products to market. This shortage is particularly problematic for innovative wearable technologies that may not fit neatly into established regulatory categories.
Software as a Medical Device (SaMD) regulations present additional challenges, as many wearable biosensors rely on sophisticated algorithms and mobile applications. The MDR has expanded the scope of software regulation, requiring more comprehensive validation and verification of software components, including those utilizing artificial intelligence or machine learning algorithms.
Cross-border compliance issues further complicate the regulatory landscape. Despite the harmonization efforts within the European Union, national interpretations and additional requirements still exist in various member states. This regulatory fragmentation increases compliance costs and complexity, particularly for smaller companies and startups.
The rapidly evolving nature of wearable technology often outpaces regulatory frameworks, creating uncertainty around novel functionalities and use cases. Regulatory bodies struggle to provide timely guidance on emerging technologies such as continuous biomarker monitoring, predictive analytics, and integrated therapeutic functions, leaving manufacturers to navigate uncharted regulatory territory.
One of the most pressing challenges is the classification ambiguity of wearable biosensors. Depending on their intended use and functionality, these devices may be classified anywhere from Class I (low risk) to Class III (high risk) medical devices, or potentially as wellness products outside medical device regulation altogether. The boundary between wellness monitoring and medical diagnosis remains particularly problematic, with many manufacturers struggling to determine the appropriate regulatory pathway.
Data protection compliance represents another significant obstacle, as wearable biosensors typically collect vast amounts of sensitive health data. The General Data Protection Regulation (GDPR) imposes strict requirements on data processing, storage, and transfer, necessitating robust data management systems and explicit user consent mechanisms. The intersection of MDR and GDPR creates a complex compliance matrix that many companies find difficult to navigate.
The conformity assessment procedures have become more rigorous under the MDR, requiring involvement of Notified Bodies for a wider range of devices. However, the limited number of designated Notified Bodies has created bottlenecks in the certification process, leading to delays in bringing products to market. This shortage is particularly problematic for innovative wearable technologies that may not fit neatly into established regulatory categories.
Software as a Medical Device (SaMD) regulations present additional challenges, as many wearable biosensors rely on sophisticated algorithms and mobile applications. The MDR has expanded the scope of software regulation, requiring more comprehensive validation and verification of software components, including those utilizing artificial intelligence or machine learning algorithms.
Cross-border compliance issues further complicate the regulatory landscape. Despite the harmonization efforts within the European Union, national interpretations and additional requirements still exist in various member states. This regulatory fragmentation increases compliance costs and complexity, particularly for smaller companies and startups.
The rapidly evolving nature of wearable technology often outpaces regulatory frameworks, creating uncertainty around novel functionalities and use cases. Regulatory bodies struggle to provide timely guidance on emerging technologies such as continuous biomarker monitoring, predictive analytics, and integrated therapeutic functions, leaving manufacturers to navigate uncharted regulatory territory.
Compliance Frameworks and Technical Standards
01 Wearable biosensors for health monitoring
Wearable biosensors can be designed to continuously monitor various health parameters such as heart rate, blood pressure, body temperature, and glucose levels. These devices typically use non-invasive or minimally invasive methods to collect physiological data from the user. The sensors can be integrated into everyday items like watches, patches, or clothing, allowing for real-time health monitoring without disrupting daily activities. These biosensors often connect to smartphones or other devices to provide users with actionable health insights.- Wearable biosensors for health monitoring: Wearable biosensors can be designed to continuously monitor various health parameters such as heart rate, blood pressure, body temperature, and glucose levels. These devices typically use non-invasive or minimally invasive methods to collect physiological data from the user's body. The collected data can be analyzed in real-time to provide insights into the user's health status and alert them to potential health issues before they become serious problems.
- Flexible and stretchable biosensor technologies: Advanced materials and fabrication techniques enable the development of flexible and stretchable biosensors that can conform to the body's contours. These sensors incorporate conductive polymers, nanomaterials, and stretchable substrates to maintain functionality while bending or stretching. This flexibility improves user comfort during extended wear periods and ensures consistent contact with the skin for accurate measurements, making them suitable for integration into clothing or direct application to the skin.
- Biochemical sensing mechanisms: Wearable biosensors employ various biochemical sensing mechanisms to detect and measure biological markers in bodily fluids such as sweat, tears, or interstitial fluid. These mechanisms include electrochemical sensors, optical sensors, and enzymatic reactions that can detect specific biomarkers like glucose, lactate, electrolytes, and stress hormones. The sensing components are miniaturized and optimized for sensitivity, specificity, and stability to provide accurate measurements in real-world conditions.
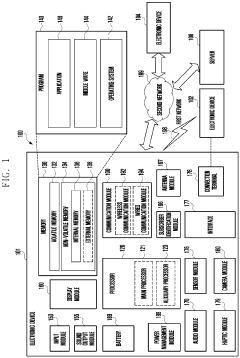
- Data processing and wireless communication: Modern wearable biosensors incorporate advanced data processing capabilities and wireless communication technologies to transmit collected data to smartphones, computers, or cloud platforms. These systems use algorithms to filter noise, calibrate measurements, and identify patterns in the data. Wireless technologies such as Bluetooth Low Energy, NFC, or cellular connectivity enable real-time data transmission while optimizing power consumption to extend battery life, making continuous monitoring feasible.
- Integration with medical systems and AI analytics: Wearable biosensors are increasingly being integrated with healthcare systems and artificial intelligence for enhanced diagnostics and personalized medicine. These integrated systems can automatically share data with electronic health records, alert healthcare providers to abnormal readings, and provide personalized health recommendations. AI algorithms analyze the continuous data streams to identify subtle patterns that may indicate early disease onset or treatment effectiveness, potentially transforming preventive healthcare and chronic disease management.
02 Electrochemical biosensors for analyte detection
Electrochemical biosensors utilize electrical signals to detect and measure specific analytes in biological samples. These sensors typically consist of electrodes modified with biorecognition elements such as enzymes, antibodies, or nucleic acids that selectively bind to target molecules. When the target analyte interacts with the biorecognition element, it generates an electrical signal proportional to the analyte concentration. These biosensors can be miniaturized and incorporated into wearable devices for continuous monitoring of various biomarkers in bodily fluids like sweat, tears, or interstitial fluid.Expand Specific Solutions03 Flexible and stretchable biosensor materials
Advanced materials that are flexible, stretchable, and biocompatible are essential for developing comfortable and effective wearable biosensors. These materials include conductive polymers, carbon-based nanomaterials, and soft elastomers that can conform to the body's contours while maintaining their sensing capabilities. The flexibility allows the sensors to maintain contact with the skin during movement, ensuring continuous and accurate data collection. These materials can also be engineered to be breathable and hypoallergenic, reducing skin irritation during prolonged wear.Expand Specific Solutions04 Data processing and wireless communication systems
Wearable biosensors incorporate sophisticated data processing algorithms and wireless communication systems to analyze and transmit the collected physiological data. These systems often use low-power microprocessors to filter noise, process signals, and extract meaningful health information from raw sensor data. Wireless technologies such as Bluetooth Low Energy (BLE), Near Field Communication (NFC), or Wi-Fi enable the seamless transmission of data to smartphones, cloud platforms, or healthcare providers. Advanced encryption protocols ensure the security and privacy of sensitive health information during transmission and storage.Expand Specific Solutions05 Implantable and minimally invasive biosensors
Some biosensors are designed to be implanted under the skin or inserted into the body with minimal invasiveness for more accurate and continuous monitoring of internal biomarkers. These sensors can directly measure analytes in interstitial fluid, blood, or other bodily fluids that are not accessible through completely non-invasive methods. Implantable biosensors often use biocompatible materials and coatings to reduce foreign body responses and extend their functional lifetime within the body. They may incorporate drug-eluting components to prevent inflammation or biofouling, which can affect sensor performance over time.Expand Specific Solutions
Key Regulatory Bodies and Industry Stakeholders
The European wearable biosensor regulatory landscape is evolving rapidly, currently positioned in a growth phase with increasing market adoption. The market is expanding at approximately 15-20% annually, driven by healthcare digitalization and remote monitoring demands. Technologically, the field shows varying maturity levels, with companies at different development stages. Samsung Electronics and Philips lead with established regulatory expertise and comprehensive compliance frameworks, while Abbott Laboratories and Roche Diabetes Care demonstrate strong specialized clinical validation protocols. Academic institutions like MIT and University of California contribute significant research advancements. Emerging players such as Biorasis and Prevayl Innovations are navigating the complex European MDR and IVDR requirements, focusing on specialized biosensor applications while building their regulatory capabilities.
Koninklijke Philips NV
Technical Solution: Philips has implemented a sophisticated regulatory compliance framework for their wearable biosensors in the European market, centered around their "Design for Compliance" methodology. This approach integrates regulatory requirements into the earliest stages of product development rather than treating compliance as an end-stage verification process. Their wearable biosensors undergo rigorous classification assessment under the MDR, with most of their advanced monitoring devices falling under Class IIa or IIb. Philips has developed specialized technical documentation systems that maintain living documents throughout the product lifecycle, allowing for continuous updates as regulatory interpretations evolve. Their compliance strategy includes comprehensive risk management processes that specifically address the unique challenges of continuous monitoring biosensors, including signal accuracy degradation over time and potential for false alarms. Philips has also established dedicated post-market surveillance teams that collect real-world performance data to feed back into their quality management system, creating a closed-loop improvement process that anticipates regulatory concerns before they become compliance issues.
Strengths: Philips' early integration of regulatory considerations into product design reduces compliance costs and time-to-market delays. Their extensive experience with European notified bodies facilitates smoother certification processes. Weaknesses: Their focus on healthcare-grade wearables creates higher regulatory burdens compared to consumer-grade competitors, potentially limiting market agility and increasing costs that must be passed to customers or healthcare systems.
Polar Electro Oy
Technical Solution: Polar Electro has developed a distinctive regulatory compliance strategy for their wearable biosensors in Europe that bridges the gap between consumer fitness devices and medical-grade monitoring equipment. Their approach centers on a tiered compliance framework that allows their products to be appropriately classified based on their intended use, with some advanced heart rate monitoring systems qualifying as medical devices while others remain in the consumer product category. Polar has implemented specialized technical documentation systems that maintain clear boundaries between health and wellness claims versus medical diagnostic capabilities, allowing them to navigate the regulatory gray areas that often challenge wearable biosensor manufacturers. Their compliance process includes targeted clinical validation studies designed to meet the specific requirements of European conformity assessment procedures while maintaining the usability expected in consumer wearables. Polar has also developed innovative approaches to user information and labeling that satisfy regulatory requirements while maintaining brand consistency and user experience. Their regulatory strategy includes close collaboration with European sports medicine research institutions to establish scientifically validated standards for physiological monitoring that can inform both product development and regulatory submissions.
Strengths: Polar's experience in both consumer and medical markets provides unique insights into navigating the regulatory boundaries between wellness and healthcare applications. Their established presence in European markets gives them deep understanding of national variations in regulatory interpretation. Weaknesses: The dual-market approach creates challenges in maintaining consistent regulatory positioning across their product portfolio and may result in more conservative feature implementation to avoid triggering higher regulatory requirements.
Critical EU MDR and IVDR Requirements Analysis
Wearable biosensors and applications thereof
PatentActiveUS20230060118A9
Innovation
- Development of highly sensitive In2O3 nanoribbon transistor biosensors with integrated on-chip gold gate electrodes, functionalized with glucose oxidase, chitosan, and single-walled carbon nanotubes, capable of detecting glucose concentrations between 10 nM to 1 mM in external body fluids without breaking the skin, and integrated into flexible, conformable devices like skin patches and contact lenses.
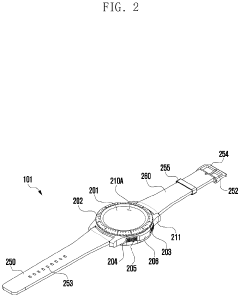
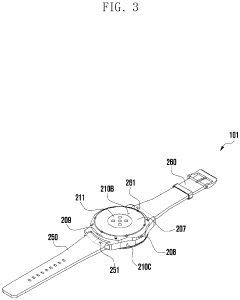
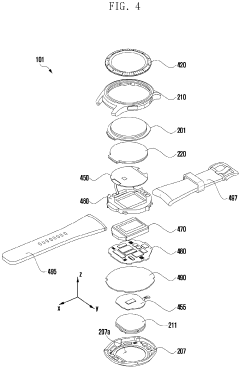
Wearable electronic device including plurality of biometric sensors
PatentPendingEP4275599A1
Innovation
- The wearable electronic device employs a configuration where a first biosensor with a light transmitter on one substrate and a light receiver on an overlapping second substrate calculates bio signals, allowing for a miniaturized design by separating the components while maintaining effective signal transmission and reception.
Data Privacy and GDPR Implications
The General Data Protection Regulation (GDPR) represents the cornerstone of data privacy legislation in Europe, with profound implications for wearable biosensor technologies. These devices continuously collect sensitive health data, including heart rate, blood glucose levels, sleep patterns, and other physiological metrics, placing them squarely within GDPR's regulatory scope. Under this framework, biometric data is classified as "special category data," requiring explicit consent and enhanced protection measures.
Wearable biosensor manufacturers must implement privacy by design principles from the earliest development stages. This includes data minimization strategies, ensuring only necessary health information is collected, and incorporating robust encryption protocols for both data storage and transmission. The principle of purpose limitation further restricts companies from repurposing collected health data without obtaining additional user consent.
GDPR's transparency requirements mandate clear communication with users about data collection practices. Manufacturers must provide comprehensive privacy notices detailing what data is collected, how it's processed, where it's stored, and the specific purposes for which it will be used. These notices must be presented in accessible language, avoiding technical jargon that might confuse average consumers.
Data subject rights present unique challenges for wearable biosensor companies. Users can request access to their complete health data records, demand data portability to transfer information between service providers, and exercise their right to be forgotten. Implementing these rights requires sophisticated data management systems capable of isolating individual user data across complex storage infrastructures.
Cross-border data transfers introduce additional compliance hurdles. Following the invalidation of the Privacy Shield framework by the Schrems II decision, companies transferring European users' health data outside the EEA must implement supplementary measures beyond standard contractual clauses. This may include enhanced encryption, pseudonymization techniques, or establishing regional data processing centers within Europe.
Recent enforcement actions demonstrate regulatory authorities' growing focus on health technology compliance. Notable cases include fines against wearable manufacturers for insufficient consent mechanisms and inadequate data security measures. The European Data Protection Board has also issued specific guidance for health applications, emphasizing the need for granular consent options and strict access controls for sensitive biometric information.
Looking forward, the proposed European Health Data Space (EHDS) regulation will likely introduce additional requirements specifically targeting health technology providers, potentially creating a more standardized but complex regulatory environment for wearable biosensor manufacturers operating in the European market.
Wearable biosensor manufacturers must implement privacy by design principles from the earliest development stages. This includes data minimization strategies, ensuring only necessary health information is collected, and incorporating robust encryption protocols for both data storage and transmission. The principle of purpose limitation further restricts companies from repurposing collected health data without obtaining additional user consent.
GDPR's transparency requirements mandate clear communication with users about data collection practices. Manufacturers must provide comprehensive privacy notices detailing what data is collected, how it's processed, where it's stored, and the specific purposes for which it will be used. These notices must be presented in accessible language, avoiding technical jargon that might confuse average consumers.
Data subject rights present unique challenges for wearable biosensor companies. Users can request access to their complete health data records, demand data portability to transfer information between service providers, and exercise their right to be forgotten. Implementing these rights requires sophisticated data management systems capable of isolating individual user data across complex storage infrastructures.
Cross-border data transfers introduce additional compliance hurdles. Following the invalidation of the Privacy Shield framework by the Schrems II decision, companies transferring European users' health data outside the EEA must implement supplementary measures beyond standard contractual clauses. This may include enhanced encryption, pseudonymization techniques, or establishing regional data processing centers within Europe.
Recent enforcement actions demonstrate regulatory authorities' growing focus on health technology compliance. Notable cases include fines against wearable manufacturers for insufficient consent mechanisms and inadequate data security measures. The European Data Protection Board has also issued specific guidance for health applications, emphasizing the need for granular consent options and strict access controls for sensitive biometric information.
Looking forward, the proposed European Health Data Space (EHDS) regulation will likely introduce additional requirements specifically targeting health technology providers, potentially creating a more standardized but complex regulatory environment for wearable biosensor manufacturers operating in the European market.
Post-Market Surveillance Requirements
Post-market surveillance (PMS) for wearable biosensors in Europe has become increasingly stringent under the Medical Device Regulation (MDR 2017/745) and In Vitro Diagnostic Regulation (IVDR 2017/746). Manufacturers must implement comprehensive surveillance systems that actively collect and analyze data on device performance throughout the product lifecycle.
The MDR specifically requires manufacturers to establish, document, implement, and maintain a post-market surveillance system proportionate to the risk class and appropriate for the device type. For wearable biosensors, this typically involves continuous monitoring of real-world performance data, including adverse events, technical malfunctions, and user feedback.
A critical component of PMS for wearable biosensors is the Post-Market Clinical Follow-up (PMCF), which requires systematic data collection to confirm safety and performance under actual conditions of use. Manufacturers must conduct PMCF studies and surveys, particularly for higher-risk biosensors that monitor critical physiological parameters or deliver therapeutic interventions.
The regulatory framework mandates periodic safety update reports (PSURs) for class IIa, IIb, and III devices, with reporting frequencies varying based on risk classification. Most advanced wearable biosensors fall into class IIa or higher, necessitating regular submission of these reports to notified bodies.
Vigilance reporting represents another crucial aspect of post-market requirements. Manufacturers must report serious incidents and field safety corrective actions (FSCAs) to competent authorities within strict timeframes: 2 days for serious public health threats, 10 days for death or serious deterioration, and 15 days for other reportable incidents.
The MDR has introduced more stringent traceability requirements through the Unique Device Identification (UDI) system. Wearable biosensor manufacturers must ensure their devices carry appropriate UDI carriers and register relevant information in the European database on medical devices (EUDAMED).
For software-enabled biosensors, which constitute the majority of modern wearable health technologies, post-market surveillance must include monitoring for cybersecurity vulnerabilities and software bugs. Manufacturers must implement processes for timely security patches and updates while maintaining regulatory compliance for each software version.
The trend toward remote monitoring capabilities in wearable biosensors introduces additional post-market considerations regarding data privacy and security under the General Data Protection Regulation (GDPR). Manufacturers must continuously monitor compliance with both medical device regulations and data protection requirements.
The MDR specifically requires manufacturers to establish, document, implement, and maintain a post-market surveillance system proportionate to the risk class and appropriate for the device type. For wearable biosensors, this typically involves continuous monitoring of real-world performance data, including adverse events, technical malfunctions, and user feedback.
A critical component of PMS for wearable biosensors is the Post-Market Clinical Follow-up (PMCF), which requires systematic data collection to confirm safety and performance under actual conditions of use. Manufacturers must conduct PMCF studies and surveys, particularly for higher-risk biosensors that monitor critical physiological parameters or deliver therapeutic interventions.
The regulatory framework mandates periodic safety update reports (PSURs) for class IIa, IIb, and III devices, with reporting frequencies varying based on risk classification. Most advanced wearable biosensors fall into class IIa or higher, necessitating regular submission of these reports to notified bodies.
Vigilance reporting represents another crucial aspect of post-market requirements. Manufacturers must report serious incidents and field safety corrective actions (FSCAs) to competent authorities within strict timeframes: 2 days for serious public health threats, 10 days for death or serious deterioration, and 15 days for other reportable incidents.
The MDR has introduced more stringent traceability requirements through the Unique Device Identification (UDI) system. Wearable biosensor manufacturers must ensure their devices carry appropriate UDI carriers and register relevant information in the European database on medical devices (EUDAMED).
For software-enabled biosensors, which constitute the majority of modern wearable health technologies, post-market surveillance must include monitoring for cybersecurity vulnerabilities and software bugs. Manufacturers must implement processes for timely security patches and updates while maintaining regulatory compliance for each software version.
The trend toward remote monitoring capabilities in wearable biosensors introduces additional post-market considerations regarding data privacy and security under the General Data Protection Regulation (GDPR). Manufacturers must continuously monitor compliance with both medical device regulations and data protection requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!