Wearable Biosensors and Their Role in Standardized Testing
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Wearable Biosensor Evolution and Objectives
Wearable biosensors have undergone a remarkable evolution over the past two decades, transforming from rudimentary step counters to sophisticated multi-parameter monitoring systems capable of continuous health assessment. The journey began in the early 2000s with simple pedometers and heart rate monitors, primarily targeting fitness enthusiasts. By 2010, these devices had evolved to incorporate accelerometers and gyroscopes, enabling more accurate activity tracking and sleep monitoring capabilities.
The watershed moment came between 2015-2018 when miniaturization of sensors, improvements in battery technology, and advances in data analytics converged to create a new generation of wearable biosensors. These devices expanded beyond physical activity metrics to include physiological parameters such as electrodermal activity, blood oxygen saturation, and even preliminary electrocardiogram capabilities for consumer markets.
Recent technological breakthroughs have further accelerated this evolution, with the integration of flexible electronics and novel materials enabling more comfortable, less obtrusive form factors. Modern biosensors now incorporate microfluidic systems for sweat analysis, optical sensors for blood composition assessment, and advanced algorithms capable of detecting subtle physiological changes that may indicate health anomalies before clinical symptoms appear.
In the context of standardized testing environments, wearable biosensors represent a paradigm shift in how we can understand cognitive performance under assessment conditions. The primary objective of implementing these technologies in testing scenarios is to establish quantifiable physiological correlates of cognitive states such as stress, attention, and mental workload—factors known to significantly impact test performance but traditionally difficult to measure objectively.
Secondary objectives include developing adaptive testing protocols that respond to a test-taker's physiological state, potentially creating more equitable assessment conditions across diverse populations. There is also significant interest in using these technologies to identify testing environments and conditions that optimize cognitive performance while minimizing detrimental stress responses.
The long-term technological goal is to develop a comprehensive, non-invasive biosensing ecosystem that can reliably measure cognitive load, stress responses, attention levels, and emotional states in real-time during standardized testing. This system would ideally be unobtrusive enough not to interfere with the testing process while providing actionable insights to both test administrators and test-takers.
As these technologies mature, the ultimate objective extends beyond mere measurement to creating a more holistic understanding of the complex interplay between physiological states and cognitive performance, potentially revolutionizing our approach to educational and professional assessment methodologies.
The watershed moment came between 2015-2018 when miniaturization of sensors, improvements in battery technology, and advances in data analytics converged to create a new generation of wearable biosensors. These devices expanded beyond physical activity metrics to include physiological parameters such as electrodermal activity, blood oxygen saturation, and even preliminary electrocardiogram capabilities for consumer markets.
Recent technological breakthroughs have further accelerated this evolution, with the integration of flexible electronics and novel materials enabling more comfortable, less obtrusive form factors. Modern biosensors now incorporate microfluidic systems for sweat analysis, optical sensors for blood composition assessment, and advanced algorithms capable of detecting subtle physiological changes that may indicate health anomalies before clinical symptoms appear.
In the context of standardized testing environments, wearable biosensors represent a paradigm shift in how we can understand cognitive performance under assessment conditions. The primary objective of implementing these technologies in testing scenarios is to establish quantifiable physiological correlates of cognitive states such as stress, attention, and mental workload—factors known to significantly impact test performance but traditionally difficult to measure objectively.
Secondary objectives include developing adaptive testing protocols that respond to a test-taker's physiological state, potentially creating more equitable assessment conditions across diverse populations. There is also significant interest in using these technologies to identify testing environments and conditions that optimize cognitive performance while minimizing detrimental stress responses.
The long-term technological goal is to develop a comprehensive, non-invasive biosensing ecosystem that can reliably measure cognitive load, stress responses, attention levels, and emotional states in real-time during standardized testing. This system would ideally be unobtrusive enough not to interfere with the testing process while providing actionable insights to both test administrators and test-takers.
As these technologies mature, the ultimate objective extends beyond mere measurement to creating a more holistic understanding of the complex interplay between physiological states and cognitive performance, potentially revolutionizing our approach to educational and professional assessment methodologies.
Market Analysis for Standardized Testing Biosensors
The global market for wearable biosensors in standardized testing is experiencing significant growth, driven by increasing demand for objective health data and technological advancements in sensor miniaturization. Current market valuations indicate the wearable biosensor sector reached approximately $13.2 billion in 2022, with projections suggesting a compound annual growth rate of 19.8% through 2030, potentially reaching $56.7 billion.
Within the standardized testing segment specifically, biosensors are gaining traction across multiple sectors. Healthcare represents the largest market share at 38%, where these devices are revolutionizing clinical trials and patient monitoring protocols. Education follows at 24%, with growing adoption for cognitive performance assessment and stress monitoring during examinations. The corporate sector accounts for 21%, utilizing these technologies for employee wellness programs and performance optimization.
Regional analysis reveals North America currently dominates the market with 42% share, benefiting from advanced healthcare infrastructure and higher technology adoption rates. Asia-Pacific represents the fastest-growing region with 23% annual growth, driven by expanding healthcare digitization initiatives in China, Japan, and South Korea. Europe holds 28% market share with particularly strong adoption in pharmaceutical research applications.
Consumer demand patterns indicate increasing preference for non-invasive, continuous monitoring capabilities, with 67% of potential users citing comfort and ease-of-use as primary adoption factors. Price sensitivity remains a significant consideration, with optimal price points for consumer-grade testing biosensors falling between $100-250 depending on functionality.
Key market drivers include regulatory shifts toward remote patient monitoring, increasing healthcare costs driving preventative care solutions, and growing consumer interest in personalized health insights. The COVID-19 pandemic has accelerated market growth by approximately 27% above pre-pandemic projections, highlighting the value of remote physiological monitoring capabilities.
Market barriers include concerns regarding data privacy (cited by 58% of potential users), accuracy limitations in current-generation sensors, and interoperability challenges with existing standardized testing platforms. Additionally, reimbursement uncertainties in healthcare applications and varying regulatory requirements across regions create market fragmentation challenges.
Emerging market opportunities exist in specialized testing environments such as athletic performance assessment, neurological condition monitoring, and workplace safety compliance. The integration of artificial intelligence with biosensor data represents a particularly high-growth segment, with 89% of industry stakeholders identifying AI-enhanced analytics as critical for future market development.
Within the standardized testing segment specifically, biosensors are gaining traction across multiple sectors. Healthcare represents the largest market share at 38%, where these devices are revolutionizing clinical trials and patient monitoring protocols. Education follows at 24%, with growing adoption for cognitive performance assessment and stress monitoring during examinations. The corporate sector accounts for 21%, utilizing these technologies for employee wellness programs and performance optimization.
Regional analysis reveals North America currently dominates the market with 42% share, benefiting from advanced healthcare infrastructure and higher technology adoption rates. Asia-Pacific represents the fastest-growing region with 23% annual growth, driven by expanding healthcare digitization initiatives in China, Japan, and South Korea. Europe holds 28% market share with particularly strong adoption in pharmaceutical research applications.
Consumer demand patterns indicate increasing preference for non-invasive, continuous monitoring capabilities, with 67% of potential users citing comfort and ease-of-use as primary adoption factors. Price sensitivity remains a significant consideration, with optimal price points for consumer-grade testing biosensors falling between $100-250 depending on functionality.
Key market drivers include regulatory shifts toward remote patient monitoring, increasing healthcare costs driving preventative care solutions, and growing consumer interest in personalized health insights. The COVID-19 pandemic has accelerated market growth by approximately 27% above pre-pandemic projections, highlighting the value of remote physiological monitoring capabilities.
Market barriers include concerns regarding data privacy (cited by 58% of potential users), accuracy limitations in current-generation sensors, and interoperability challenges with existing standardized testing platforms. Additionally, reimbursement uncertainties in healthcare applications and varying regulatory requirements across regions create market fragmentation challenges.
Emerging market opportunities exist in specialized testing environments such as athletic performance assessment, neurological condition monitoring, and workplace safety compliance. The integration of artificial intelligence with biosensor data represents a particularly high-growth segment, with 89% of industry stakeholders identifying AI-enhanced analytics as critical for future market development.
Technical Challenges in Biosensor Standardization
Despite significant advancements in wearable biosensor technology, standardization remains one of the most formidable challenges in the field. The primary obstacle stems from the vast diversity of biosensor types, ranging from electrochemical and optical to piezoelectric and thermal sensors, each operating on different principles and generating distinct data formats. This heterogeneity makes establishing universal standards exceptionally difficult, as protocols suitable for one sensor type may be entirely inappropriate for another.
Measurement consistency presents another critical challenge. Environmental factors such as temperature, humidity, and electromagnetic interference can significantly impact sensor readings, leading to variability in results across different testing environments. This inconsistency undermines the reliability of biosensor data, particularly in standardized testing scenarios where reproducibility is paramount.
Data calibration and validation methodologies also lack standardization across the industry. Different manufacturers employ proprietary algorithms and reference values, resulting in discrepancies when comparing results from different devices. The absence of universally accepted calibration protocols makes it challenging to establish baseline measurements and reference ranges for physiological parameters monitored by wearable biosensors.
Interoperability issues further complicate standardization efforts. The proliferation of proprietary data formats and communication protocols creates significant barriers to data exchange between different biosensor systems. This fragmentation hinders the integration of biosensor data into broader healthcare information systems and limits the potential for comprehensive data analysis across platforms.
Regulatory frameworks for wearable biosensors remain inconsistent globally, with varying requirements for accuracy, precision, and reliability. The FDA in the United States, the EMA in Europe, and regulatory bodies in other regions have different approaches to validating biosensor performance, creating a complex landscape for manufacturers seeking international compliance.
Biological variability among users presents additional standardization challenges. Factors such as skin properties, body composition, and individual physiological differences can affect sensor performance, necessitating personalized calibration approaches that are difficult to standardize. This variability is particularly problematic for non-invasive sensors that rely on indirect measurements of biomarkers.
Technical limitations in miniaturization and power management also impact standardization efforts. As sensors become increasingly compact and energy-efficient, maintaining consistent performance becomes more challenging, especially over extended periods of continuous monitoring. Battery life constraints and power management strategies can introduce additional variables that affect measurement consistency.
Measurement consistency presents another critical challenge. Environmental factors such as temperature, humidity, and electromagnetic interference can significantly impact sensor readings, leading to variability in results across different testing environments. This inconsistency undermines the reliability of biosensor data, particularly in standardized testing scenarios where reproducibility is paramount.
Data calibration and validation methodologies also lack standardization across the industry. Different manufacturers employ proprietary algorithms and reference values, resulting in discrepancies when comparing results from different devices. The absence of universally accepted calibration protocols makes it challenging to establish baseline measurements and reference ranges for physiological parameters monitored by wearable biosensors.
Interoperability issues further complicate standardization efforts. The proliferation of proprietary data formats and communication protocols creates significant barriers to data exchange between different biosensor systems. This fragmentation hinders the integration of biosensor data into broader healthcare information systems and limits the potential for comprehensive data analysis across platforms.
Regulatory frameworks for wearable biosensors remain inconsistent globally, with varying requirements for accuracy, precision, and reliability. The FDA in the United States, the EMA in Europe, and regulatory bodies in other regions have different approaches to validating biosensor performance, creating a complex landscape for manufacturers seeking international compliance.
Biological variability among users presents additional standardization challenges. Factors such as skin properties, body composition, and individual physiological differences can affect sensor performance, necessitating personalized calibration approaches that are difficult to standardize. This variability is particularly problematic for non-invasive sensors that rely on indirect measurements of biomarkers.
Technical limitations in miniaturization and power management also impact standardization efforts. As sensors become increasingly compact and energy-efficient, maintaining consistent performance becomes more challenging, especially over extended periods of continuous monitoring. Battery life constraints and power management strategies can introduce additional variables that affect measurement consistency.
Current Standardization Approaches for Biosensor Testing
01 Wearable biosensors for health monitoring
Wearable biosensors designed for continuous health monitoring can track various physiological parameters such as heart rate, blood pressure, body temperature, and oxygen saturation. These devices enable real-time health tracking and early detection of abnormalities, allowing for timely medical intervention. The sensors are typically integrated into comfortable wearable formats like patches, wristbands, or clothing to facilitate long-term use while maintaining accuracy in data collection.- Wearable biosensors for health monitoring: Wearable biosensors designed for continuous health monitoring can track various physiological parameters such as heart rate, blood pressure, body temperature, and glucose levels. These devices enable real-time health data collection and analysis, allowing for early detection of health issues and personalized healthcare management. The sensors are typically integrated into comfortable, non-invasive wearable formats that can be used for extended periods in daily life.
- Sweat-based biosensing technologies: Biosensors that analyze sweat composition can provide valuable insights into a person's health status without invasive procedures. These sensors detect various biomarkers in sweat, including electrolytes, metabolites, and hormones, which can indicate hydration levels, stress, and potential health conditions. The technology typically involves flexible, skin-adherent patches with integrated microfluidic channels and sensing elements that capture and analyze sweat in real-time.
- Implantable and minimally invasive biosensors: Advanced biosensing technologies that can be implanted under the skin or introduced into the body with minimal invasiveness. These sensors provide continuous monitoring of internal biomarkers such as glucose, lactate, or specific disease markers that cannot be easily detected from the skin surface. The designs focus on biocompatibility, longevity, and wireless communication capabilities to transmit data to external devices for analysis and alert generation.
- Flexible and stretchable biosensor materials: Novel materials and fabrication techniques for creating biosensors that can conform to the body's contours and withstand movement and deformation. These include conductive polymers, nanomaterials, and hybrid composites that maintain sensing functionality while being stretched or bent. The flexible nature of these sensors improves comfort, enables better skin contact for more accurate measurements, and allows for integration into various form factors such as patches, textiles, or accessories.
- Data processing and connectivity for wearable biosensors: Systems and methods for processing biosensor data and connecting wearable devices to healthcare networks or personal devices. These technologies include algorithms for signal processing, noise reduction, and biomarker identification, as well as communication protocols for secure data transmission. The integration with smartphones, cloud platforms, and healthcare systems enables comprehensive health monitoring, personalized feedback, and timely medical interventions based on the collected biosensor data.
02 Biochemical sensing technologies
Advanced biochemical sensing technologies in wearable devices enable non-invasive or minimally invasive monitoring of various biomarkers in bodily fluids such as sweat, tears, or interstitial fluid. These sensors can detect glucose levels, electrolytes, metabolites, and other biochemical indicators, providing valuable information about the user's metabolic state and overall health condition without requiring traditional blood sampling methods.Expand Specific Solutions03 Flexible and stretchable biosensor materials
Development of flexible and stretchable materials for biosensors allows for better conformability to the human body, improving comfort and signal quality. These materials include conductive polymers, nanomaterials, and hybrid composites that maintain functionality during movement and deformation. The flexibility enables integration into various form factors such as adhesive patches, textiles, or skin-like interfaces that can be worn for extended periods without causing discomfort or skin irritation.Expand Specific Solutions04 Data processing and wireless communication systems
Wearable biosensors incorporate advanced data processing algorithms and wireless communication systems to analyze physiological data in real-time and transmit it to connected devices or healthcare providers. These systems often utilize machine learning techniques to improve accuracy, reduce noise, and identify patterns indicative of health conditions. The wireless connectivity enables remote monitoring, integration with electronic health records, and the development of comprehensive health management platforms.Expand Specific Solutions05 Energy harvesting and power management
Innovative energy harvesting technologies and power management systems extend the operational lifetime of wearable biosensors. These include harvesting energy from body heat, movement, or ambient light, as well as optimizing power consumption through efficient circuit design and operation modes. Advanced battery technologies and wireless charging capabilities further enhance the usability of these devices by reducing the frequency of recharging or battery replacement, making them more practical for continuous health monitoring applications.Expand Specific Solutions
Leading Companies in Wearable Biosensor Industry
The wearable biosensor market for standardized testing is in a growth phase, characterized by increasing adoption across healthcare and research sectors. The market size is expanding rapidly, driven by demand for continuous health monitoring and remote diagnostics. Technologically, the field shows varying maturity levels, with established players like Samsung Electronics, Google, and LG Electronics leading commercial applications, while research institutions such as MIT, Caltech, and National University of Singapore focus on next-generation innovations. Academic-industry partnerships are accelerating development, with universities collaborating with healthcare companies like PHC Holdings and Ascensia Diabetes Care to bridge research and commercialization gaps. The competitive landscape features both technology giants and specialized medical device manufacturers working to establish standards in this emerging field.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed a comprehensive wearable biosensor ecosystem specifically designed for educational and testing environments. Their technology integrates seamlessly with their Galaxy Watch platform, featuring specialized sensors that monitor heart rate variability, electrodermal activity, and blood oxygen levels during standardized testing scenarios[2]. Samsung's approach emphasizes non-invasive continuous monitoring with minimal distraction to test-takers. Their biosensors utilize proprietary algorithms to filter motion artifacts and environmental noise, ensuring accurate physiological measurements even in dynamic testing environments[4]. The company has also developed a secure cloud-based platform that allows educational institutions to anonymously aggregate and analyze physiological data across test populations, providing insights into testing conditions and cognitive load patterns[7]. Samsung's solution includes specialized SDK tools for researchers to develop custom applications that can correlate physiological signals with specific testing parameters and cognitive tasks.
Strengths: Robust consumer-grade hardware with excellent reliability and battery life; comprehensive ecosystem integration allowing for seamless data collection and analysis; strong privacy controls and data security features. Weaknesses: Less specialized for research applications compared to academic solutions; potential compatibility limitations with non-Samsung devices; relatively higher consumer cost that may limit widespread adoption in educational settings.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced wearable biosensor platforms that integrate multiple sensing modalities for comprehensive physiological monitoring during standardized testing scenarios. Their technology utilizes flexible, skin-adherent electronics with microfluidic channels that can detect various biomarkers including cortisol, glucose, and electrolytes in real-time[1]. MIT's approach combines electrochemical sensors with wireless data transmission capabilities, allowing for continuous monitoring without disrupting test-takers. The system incorporates machine learning algorithms that correlate physiological signals with cognitive states, enabling researchers to objectively measure stress levels, attention, and cognitive load during examinations[3]. MIT has also pioneered sweat-based sensing technologies that can detect minute changes in biomarker concentrations, providing insights into the test-taker's physiological response to cognitive challenges without invasive procedures[5].
Strengths: Superior integration of multiple sensing modalities with advanced data analytics; exceptional miniaturization capabilities allowing for unobtrusive monitoring; strong interdisciplinary approach combining materials science, electrical engineering, and cognitive science. Weaknesses: Higher cost implementation compared to simpler solutions; requires specialized expertise for deployment and data interpretation; potential privacy concerns with comprehensive physiological data collection.
Key Patents and Research in Biosensor Calibration
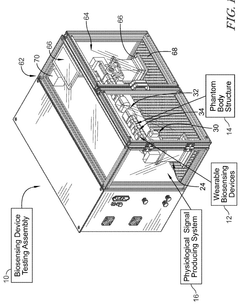
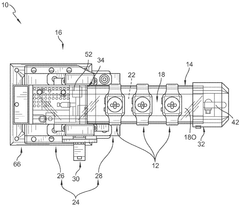
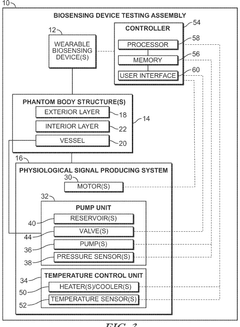
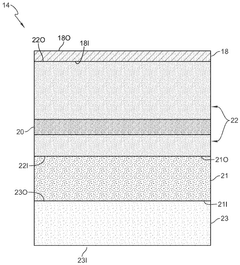
Phantom testing assembly for wearable biosensing devices
PatentWO2025207894A1
Innovation
- A biosensing device testing assembly comprising a phantom body structure with tunable compliance layers and variable vessel depths, a physiological signal producing system to simulate physiological signals, and a controller to mimic real-world conditions, allowing evaluation of wearable biosensing devices under dynamic conditions.
Wearable biosensor device and method for detection and measurment of BIO-molecules and BIO-particles
PatentPendingCA3228054A1
Innovation
- A wearable device with a fluid collection system that collects sweat from the skin using a main channel and inlet channels, preserving molecular contents through vents to store and analyze biomarkers, combined with a biosensor for digital monitoring and a potentiostat readout device for data analysis.
Data Security in Wearable Biosensor Applications
As wearable biosensors increasingly integrate into standardized testing environments, data security emerges as a critical concern. These devices collect vast amounts of sensitive biometric data including heart rate, blood pressure, skin conductance, and even neural activity, necessitating robust security frameworks to protect user privacy and maintain testing integrity.
The primary security vulnerabilities in wearable biosensor applications stem from multiple attack vectors: device-level vulnerabilities, transmission interception, and server-side breaches. Device-level security concerns include firmware exploitation and physical tampering, while data transmission presents opportunities for man-in-the-middle attacks. Server infrastructure storing aggregated testing data represents a high-value target for malicious actors seeking access to comprehensive biometric profiles.
Regulatory frameworks governing biosensor data security vary significantly across jurisdictions. The European Union's GDPR imposes strict requirements for biometric data handling, while the United States relies on a patchwork of federal and state regulations including HIPAA for health-related applications. These disparate approaches create compliance challenges for global deployment of biosensor-based standardized testing systems.
Current security solutions implement multi-layered approaches combining hardware and software protections. Advanced encryption protocols secure data both at rest and in transit, while secure enclaves and trusted execution environments protect processing operations. Biometric authentication mechanisms increasingly serve dual purposes—both as testing metrics and security controls—creating an elegant security-by-design approach specific to this technology domain.
Anonymization and data minimization techniques represent essential strategies for biosensor testing applications. Differential privacy implementations allow meaningful statistical analysis while protecting individual test participants. Federated learning approaches enable model development without centralizing sensitive biometric data, reducing breach impact potential while maintaining testing validity.
The standardization landscape remains fragmented, with IEEE, ISO, and industry consortia developing competing security frameworks. The NIST Cybersecurity Framework provides general guidance, while specialized standards like ISO/IEEE 11073 address health device communication security. This fragmentation creates interoperability challenges but also drives innovation through competitive standardization efforts.
Future security directions point toward blockchain-based consent management systems that provide test-takers granular control over their biometric data usage. Zero-knowledge proofs offer promising approaches for verifying test results without exposing underlying biometric data. As quantum computing threatens current cryptographic protections, post-quantum cryptography research specifically targeting wearable constraints represents a critical research direction for maintaining long-term security in biosensor-based standardized testing.
The primary security vulnerabilities in wearable biosensor applications stem from multiple attack vectors: device-level vulnerabilities, transmission interception, and server-side breaches. Device-level security concerns include firmware exploitation and physical tampering, while data transmission presents opportunities for man-in-the-middle attacks. Server infrastructure storing aggregated testing data represents a high-value target for malicious actors seeking access to comprehensive biometric profiles.
Regulatory frameworks governing biosensor data security vary significantly across jurisdictions. The European Union's GDPR imposes strict requirements for biometric data handling, while the United States relies on a patchwork of federal and state regulations including HIPAA for health-related applications. These disparate approaches create compliance challenges for global deployment of biosensor-based standardized testing systems.
Current security solutions implement multi-layered approaches combining hardware and software protections. Advanced encryption protocols secure data both at rest and in transit, while secure enclaves and trusted execution environments protect processing operations. Biometric authentication mechanisms increasingly serve dual purposes—both as testing metrics and security controls—creating an elegant security-by-design approach specific to this technology domain.
Anonymization and data minimization techniques represent essential strategies for biosensor testing applications. Differential privacy implementations allow meaningful statistical analysis while protecting individual test participants. Federated learning approaches enable model development without centralizing sensitive biometric data, reducing breach impact potential while maintaining testing validity.
The standardization landscape remains fragmented, with IEEE, ISO, and industry consortia developing competing security frameworks. The NIST Cybersecurity Framework provides general guidance, while specialized standards like ISO/IEEE 11073 address health device communication security. This fragmentation creates interoperability challenges but also drives innovation through competitive standardization efforts.
Future security directions point toward blockchain-based consent management systems that provide test-takers granular control over their biometric data usage. Zero-knowledge proofs offer promising approaches for verifying test results without exposing underlying biometric data. As quantum computing threatens current cryptographic protections, post-quantum cryptography research specifically targeting wearable constraints represents a critical research direction for maintaining long-term security in biosensor-based standardized testing.
Regulatory Framework for Medical-Grade Biosensors
The regulatory landscape for medical-grade wearable biosensors is complex and multifaceted, requiring careful navigation by manufacturers and healthcare providers. At the international level, the International Medical Device Regulators Forum (IMDRF) provides harmonized guidelines that influence regional frameworks. These guidelines establish fundamental principles for safety, efficacy, and quality that biosensor technologies must adhere to before market approval.
In the United States, the Food and Drug Administration (FDA) classifies wearable biosensors based on their intended use and risk profile. Class I devices face minimal regulatory control, while Class II devices require 510(k) clearance demonstrating substantial equivalence to predicate devices. Class III biosensors, which may include implantable or life-supporting technologies, undergo the most rigorous premarket approval process requiring clinical trial evidence.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which significantly increased requirements for clinical evidence, post-market surveillance, and unique device identification. These regulations have particular implications for AI-enabled biosensors, requiring manufacturers to demonstrate ongoing algorithm validation and performance monitoring.
Standardization bodies such as ISO and IEC have developed specific standards addressing biosensor performance metrics, including ISO 13485 for quality management systems and IEC 60601 for electrical safety. These standards provide crucial benchmarks for ensuring consistent performance across different testing environments and are often incorporated into regulatory requirements by reference.
Data privacy regulations present another critical dimension, with HIPAA in the US and GDPR in Europe imposing strict requirements on the collection, storage, and transmission of biometric data. These regulations necessitate robust encryption protocols, clear consent mechanisms, and comprehensive data management policies for wearable biosensor implementations in standardized testing environments.
Emerging regulatory considerations include the development of frameworks for continuous monitoring devices, remote patient monitoring, and the integration of biosensor data with electronic health records. Regulatory bodies are increasingly focusing on interoperability standards to ensure seamless data exchange between different healthcare systems and devices.
The pathway to regulatory compliance typically involves extensive documentation, including technical files, risk management reports, clinical evaluation reports, and post-market surveillance plans. For standardized testing applications, additional validation may be required to demonstrate the reliability and reproducibility of biosensor measurements across diverse populations and testing conditions.
In the United States, the Food and Drug Administration (FDA) classifies wearable biosensors based on their intended use and risk profile. Class I devices face minimal regulatory control, while Class II devices require 510(k) clearance demonstrating substantial equivalence to predicate devices. Class III biosensors, which may include implantable or life-supporting technologies, undergo the most rigorous premarket approval process requiring clinical trial evidence.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which significantly increased requirements for clinical evidence, post-market surveillance, and unique device identification. These regulations have particular implications for AI-enabled biosensors, requiring manufacturers to demonstrate ongoing algorithm validation and performance monitoring.
Standardization bodies such as ISO and IEC have developed specific standards addressing biosensor performance metrics, including ISO 13485 for quality management systems and IEC 60601 for electrical safety. These standards provide crucial benchmarks for ensuring consistent performance across different testing environments and are often incorporated into regulatory requirements by reference.
Data privacy regulations present another critical dimension, with HIPAA in the US and GDPR in Europe imposing strict requirements on the collection, storage, and transmission of biometric data. These regulations necessitate robust encryption protocols, clear consent mechanisms, and comprehensive data management policies for wearable biosensor implementations in standardized testing environments.
Emerging regulatory considerations include the development of frameworks for continuous monitoring devices, remote patient monitoring, and the integration of biosensor data with electronic health records. Regulatory bodies are increasingly focusing on interoperability standards to ensure seamless data exchange between different healthcare systems and devices.
The pathway to regulatory compliance typically involves extensive documentation, including technical files, risk management reports, clinical evaluation reports, and post-market surveillance plans. For standardized testing applications, additional validation may be required to demonstrate the reliability and reproducibility of biosensor measurements across diverse populations and testing conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!