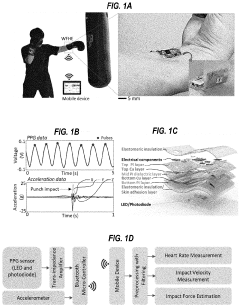
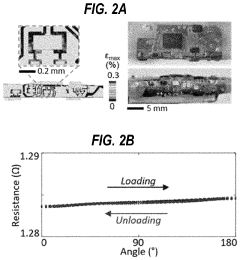
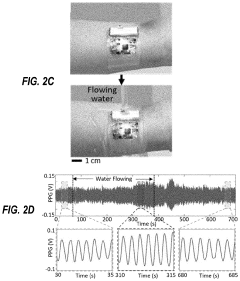
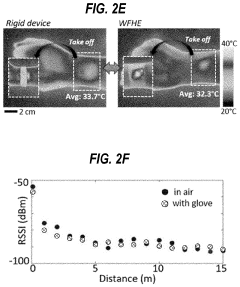
How Are Standards Shaping the Wearable Biosensor Market
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Wearable Biosensor Standards Evolution and Objectives
Wearable biosensor standards have evolved significantly over the past decade, transitioning from fragmented proprietary approaches to increasingly harmonized frameworks. Initially, early wearable biosensors operated within isolated ecosystems with minimal interoperability, creating market fragmentation and limiting adoption. The turning point came around 2014-2015 when industry leaders recognized the need for standardization to enable market growth and technological advancement.
The evolution of these standards has followed a trajectory from basic connectivity protocols to sophisticated data exchange frameworks. Early standards focused primarily on physical connectivity and power management, while contemporary standards address complex issues including data security, clinical validation, and regulatory compliance. Organizations such as IEEE, ISO, and IEC have played pivotal roles in developing these standards, with IEEE 11073 Personal Health Device standards becoming particularly influential in establishing communication protocols for personal health devices.
The objectives of wearable biosensor standardization efforts are multifaceted and increasingly ambitious. Primary goals include ensuring interoperability across devices and platforms, establishing reliable data quality metrics, and creating frameworks for clinical validation. These standards aim to bridge the gap between consumer-grade wearables and medical-grade devices, enabling more seamless integration of biosensor data into healthcare systems.
Another critical objective is addressing the growing concerns around data privacy and security. Standards organizations are working to establish robust protocols for data encryption, user consent management, and secure data transmission that comply with regulations like GDPR and HIPAA. This focus on privacy has become increasingly important as biosensors collect more sensitive health information.
Technical performance benchmarking represents another key standardization objective. The industry is moving toward consensus on how to measure and report accuracy, precision, and reliability of various biosensor modalities including heart rate monitoring, blood oxygen saturation, and emerging biomarkers. These benchmarks are essential for enabling fair comparison between competing products and establishing minimum performance thresholds.
Looking forward, standardization efforts are expanding to address emerging technologies such as continuous glucose monitoring, sweat analysis, and non-invasive blood pressure monitoring. The development of these standards is increasingly collaborative, involving not only technology companies but also healthcare providers, regulatory bodies, and patient advocacy groups to ensure standards meet the needs of all stakeholders in the ecosystem.
The evolution of these standards has followed a trajectory from basic connectivity protocols to sophisticated data exchange frameworks. Early standards focused primarily on physical connectivity and power management, while contemporary standards address complex issues including data security, clinical validation, and regulatory compliance. Organizations such as IEEE, ISO, and IEC have played pivotal roles in developing these standards, with IEEE 11073 Personal Health Device standards becoming particularly influential in establishing communication protocols for personal health devices.
The objectives of wearable biosensor standardization efforts are multifaceted and increasingly ambitious. Primary goals include ensuring interoperability across devices and platforms, establishing reliable data quality metrics, and creating frameworks for clinical validation. These standards aim to bridge the gap between consumer-grade wearables and medical-grade devices, enabling more seamless integration of biosensor data into healthcare systems.
Another critical objective is addressing the growing concerns around data privacy and security. Standards organizations are working to establish robust protocols for data encryption, user consent management, and secure data transmission that comply with regulations like GDPR and HIPAA. This focus on privacy has become increasingly important as biosensors collect more sensitive health information.
Technical performance benchmarking represents another key standardization objective. The industry is moving toward consensus on how to measure and report accuracy, precision, and reliability of various biosensor modalities including heart rate monitoring, blood oxygen saturation, and emerging biomarkers. These benchmarks are essential for enabling fair comparison between competing products and establishing minimum performance thresholds.
Looking forward, standardization efforts are expanding to address emerging technologies such as continuous glucose monitoring, sweat analysis, and non-invasive blood pressure monitoring. The development of these standards is increasingly collaborative, involving not only technology companies but also healthcare providers, regulatory bodies, and patient advocacy groups to ensure standards meet the needs of all stakeholders in the ecosystem.
Market Demand Analysis for Standardized Biosensors
The wearable biosensor market is experiencing unprecedented growth, driven by increasing health consciousness and the rising prevalence of chronic diseases worldwide. Current market analysis indicates robust demand for standardized biosensors across healthcare, fitness, and consumer electronics sectors. The global wearable biosensor market, valued at approximately $13 billion in 2022, is projected to reach $33 billion by 2027, representing a compound annual growth rate of 19.7% during this forecast period.
Consumer demand for health monitoring capabilities has surged dramatically following the COVID-19 pandemic, with particular emphasis on continuous vital sign monitoring and early disease detection. Market research reveals that over 60% of consumers now express interest in wearable devices that can monitor multiple health parameters simultaneously, compared to just 35% in pre-pandemic surveys.
Healthcare providers represent another significant market segment, with hospitals and clinics increasingly adopting standardized biosensor technologies to enable remote patient monitoring and reduce hospitalization rates. The telehealth market expansion has created substantial demand for interoperable biosensors that can seamlessly integrate with existing healthcare information systems.
Regulatory bodies and insurance companies are emerging as influential market drivers, with many insurers now offering incentives for preventive health monitoring through standardized wearable devices. This trend is particularly evident in markets like North America and Europe, where insurance-backed wellness programs have grown by 27% annually since 2020.
Regional analysis shows varying adoption patterns, with North America currently leading market share at 42%, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region demonstrates the fastest growth trajectory, with China and India representing particularly promising markets due to their large populations and rapidly expanding middle classes with increasing disposable income.
Consumer preferences are evolving toward multi-functional devices with longer battery life, improved accuracy, and enhanced data security. Market surveys indicate that 73% of potential buyers consider standardization and interoperability as "very important" or "extremely important" factors in purchasing decisions, highlighting the critical role of standards in market development.
Industry forecasts suggest that standardized biosensors for glucose monitoring, heart rate variability, and stress measurement will see particularly strong demand growth in the coming years. The aging global population and increasing incidence of lifestyle-related diseases further amplify market potential, with an estimated 40% of adults over 65 expected to use some form of wearable health monitoring device by 2025.
Consumer demand for health monitoring capabilities has surged dramatically following the COVID-19 pandemic, with particular emphasis on continuous vital sign monitoring and early disease detection. Market research reveals that over 60% of consumers now express interest in wearable devices that can monitor multiple health parameters simultaneously, compared to just 35% in pre-pandemic surveys.
Healthcare providers represent another significant market segment, with hospitals and clinics increasingly adopting standardized biosensor technologies to enable remote patient monitoring and reduce hospitalization rates. The telehealth market expansion has created substantial demand for interoperable biosensors that can seamlessly integrate with existing healthcare information systems.
Regulatory bodies and insurance companies are emerging as influential market drivers, with many insurers now offering incentives for preventive health monitoring through standardized wearable devices. This trend is particularly evident in markets like North America and Europe, where insurance-backed wellness programs have grown by 27% annually since 2020.
Regional analysis shows varying adoption patterns, with North America currently leading market share at 42%, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region demonstrates the fastest growth trajectory, with China and India representing particularly promising markets due to their large populations and rapidly expanding middle classes with increasing disposable income.
Consumer preferences are evolving toward multi-functional devices with longer battery life, improved accuracy, and enhanced data security. Market surveys indicate that 73% of potential buyers consider standardization and interoperability as "very important" or "extremely important" factors in purchasing decisions, highlighting the critical role of standards in market development.
Industry forecasts suggest that standardized biosensors for glucose monitoring, heart rate variability, and stress measurement will see particularly strong demand growth in the coming years. The aging global population and increasing incidence of lifestyle-related diseases further amplify market potential, with an estimated 40% of adults over 65 expected to use some form of wearable health monitoring device by 2025.
Current Standards Landscape and Technical Challenges
The wearable biosensor market currently operates within a complex standards landscape that varies significantly across regions and device categories. ISO 13485, which specifies requirements for quality management systems in medical devices, serves as a foundational standard for manufacturers seeking to ensure consistent quality and regulatory compliance. However, its implementation across different jurisdictions remains inconsistent, creating challenges for global market players.
FDA regulations in the United States, particularly 21 CFR Part 820 for quality system regulation, have established stringent requirements for wearable biosensors classified as medical devices. Meanwhile, the European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced more rigorous classification systems and conformity assessment procedures, significantly impacting market entry strategies for wearable biosensor manufacturers.
Technical interoperability presents another significant challenge, with IEEE 11073 standards for health informatics attempting to address data exchange protocols between personal health devices. Despite these efforts, the fragmentation of communication protocols continues to hinder seamless integration across healthcare ecosystems. Bluetooth Low Energy (BLE) has emerged as a dominant wireless standard, yet proprietary implementations often limit cross-platform compatibility.
Data security and privacy standards represent perhaps the most critical challenge facing the industry. While regulations like GDPR in Europe and HIPAA in the US provide overarching frameworks, specific technical standards for securing biosensor data remain underdeveloped. The ISO/IEC 27001 for information security management systems offers general guidance, but lacks biosensor-specific protocols for the unique vulnerabilities these devices present.
Calibration and accuracy standards constitute another major technical hurdle. The lack of universally accepted benchmarks for physiological measurements creates significant variability in device performance claims. While ISO 80601-2-61 addresses pulse oximetry specifically, many other biosensor modalities operate without standardized accuracy parameters, complicating clinical validation and comparison between competing products.
Material biocompatibility standards, primarily governed by ISO 10993 series, face implementation challenges as novel materials and form factors emerge. Extended skin contact requirements for wearable devices demand specialized testing protocols that existing standards do not fully address, creating regulatory uncertainty for innovative designs.
The rapid pace of technological innovation consistently outstrips standards development, creating a persistent gap between market capabilities and regulatory frameworks. This disconnect particularly affects emerging biosensor technologies like continuous glucose monitoring, sweat analysis, and non-invasive blood pressure monitoring, where technical standards remain in nascent stages despite accelerating commercial development.
FDA regulations in the United States, particularly 21 CFR Part 820 for quality system regulation, have established stringent requirements for wearable biosensors classified as medical devices. Meanwhile, the European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced more rigorous classification systems and conformity assessment procedures, significantly impacting market entry strategies for wearable biosensor manufacturers.
Technical interoperability presents another significant challenge, with IEEE 11073 standards for health informatics attempting to address data exchange protocols between personal health devices. Despite these efforts, the fragmentation of communication protocols continues to hinder seamless integration across healthcare ecosystems. Bluetooth Low Energy (BLE) has emerged as a dominant wireless standard, yet proprietary implementations often limit cross-platform compatibility.
Data security and privacy standards represent perhaps the most critical challenge facing the industry. While regulations like GDPR in Europe and HIPAA in the US provide overarching frameworks, specific technical standards for securing biosensor data remain underdeveloped. The ISO/IEC 27001 for information security management systems offers general guidance, but lacks biosensor-specific protocols for the unique vulnerabilities these devices present.
Calibration and accuracy standards constitute another major technical hurdle. The lack of universally accepted benchmarks for physiological measurements creates significant variability in device performance claims. While ISO 80601-2-61 addresses pulse oximetry specifically, many other biosensor modalities operate without standardized accuracy parameters, complicating clinical validation and comparison between competing products.
Material biocompatibility standards, primarily governed by ISO 10993 series, face implementation challenges as novel materials and form factors emerge. Extended skin contact requirements for wearable devices demand specialized testing protocols that existing standards do not fully address, creating regulatory uncertainty for innovative designs.
The rapid pace of technological innovation consistently outstrips standards development, creating a persistent gap between market capabilities and regulatory frameworks. This disconnect particularly affects emerging biosensor technologies like continuous glucose monitoring, sweat analysis, and non-invasive blood pressure monitoring, where technical standards remain in nascent stages despite accelerating commercial development.
Prevalent Standardization Frameworks and Approaches
01 Wearable biosensors for continuous health monitoring
Wearable biosensors enable continuous monitoring of various health parameters such as heart rate, blood glucose, and physical activity. These devices can be worn on different parts of the body and provide real-time data collection and analysis. The continuous monitoring capability allows for early detection of health issues and personalized healthcare management, making them valuable tools for both preventive care and chronic disease management.- Wearable biosensors for continuous health monitoring: Wearable biosensors enable continuous monitoring of various health parameters in real-time. These devices can track vital signs, biochemical markers, and physiological signals without interrupting daily activities. The technology incorporates miniaturized sensors that can be worn on different body parts to provide ongoing health data collection, allowing for early detection of health issues and personalized healthcare management.
- Sweat-based biosensing technologies: Sweat-based biosensors analyze biomarkers present in human sweat to provide non-invasive health monitoring. These sensors detect various analytes including electrolytes, metabolites, and hormones through specialized sensing materials and transduction mechanisms. The technology enables continuous monitoring of physiological status without blood sampling, making it particularly suitable for athletic performance tracking, hydration monitoring, and early disease detection.
- Flexible and stretchable biosensor platforms: Flexible and stretchable biosensor platforms conform to body contours for improved comfort and signal quality. These sensors utilize advanced materials such as conductive polymers, nanomaterials, and elastomeric substrates to maintain functionality during movement and deformation. The technology enables better skin contact, reduced motion artifacts, and enhanced user compliance for long-term health monitoring applications.
- Implantable and minimally invasive biosensors: Implantable and minimally invasive biosensors provide continuous monitoring from within the body. These devices can be inserted subcutaneously or implanted in specific tissues to measure biomarkers that are difficult to detect externally. The technology incorporates biocompatible materials, wireless data transmission, and long-lasting power solutions to enable extended monitoring periods with minimal intervention, particularly beneficial for chronic disease management.
- Data integration and analysis systems for wearable biosensors: Data integration and analysis systems process the large volumes of information generated by wearable biosensors. These systems employ advanced algorithms, machine learning, and artificial intelligence to interpret biosensor data, identify patterns, and generate actionable insights. The technology enables meaningful interpretation of complex physiological data, facilitates remote monitoring capabilities, and supports clinical decision-making through comprehensive health dashboards and alert systems.
02 Sweat-based biosensing technologies
Sweat-based biosensors analyze biomarkers present in human sweat to provide non-invasive health monitoring. These sensors can detect various analytes including electrolytes, metabolites, and hormones through specialized sensing mechanisms. The technology incorporates microfluidic channels and electrochemical detection methods to capture, transport, and analyze sweat samples efficiently. These devices offer advantages in sports performance monitoring, hydration status assessment, and certain disease management applications.Expand Specific Solutions03 Implantable and minimally invasive biosensor systems
Implantable and minimally invasive biosensor systems are designed to be placed under the skin or within body tissues for long-term monitoring of physiological parameters. These systems often incorporate biocompatible materials to reduce foreign body responses and extend operational lifespan. The sensors can transmit data wirelessly to external devices for continuous health monitoring without requiring frequent replacement. Applications include glucose monitoring for diabetes management, cardiac function assessment, and therapeutic drug monitoring.Expand Specific Solutions04 Smart textile and flexible substrate biosensors
Biosensors integrated into textiles and flexible substrates enable comfortable, unobtrusive health monitoring through everyday clothing and accessories. These systems incorporate conductive fibers, printed electronics, and stretchable materials that maintain functionality during body movement and regular wear. The technology allows for washable, durable sensing platforms that can monitor multiple physiological parameters simultaneously. Applications include athletic performance monitoring, occupational safety assessment, and remote patient monitoring for elderly care.Expand Specific Solutions05 Data processing and AI integration in biosensor systems
Advanced data processing and artificial intelligence integration enhance the capabilities of wearable biosensor systems by improving signal processing, pattern recognition, and predictive analytics. These technologies filter noise from biosensor signals, identify meaningful health trends, and generate personalized insights based on collected data. Machine learning algorithms can detect subtle changes in physiological parameters that may indicate early disease onset or health deterioration. The systems often include secure data transmission protocols and cloud-based storage solutions for comprehensive health data management.Expand Specific Solutions
Key Standards Organizations and Industry Leaders
The wearable biosensor market is evolving rapidly, with standards playing a crucial role in shaping its competitive landscape. Currently in a growth phase, this market is expanding at a significant rate as healthcare monitoring shifts toward personalized, continuous solutions. Major players like Samsung Electronics and Google are driving innovation through proprietary technologies while collaborating on interoperability standards. Academic institutions such as University of California and Caltech are contributing fundamental research, while specialized companies like Early Warning, Biosense Webster, and Braveheart Wireless focus on niche applications. The market is witnessing increased standardization efforts around data security, interoperability, and clinical validation, which are essential for widespread adoption and regulatory approval across healthcare environments.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed a comprehensive wearable biosensor ecosystem centered around their Samsung Health platform. Their technology integrates various biosensors into smartwatches and fitness bands that comply with key industry standards including IEEE 11073 for personal health device communication and Bluetooth Low Energy (BLE) standards. Samsung's Galaxy Watch series incorporates advanced photoplethysmography (PPG) sensors for heart rate monitoring, electrocardiogram (ECG) capabilities that have received FDA clearance, and blood oxygen (SpO2) monitoring - all adhering to medical-grade accuracy standards. The company has also pioneered the implementation of ISO 13485 quality management systems for their medical-adjacent wearable devices, ensuring regulatory compliance across global markets. Samsung's biosensor technology leverages AI algorithms to analyze collected biometric data while maintaining GDPR and HIPAA compliance for data security and privacy.
Strengths: Strong vertical integration allowing hardware-software optimization; extensive global distribution network; established consumer trust in health features. Weaknesses: Primarily focused on consumer rather than clinical applications; ecosystem somewhat closed compared to open standards advocates; higher price points limiting accessibility in developing markets.
Google LLC
Technical Solution: Google has developed a standards-focused approach to wearable biosensors through its Fitbit acquisition and Wear OS platform. The company has been instrumental in establishing the Open mHealth standard, which provides a common schema for health data interoperability across devices and applications. Google's wearable biosensor technology incorporates continuous heart rate monitoring, sleep tracking, and activity recognition algorithms that comply with IEEE 11073 standards for personal health device communication. Their Fitbit ECG app received FDA clearance by meeting regulatory standards for software as a medical device (SaMD). Google has also pioneered work in standardizing AI-based health metrics through their Health Connect API, which serves as a centralized hub for health and fitness data across Android devices and apps. The company actively participates in the Consumer Technology Association's Health and Fitness Technology Division to develop industry standards for consumer wearables, particularly focusing on accuracy standards for heart rate monitoring and sleep tracking technologies.
Strengths: Extensive data processing capabilities; strong software ecosystem integration; leadership in open standards development for health data interoperability. Weaknesses: Less hardware manufacturing experience compared to dedicated device makers; privacy concerns affecting consumer trust in health data collection; relatively recent entry into medical-grade biosensor market.
Critical Standards and Technical Specifications Analysis
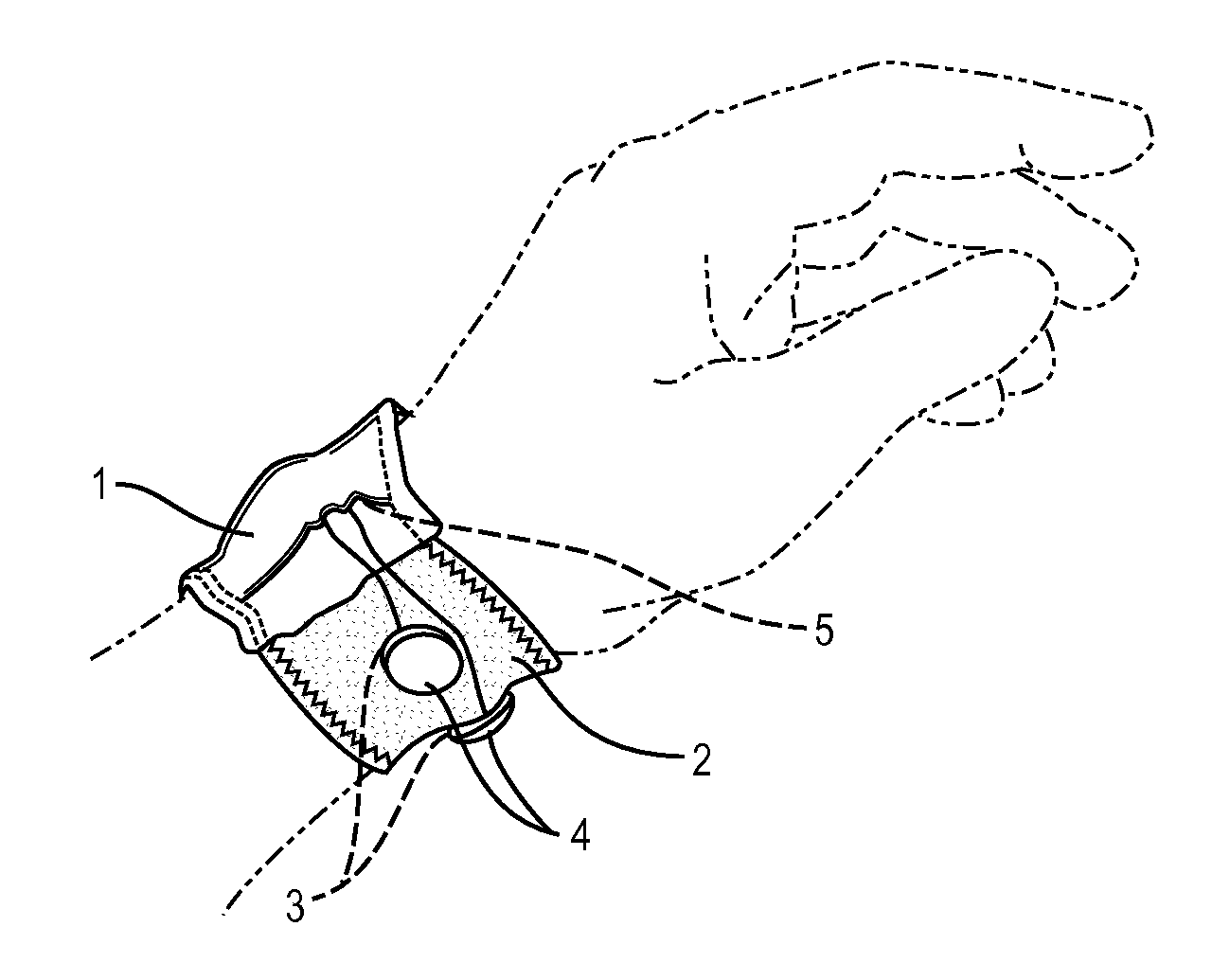
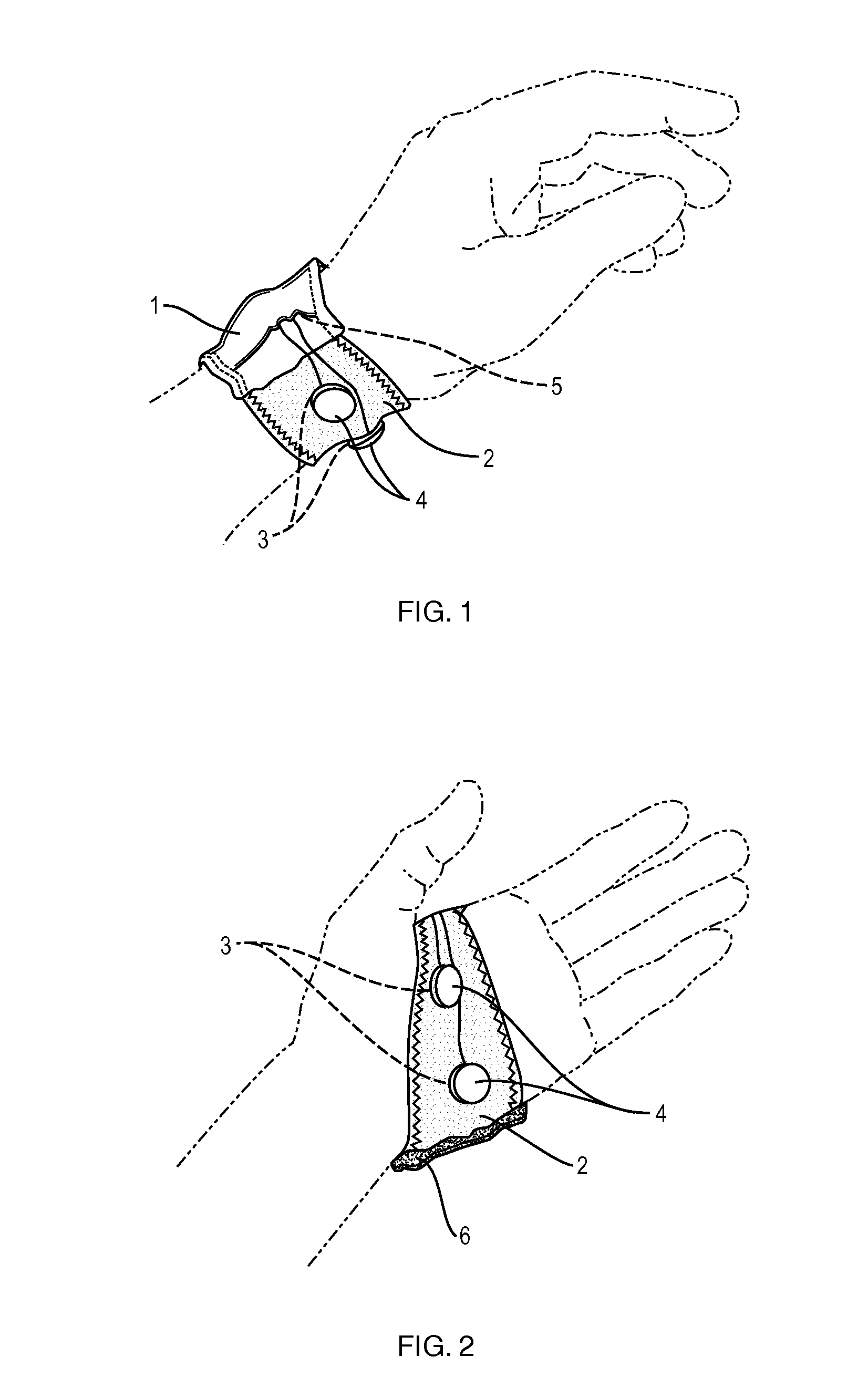
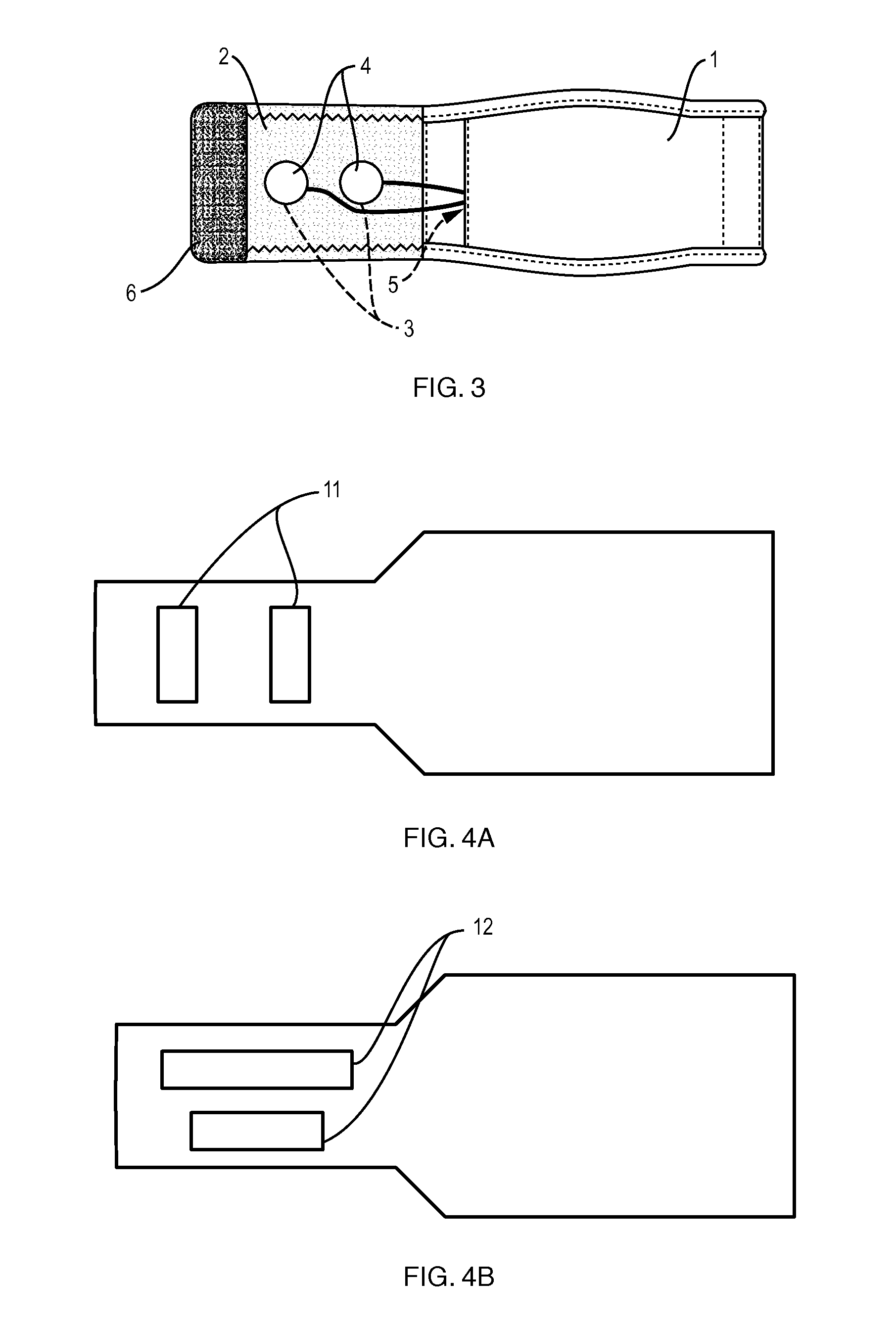
Washable wearable biosensor
PatentActiveUS8140143B2
Innovation
- A washable, wearable biosensor system using breathable materials and flexible textile-based form factors with non-traditional electrode placement, supporting real-time data collection and analysis of physiological parameters like photoplethysmography, skin conductance, motion, and temperature, with channel-sharing wireless protocols for multiple users and integrated motion sensors to reduce noise and improve data integrity.
Flexible biosensors and methods of using same to estimate heart rate
PatentPendingUS20220192515A1
Innovation
- A flexible wearable biosensor with an electrical circuit encapsulated in an elastomer, capable of bending 180 degrees, that generates photoplethysmogram (PPG) and acceleration signals, includes LEDs and a photodiode for PPG measurement, a rechargeable battery, and a wireless transceiver for seamless skin contact and data transmission, addressing the issues of rigidity and practicality.
Interoperability and Data Exchange Protocols
Interoperability remains one of the most critical challenges in the wearable biosensor market. As the ecosystem expands with diverse devices from multiple manufacturers, the need for standardized data exchange protocols has become increasingly urgent. Currently, several key protocols dominate the landscape, including Bluetooth Low Energy (BLE), which has emerged as the de facto standard for short-range communication between wearable devices and smartphones. The Bluetooth Special Interest Group (SIG) has developed specific profiles for healthcare applications, such as the Heart Rate Profile and Glucose Profile, enabling consistent data formatting across compatible devices.
Beyond Bluetooth, other significant protocols include ANT+, widely used in fitness devices, and Near Field Communication (NFC) for secure, short-range data transfer. The IEEE 11073 standards family specifically addresses personal health device communication, providing detailed frameworks for data exchange between medical devices and external computer systems. These standards define how physiological data should be structured, encoded, and transmitted, ensuring consistency across different platforms.
The Fast Healthcare Interoperability Resources (FHIR) standard has gained substantial traction for integrating wearable data into healthcare information systems. Developed by Health Level Seven International (HL7), FHIR provides a modern, API-based approach to health data exchange that facilitates the integration of wearable biosensor data into electronic health records and clinical decision support systems.
Regulatory bodies have recognized the importance of interoperability standards. The FDA has increasingly emphasized the need for standardized data formats in its guidance for digital health technologies. Similarly, the European Medical Device Regulation (MDR) includes provisions that encourage interoperable solutions for medical devices, including wearable biosensors.
Despite these advances, fragmentation remains a significant challenge. Proprietary protocols continue to exist alongside open standards, creating barriers to seamless data exchange. This fragmentation has led to the emergence of middleware solutions and data aggregation platforms that translate between different protocols, acting as bridges between otherwise incompatible systems.
Cloud-based interoperability solutions have emerged as a promising approach to address these challenges. Platforms like Apple HealthKit, Google Fit, and Samsung Health serve as centralized repositories that can collect, normalize, and share data from various wearable devices, regardless of their native protocols. These platforms implement standardized APIs that developers can use to access unified health data, potentially overcoming device-specific limitations.
The future of interoperability in wearable biosensors likely lies in the continued development and adoption of open standards, with particular emphasis on security and privacy considerations. As these devices increasingly generate sensitive health data, protocols must not only facilitate data exchange but also ensure appropriate protection measures are implemented consistently across the ecosystem.
Beyond Bluetooth, other significant protocols include ANT+, widely used in fitness devices, and Near Field Communication (NFC) for secure, short-range data transfer. The IEEE 11073 standards family specifically addresses personal health device communication, providing detailed frameworks for data exchange between medical devices and external computer systems. These standards define how physiological data should be structured, encoded, and transmitted, ensuring consistency across different platforms.
The Fast Healthcare Interoperability Resources (FHIR) standard has gained substantial traction for integrating wearable data into healthcare information systems. Developed by Health Level Seven International (HL7), FHIR provides a modern, API-based approach to health data exchange that facilitates the integration of wearable biosensor data into electronic health records and clinical decision support systems.
Regulatory bodies have recognized the importance of interoperability standards. The FDA has increasingly emphasized the need for standardized data formats in its guidance for digital health technologies. Similarly, the European Medical Device Regulation (MDR) includes provisions that encourage interoperable solutions for medical devices, including wearable biosensors.
Despite these advances, fragmentation remains a significant challenge. Proprietary protocols continue to exist alongside open standards, creating barriers to seamless data exchange. This fragmentation has led to the emergence of middleware solutions and data aggregation platforms that translate between different protocols, acting as bridges between otherwise incompatible systems.
Cloud-based interoperability solutions have emerged as a promising approach to address these challenges. Platforms like Apple HealthKit, Google Fit, and Samsung Health serve as centralized repositories that can collect, normalize, and share data from various wearable devices, regardless of their native protocols. These platforms implement standardized APIs that developers can use to access unified health data, potentially overcoming device-specific limitations.
The future of interoperability in wearable biosensors likely lies in the continued development and adoption of open standards, with particular emphasis on security and privacy considerations. As these devices increasingly generate sensitive health data, protocols must not only facilitate data exchange but also ensure appropriate protection measures are implemented consistently across the ecosystem.
Regulatory Compliance and Certification Pathways
The regulatory landscape for wearable biosensors is complex and multifaceted, requiring manufacturers to navigate various certification pathways across different global markets. In the United States, the Food and Drug Administration (FDA) classifies wearable biosensors based on their intended use and risk level, with most falling under Class I (low risk) or Class II (moderate risk) medical devices. For Class II devices, manufacturers typically need to submit a 510(k) premarket notification demonstrating substantial equivalence to a legally marketed device, while novel technologies may require more rigorous Premarket Approval (PMA).
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have significantly impacted the wearable biosensor market since their implementation. These regulations have introduced more stringent requirements for clinical evidence, post-market surveillance, and technical documentation. Manufacturers must obtain CE marking through conformity assessment procedures involving Notified Bodies for higher-risk devices, demonstrating compliance with General Safety and Performance Requirements (GSPRs).
International standards play a crucial role in harmonizing regulatory approaches across different jurisdictions. ISO 13485 for quality management systems in medical devices serves as a foundational standard for manufacturers seeking global market access. For specific technical aspects, standards such as IEC 60601 for electrical safety, ISO 10993 for biocompatibility, and IEEE 11073 for interoperability provide essential frameworks for compliance and certification.
Emerging markets present additional regulatory considerations. China's National Medical Products Administration (NMPA) has implemented reforms aligning more closely with international standards while maintaining unique requirements. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) employs a risk-based classification system similar to other major markets but with distinct approval pathways.
The certification timeline for wearable biosensors varies significantly based on device classification and market. Low-risk devices may achieve certification in 3-6 months, while novel high-risk technologies can require 18-36 months for full approval. This timeline variability creates strategic challenges for manufacturers planning global market entry strategies.
Cost considerations for regulatory compliance are substantial, including direct fees to regulatory bodies, costs for clinical studies, quality management system implementation, and ongoing compliance maintenance. These expenses can range from tens of thousands to millions of dollars depending on device complexity and target markets, creating potential barriers to entry for smaller innovators.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have significantly impacted the wearable biosensor market since their implementation. These regulations have introduced more stringent requirements for clinical evidence, post-market surveillance, and technical documentation. Manufacturers must obtain CE marking through conformity assessment procedures involving Notified Bodies for higher-risk devices, demonstrating compliance with General Safety and Performance Requirements (GSPRs).
International standards play a crucial role in harmonizing regulatory approaches across different jurisdictions. ISO 13485 for quality management systems in medical devices serves as a foundational standard for manufacturers seeking global market access. For specific technical aspects, standards such as IEC 60601 for electrical safety, ISO 10993 for biocompatibility, and IEEE 11073 for interoperability provide essential frameworks for compliance and certification.
Emerging markets present additional regulatory considerations. China's National Medical Products Administration (NMPA) has implemented reforms aligning more closely with international standards while maintaining unique requirements. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) employs a risk-based classification system similar to other major markets but with distinct approval pathways.
The certification timeline for wearable biosensors varies significantly based on device classification and market. Low-risk devices may achieve certification in 3-6 months, while novel high-risk technologies can require 18-36 months for full approval. This timeline variability creates strategic challenges for manufacturers planning global market entry strategies.
Cost considerations for regulatory compliance are substantial, including direct fees to regulatory bodies, costs for clinical studies, quality management system implementation, and ongoing compliance maintenance. These expenses can range from tens of thousands to millions of dollars depending on device complexity and target markets, creating potential barriers to entry for smaller innovators.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!