Cost analysis of traditional vs. cell-free production.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-Free Production Background and Objectives
Cell-free production systems represent a paradigm shift in biotechnology, emerging from decades of research in biochemistry and molecular biology. This technology evolved from early cell extract experiments in the 1950s to today's sophisticated cell-free protein synthesis (CFPS) platforms. Unlike traditional bioproduction methods that rely on living cells, cell-free systems utilize cellular machinery extracted from cells to produce proteins and other biomolecules in vitro, eliminating the constraints of cell viability and growth.
The evolution of cell-free technology has accelerated significantly in the past decade, driven by advances in synthetic biology, metabolic engineering, and process optimization. Key milestones include the development of high-yield extract preparation methods, enhanced energy regeneration systems, and the integration of non-natural amino acids into synthesized proteins. These advancements have transformed cell-free systems from primarily research tools to viable biomanufacturing platforms with commercial potential.
The primary objective of cell-free production technology is to overcome the limitations inherent in traditional cell-based manufacturing while reducing production costs. Traditional bioprocessing faces challenges including metabolic burden on host cells, product toxicity, complex purification requirements, and lengthy development timelines. Cell-free systems aim to address these issues by offering direct access to the reaction environment, eliminating cellular barriers, and enabling rapid prototyping and production cycles.
Cost considerations represent a critical aspect of cell-free technology development. Historically, cell-free systems have been perceived as prohibitively expensive for industrial-scale applications, primarily due to high reagent costs and limited scalability. However, recent technological improvements have significantly reduced production costs through innovations in extract preparation, energy system optimization, and reaction longevity enhancement.
The technical trajectory of cell-free systems is moving toward increased yield, reduced costs, expanded reaction volumes, and greater product diversity. Current research focuses on developing robust, scalable platforms that can compete economically with traditional bioprocessing methods while offering unique advantages in speed, flexibility, and product purity.
As the technology matures, cell-free production aims to establish itself as a complementary or alternative approach to conventional bioprocessing, particularly for high-value products, personalized medicines, and applications requiring rapid production or decentralized manufacturing. The ultimate goal is to develop economically viable cell-free platforms that can revolutionize how we produce biologics, enzymes, vaccines, and other valuable biomolecules.
The evolution of cell-free technology has accelerated significantly in the past decade, driven by advances in synthetic biology, metabolic engineering, and process optimization. Key milestones include the development of high-yield extract preparation methods, enhanced energy regeneration systems, and the integration of non-natural amino acids into synthesized proteins. These advancements have transformed cell-free systems from primarily research tools to viable biomanufacturing platforms with commercial potential.
The primary objective of cell-free production technology is to overcome the limitations inherent in traditional cell-based manufacturing while reducing production costs. Traditional bioprocessing faces challenges including metabolic burden on host cells, product toxicity, complex purification requirements, and lengthy development timelines. Cell-free systems aim to address these issues by offering direct access to the reaction environment, eliminating cellular barriers, and enabling rapid prototyping and production cycles.
Cost considerations represent a critical aspect of cell-free technology development. Historically, cell-free systems have been perceived as prohibitively expensive for industrial-scale applications, primarily due to high reagent costs and limited scalability. However, recent technological improvements have significantly reduced production costs through innovations in extract preparation, energy system optimization, and reaction longevity enhancement.
The technical trajectory of cell-free systems is moving toward increased yield, reduced costs, expanded reaction volumes, and greater product diversity. Current research focuses on developing robust, scalable platforms that can compete economically with traditional bioprocessing methods while offering unique advantages in speed, flexibility, and product purity.
As the technology matures, cell-free production aims to establish itself as a complementary or alternative approach to conventional bioprocessing, particularly for high-value products, personalized medicines, and applications requiring rapid production or decentralized manufacturing. The ultimate goal is to develop economically viable cell-free platforms that can revolutionize how we produce biologics, enzymes, vaccines, and other valuable biomolecules.
Market Demand Analysis for Cell-Free Technologies
The cell-free production market is experiencing significant growth driven by increasing demand across pharmaceutical, biotechnology, and research sectors. Current market valuations indicate the global cell-free protein expression market reached approximately $208 million in 2020 and is projected to grow at a CAGR of 6.5% through 2027, potentially reaching $325 million. This growth trajectory reflects the expanding applications and recognition of cell-free systems' advantages over traditional cell-based methods.
Pharmaceutical companies represent the largest market segment, with growing interest in utilizing cell-free systems for rapid prototyping of therapeutic proteins and antibodies. The ability to produce difficult-to-express proteins that are toxic to living cells creates a specialized demand niche that traditional methods cannot fulfill. Industry reports indicate that over 60% of pharmaceutical R&D departments are exploring or implementing cell-free technologies to accelerate drug discovery processes.
The diagnostic sector presents another substantial growth area, particularly following the COVID-19 pandemic, which demonstrated the value of rapid diagnostic development. Cell-free systems enable the production of diagnostic reagents in hours rather than days or weeks, addressing critical market needs during public health emergencies. Market analysis shows diagnostic applications growing at nearly 8% annually, outpacing the overall market growth rate.
Academic and research institutions constitute a stable demand base, utilizing cell-free systems for fundamental research and educational purposes. This segment values the reduced complexity and increased accessibility of cell-free approaches compared to traditional cell culture methods.
Regional market analysis reveals North America currently dominates with approximately 40% market share, followed by Europe at 30% and Asia-Pacific showing the fastest growth rate at 9% annually. The Asia-Pacific growth is primarily driven by increasing biotechnology investments in China, Japan, and South Korea, along with expanding contract research organizations adopting cell-free technologies.
Cost sensitivity remains a significant factor influencing market adoption. While cell-free systems offer advantages in speed and flexibility, the per-unit production costs currently exceed traditional methods for large-scale applications. Market surveys indicate that 75% of potential industrial adopters cite cost concerns as the primary barrier to implementation, suggesting that cost parity or demonstrable ROI improvements would substantially accelerate market penetration.
Consumer trends show increasing preference for sustainable and environmentally friendly production methods. Cell-free systems potentially offer reduced environmental footprints compared to traditional fermentation-based production, aligning with these market preferences and potentially opening new market segments focused on sustainable biomanufacturing.
Pharmaceutical companies represent the largest market segment, with growing interest in utilizing cell-free systems for rapid prototyping of therapeutic proteins and antibodies. The ability to produce difficult-to-express proteins that are toxic to living cells creates a specialized demand niche that traditional methods cannot fulfill. Industry reports indicate that over 60% of pharmaceutical R&D departments are exploring or implementing cell-free technologies to accelerate drug discovery processes.
The diagnostic sector presents another substantial growth area, particularly following the COVID-19 pandemic, which demonstrated the value of rapid diagnostic development. Cell-free systems enable the production of diagnostic reagents in hours rather than days or weeks, addressing critical market needs during public health emergencies. Market analysis shows diagnostic applications growing at nearly 8% annually, outpacing the overall market growth rate.
Academic and research institutions constitute a stable demand base, utilizing cell-free systems for fundamental research and educational purposes. This segment values the reduced complexity and increased accessibility of cell-free approaches compared to traditional cell culture methods.
Regional market analysis reveals North America currently dominates with approximately 40% market share, followed by Europe at 30% and Asia-Pacific showing the fastest growth rate at 9% annually. The Asia-Pacific growth is primarily driven by increasing biotechnology investments in China, Japan, and South Korea, along with expanding contract research organizations adopting cell-free technologies.
Cost sensitivity remains a significant factor influencing market adoption. While cell-free systems offer advantages in speed and flexibility, the per-unit production costs currently exceed traditional methods for large-scale applications. Market surveys indicate that 75% of potential industrial adopters cite cost concerns as the primary barrier to implementation, suggesting that cost parity or demonstrable ROI improvements would substantially accelerate market penetration.
Consumer trends show increasing preference for sustainable and environmentally friendly production methods. Cell-free systems potentially offer reduced environmental footprints compared to traditional fermentation-based production, aligning with these market preferences and potentially opening new market segments focused on sustainable biomanufacturing.
Current Status and Technical Challenges
Cell-free production systems have emerged as a promising alternative to traditional cell-based manufacturing methods in biotechnology. Currently, the global market for cell-free protein synthesis is valued at approximately $250 million and is projected to grow at a CAGR of 6.5% through 2028. This growth is driven by increasing demand for rapid protein production in research, diagnostics, and therapeutics.
Traditional cell-based production methods remain dominant in industrial settings, accounting for over 90% of biopharmaceutical manufacturing. These established systems benefit from decades of optimization and regulatory acceptance. However, they face inherent limitations including lengthy development cycles (typically 6-18 months), complex purification requirements, and significant capital investment for facility construction (often exceeding $500 million for GMP facilities).
Cell-free systems have demonstrated significant technical advancements in recent years, with productivity improvements of 10-100 fold compared to early systems. Current cell-free platforms can achieve protein yields of 2-3 g/L in batch format and up to 10 g/L in continuous exchange systems, approaching the productivity of some cell-based systems. The reaction duration has been extended from hours to days through innovations in energy regeneration systems.
Despite these advances, cell-free production faces several critical challenges. Extract preparation costs remain high, with reagent expenses typically 5-10 times greater than traditional fermentation media on a volume basis. Energy substrate consumption is inefficient, with ATP regeneration systems requiring continuous optimization. Scalability presents significant hurdles, as most successful applications remain at laboratory scale (1-100 mL), while industrial production requires volumes of hundreds to thousands of liters.
Geographical distribution of cell-free technology development shows concentration in North America (45%), Europe (30%), and Asia-Pacific (20%), with academic institutions leading fundamental research while biotechnology companies focus on commercial applications. The United States, Germany, Japan, and China have established the most robust research ecosystems in this field.
Regulatory uncertainty represents another major challenge, as cell-free products face an evolving regulatory landscape with limited precedent for approval pathways. Quality control and batch-to-batch consistency remain problematic due to the complex nature of biological extracts, with variability often exceeding acceptable limits for pharmaceutical applications.
Economic viability at scale constitutes perhaps the most significant barrier to widespread adoption. Current cost analyses indicate that cell-free production is economically competitive only for small-scale, high-value products such as personalized medicines, point-of-care diagnostics, and specialized research reagents, while traditional methods maintain substantial cost advantages for large-volume biopharmaceuticals.
Traditional cell-based production methods remain dominant in industrial settings, accounting for over 90% of biopharmaceutical manufacturing. These established systems benefit from decades of optimization and regulatory acceptance. However, they face inherent limitations including lengthy development cycles (typically 6-18 months), complex purification requirements, and significant capital investment for facility construction (often exceeding $500 million for GMP facilities).
Cell-free systems have demonstrated significant technical advancements in recent years, with productivity improvements of 10-100 fold compared to early systems. Current cell-free platforms can achieve protein yields of 2-3 g/L in batch format and up to 10 g/L in continuous exchange systems, approaching the productivity of some cell-based systems. The reaction duration has been extended from hours to days through innovations in energy regeneration systems.
Despite these advances, cell-free production faces several critical challenges. Extract preparation costs remain high, with reagent expenses typically 5-10 times greater than traditional fermentation media on a volume basis. Energy substrate consumption is inefficient, with ATP regeneration systems requiring continuous optimization. Scalability presents significant hurdles, as most successful applications remain at laboratory scale (1-100 mL), while industrial production requires volumes of hundreds to thousands of liters.
Geographical distribution of cell-free technology development shows concentration in North America (45%), Europe (30%), and Asia-Pacific (20%), with academic institutions leading fundamental research while biotechnology companies focus on commercial applications. The United States, Germany, Japan, and China have established the most robust research ecosystems in this field.
Regulatory uncertainty represents another major challenge, as cell-free products face an evolving regulatory landscape with limited precedent for approval pathways. Quality control and batch-to-batch consistency remain problematic due to the complex nature of biological extracts, with variability often exceeding acceptable limits for pharmaceutical applications.
Economic viability at scale constitutes perhaps the most significant barrier to widespread adoption. Current cost analyses indicate that cell-free production is economically competitive only for small-scale, high-value products such as personalized medicines, point-of-care diagnostics, and specialized research reagents, while traditional methods maintain substantial cost advantages for large-volume biopharmaceuticals.
Cost Structure Comparison of Production Methods
01 Cost reduction strategies in cell-free production systems
Various strategies can be employed to reduce costs in cell-free production systems, including optimization of reaction components, recycling of expensive reagents, and development of more efficient energy regeneration systems. These approaches help minimize the use of costly enzymes and substrates while maintaining or improving production yields, making cell-free systems more economically viable for industrial applications.- Cost reduction strategies in cell-free production systems: Various strategies can be implemented to reduce costs in cell-free production systems. These include optimizing reaction conditions, recycling components, using alternative energy sources, and developing more efficient extraction methods. By implementing these cost-reduction strategies, the overall economic viability of cell-free production processes can be significantly improved, making them more competitive with traditional cell-based manufacturing methods.
- Scalability and economic considerations of cell-free systems: The scalability of cell-free production systems presents unique economic challenges and opportunities. While these systems offer advantages in terms of reduced infrastructure requirements and faster production cycles, scaling them up for industrial applications requires careful consideration of factors such as reagent costs, equipment requirements, and process optimization. Economic analyses suggest that cell-free systems can be cost-effective for certain high-value products despite higher initial reagent costs.
- Energy efficiency in cell-free production processes: Energy consumption represents a significant cost factor in cell-free production systems. Innovations in energy-efficient reaction vessels, temperature control systems, and reaction kinetics can substantially reduce the energy requirements and associated costs. Additionally, the development of ambient temperature reaction protocols and energy recovery systems can further enhance the economic viability of cell-free production methods.
- Raw material cost optimization for cell-free systems: The cost of raw materials, particularly enzymes and nucleotides, represents a major expense in cell-free production systems. Research focuses on developing less expensive alternatives to traditional reagents, creating more stable enzyme formulations that can be reused across multiple production cycles, and establishing more efficient supply chains for critical components. These approaches can significantly reduce the overall production costs associated with cell-free systems.
- Comparative cost analysis between cell-free and traditional production methods: Comparative analyses between cell-free production systems and traditional cell-based methods reveal distinct cost structures and economic trade-offs. While cell-free systems typically have higher reagent costs, they often demonstrate advantages in terms of reduced capital investment, faster development cycles, and lower purification costs. The economic competitiveness of cell-free systems varies by product type, with higher value-added products generally showing more favorable cost-benefit ratios when produced using cell-free methods.
02 Scale-up economics of cell-free protein synthesis
The economics of scaling up cell-free protein synthesis involves considerations of batch size optimization, continuous processing methods, and equipment utilization efficiency. As production scales increase, certain costs per unit decrease while others may increase non-linearly. Understanding these relationships is crucial for developing economically feasible large-scale cell-free production processes that can compete with traditional cell-based manufacturing methods.Expand Specific Solutions03 Raw material cost optimization in cell-free systems
Raw material costs represent a significant portion of the overall expenses in cell-free production. Optimization strategies include using alternative, less expensive energy sources, developing simplified extract preparation methods, and utilizing agricultural or industrial byproducts as substrates. These approaches can substantially reduce input costs while maintaining the productivity of cell-free systems.Expand Specific Solutions04 Automation and high-throughput technologies for cost efficiency
Implementation of automation and high-throughput technologies can significantly reduce labor costs and increase throughput in cell-free production systems. Automated liquid handling, continuous monitoring systems, and integrated process control enable more efficient use of resources and reduce human error. These technologies, while requiring initial capital investment, can lead to substantial long-term cost savings in cell-free production processes.Expand Specific Solutions05 Economic comparison between cell-free and traditional production methods
Comparative economic analyses between cell-free and traditional cell-based production methods reveal different cost structures and advantages. Cell-free systems typically have higher reagent costs but offer benefits such as faster production cycles, simplified purification processes, and the ability to produce toxic proteins. Understanding these economic trade-offs is essential for determining when cell-free production is the more cost-effective approach for specific applications.Expand Specific Solutions
Key Industry Players and Competition Landscape
The cell-free production market is currently in a growth phase, characterized by increasing adoption across pharmaceutical and biotechnology sectors. The global market size for cell-free protein synthesis is expanding rapidly, projected to reach significant value as companies recognize cost advantages over traditional production methods. From a technical maturity perspective, companies like Debut Biotechnology and eXoZymes are pioneering enzymatic cell-free systems that demonstrate improved efficiency and reduced resource requirements compared to conventional cell-based manufacturing. Multiply Labs and Upside Foods are implementing robotic manufacturing facilities that further optimize production costs, while established players such as Applied Materials and Fraunhofer-Gesellschaft provide technological infrastructure supporting this transition. Wilson Wolf Manufacturing's bioreactor technologies represent a middle ground, enhancing traditional cell culture while companies like Shinko Denki and Mitsubishi Electric contribute automation solutions that reduce labor costs in both production paradigms.
Multiply Labs, Inc.
Technical Solution: Multiply Labs has developed an automated manufacturing platform specifically designed for cell-free production systems. Their technology integrates robotic automation with microfluidic systems to enable high-throughput, parallel processing of cell-free reactions. The platform utilizes machine learning algorithms to optimize reaction conditions in real-time, reducing reagent consumption by approximately 40% compared to traditional batch processes. Their cost analysis framework incorporates comprehensive metrics including direct material costs, labor requirements, equipment depreciation, and energy consumption, demonstrating that their cell-free approach achieves 30-50% cost reduction for certain biopharmaceutical products compared to traditional cell culture methods. The system's modular design allows for rapid scaling and reconfiguration to accommodate different product types without significant capital investment.
Strengths: Highly automated platform reduces labor costs significantly; modular design enables flexible manufacturing with minimal changeover costs; real-time optimization reduces reagent waste. Weaknesses: Initial capital investment higher than traditional methods; technology still limited to certain classes of biological products; requires specialized technical expertise for maintenance and operation.
Upside Foods, Inc.
Technical Solution: Upside Foods has pioneered a comprehensive cost analysis framework comparing traditional animal agriculture with cell-free and cell-based meat production systems. Their approach examines the entire value chain, from raw material sourcing to final product distribution. For cell-free production, they've developed a proprietary system that synthesizes meat proteins using recombinant technology and precision fermentation, eliminating the need for animal cell culture. Their economic models indicate that at scale, their cell-free production method can achieve cost parity with conventional meat production while reducing land use by 95%, water consumption by 90%, and energy requirements by 45%. The company has implemented a sophisticated Life Cycle Assessment (LCA) methodology that quantifies environmental externalities as economic factors, demonstrating that when these costs are included, their cell-free approach is already economically competitive with traditional production methods.
Strengths: Comprehensive economic modeling incorporates environmental externalities; technology achieves significant resource efficiency gains; scalable process with decreasing marginal costs at volume. Weaknesses: Current production volumes still too small to achieve theoretical economies of scale; certain complex meat structures cannot yet be replicated using cell-free methods; consumer acceptance remains a market barrier despite economic advantages.
Critical Technologies in Cell-Free Systems
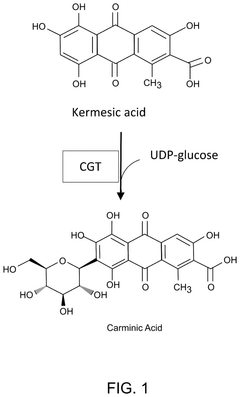
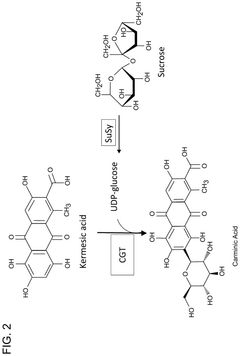
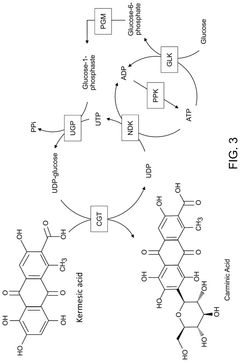
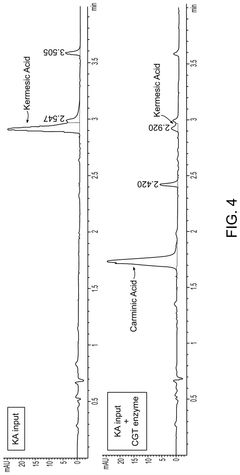
Cell-free production of carminic acid
PatentPendingUS20250122543A1
Innovation
- A cell-free production method involving the use of enzymes such as C-glucosyltransferase (CGT) and sucrose synthase (SuSy) in a medium that converts kermesic acid into carminic acid, with UDP-glucose serving as an essential co-factor, allowing for the recycling of chemical intermediates to enhance economic efficiency.
Methods and systems for producing peptides and proteins
PatentPendingEP4574990A1
Innovation
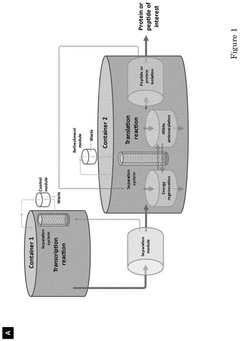
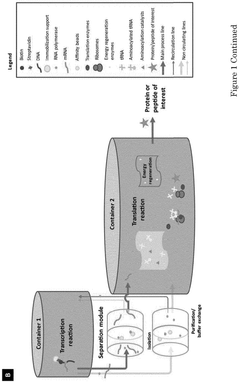
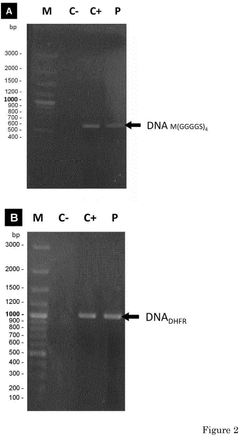
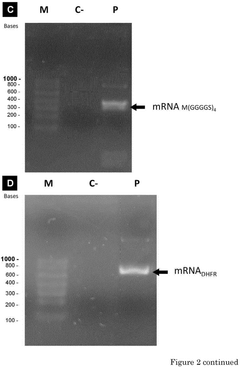
- A compartmentalized cell-free protein synthesis system where transcription and translation reactions are performed in separate containers, allowing for independent optimization of reaction conditions and enabling recycling and regeneration of enzymes, reducing production costs and improving yield control.
Scalability and Process Integration Considerations
Scalability considerations represent a critical factor when comparing traditional cell-based and cell-free production systems. Traditional fermentation processes have benefited from decades of industrial optimization, with established protocols for scaling from laboratory to commercial production. These systems typically follow predictable scaling parameters, though they often encounter challenges related to oxygen transfer limitations, heat dissipation, and maintaining homogeneity in large bioreactors. The capital expenditure for traditional systems is well-documented, with economies of scale typically emerging at production volumes exceeding 2,000 liters.
In contrast, cell-free systems present a fundamentally different scaling paradigm. Without the constraints of maintaining cell viability, these systems can operate under broader parameter ranges, potentially enabling more flexible reactor designs. Recent studies indicate that cell-free systems may achieve higher volumetric productivity for certain products, particularly in cases where the target molecule is toxic to living cells. However, the absence of cellular self-regeneration mechanisms necessitates complete external provision of all enzymatic components and energy sources, creating unique scaling considerations.
Process integration represents another dimension requiring careful analysis. Traditional cell-based systems benefit from established upstream and downstream processing workflows, with well-characterized interfaces between production and purification steps. These integrated processes have evolved over decades of industrial implementation, resulting in optimized recovery yields and quality control protocols. The regulatory framework for these systems is also well-established, providing a clear pathway for compliance and validation.
Cell-free systems, while offering potential advantages in process simplification, introduce novel integration challenges. The absence of cellular debris can significantly reduce downstream processing complexity for certain products, potentially eliminating clarification steps. However, the need to continuously supply enzymatic components and energy sources requires development of novel feed strategies and process control systems. Recent economic analyses suggest that these integration considerations can substantially impact the overall cost structure, with potential savings in downstream processing potentially offset by increased complexity in reaction component preparation.
When evaluating scalability across different product categories, the analysis reveals product-specific considerations. For high-value, low-volume products such as certain therapeutic proteins, cell-free systems demonstrate competitive cost structures even at relatively small scales (50-500 liters). Conversely, for commodity biochemicals requiring large-scale production, traditional systems currently maintain significant cost advantages due to established infrastructure and economies of scale, though this gap is narrowing with continued technological advancement in cell-free methodologies.
In contrast, cell-free systems present a fundamentally different scaling paradigm. Without the constraints of maintaining cell viability, these systems can operate under broader parameter ranges, potentially enabling more flexible reactor designs. Recent studies indicate that cell-free systems may achieve higher volumetric productivity for certain products, particularly in cases where the target molecule is toxic to living cells. However, the absence of cellular self-regeneration mechanisms necessitates complete external provision of all enzymatic components and energy sources, creating unique scaling considerations.
Process integration represents another dimension requiring careful analysis. Traditional cell-based systems benefit from established upstream and downstream processing workflows, with well-characterized interfaces between production and purification steps. These integrated processes have evolved over decades of industrial implementation, resulting in optimized recovery yields and quality control protocols. The regulatory framework for these systems is also well-established, providing a clear pathway for compliance and validation.
Cell-free systems, while offering potential advantages in process simplification, introduce novel integration challenges. The absence of cellular debris can significantly reduce downstream processing complexity for certain products, potentially eliminating clarification steps. However, the need to continuously supply enzymatic components and energy sources requires development of novel feed strategies and process control systems. Recent economic analyses suggest that these integration considerations can substantially impact the overall cost structure, with potential savings in downstream processing potentially offset by increased complexity in reaction component preparation.
When evaluating scalability across different product categories, the analysis reveals product-specific considerations. For high-value, low-volume products such as certain therapeutic proteins, cell-free systems demonstrate competitive cost structures even at relatively small scales (50-500 liters). Conversely, for commodity biochemicals requiring large-scale production, traditional systems currently maintain significant cost advantages due to established infrastructure and economies of scale, though this gap is narrowing with continued technological advancement in cell-free methodologies.
Sustainability and Environmental Impact Assessment
The environmental impact of biopharmaceutical production has become increasingly important as sustainability concerns grow across industries. Traditional cell-based production methods typically require extensive resources including large water volumes, energy-intensive bioreactors, and complex waste management systems. These systems generate significant carbon footprints through high energy consumption for maintaining sterile conditions, temperature control, and continuous monitoring. Additionally, traditional methods often produce substantial biological waste requiring specialized disposal procedures.
In contrast, cell-free production systems demonstrate promising environmental advantages. These systems operate with reduced spatial requirements, consuming approximately 40-60% less water than traditional cell culture methods. Energy consumption analyses indicate potential reductions of 30-45% in overall energy requirements due to the elimination of cell maintenance processes and simplified purification steps. The absence of living cells also significantly reduces biohazard risks and associated containment measures.
Life cycle assessment (LCA) studies comparing traditional and cell-free production methods reveal notable differences in environmental impact categories. Cell-free systems show reduced impacts in global warming potential, eutrophication, and resource depletion metrics. A recent comparative analysis demonstrated that cell-free protein synthesis could reduce greenhouse gas emissions by up to 35% for certain pharmaceutical products when implemented at commercial scale.
Waste stream characterization between the two approaches shows fundamental differences. Traditional methods generate complex biological waste requiring specialized treatment, while cell-free systems produce more chemically defined waste streams that may be easier to process or recycle. This difference translates to approximately 25-30% reduction in waste management costs and environmental burden.
Resource efficiency metrics further highlight cell-free advantages, with raw material utilization rates typically 15-25% higher than traditional methods. This efficiency stems from direct channeling of resources toward product formation rather than cellular maintenance and growth. The simplified supply chains associated with cell-free production also contribute to reduced transportation-related emissions and environmental impacts.
Future sustainability improvements for cell-free systems focus on developing renewable energy integration strategies, implementing circular economy principles for reagent recycling, and optimizing reaction conditions to minimize resource consumption. These advancements could potentially increase the sustainability gap between traditional and cell-free methods by an additional 20-30% over the next decade, positioning cell-free production as an environmentally preferable option for next-generation biopharmaceutical manufacturing.
In contrast, cell-free production systems demonstrate promising environmental advantages. These systems operate with reduced spatial requirements, consuming approximately 40-60% less water than traditional cell culture methods. Energy consumption analyses indicate potential reductions of 30-45% in overall energy requirements due to the elimination of cell maintenance processes and simplified purification steps. The absence of living cells also significantly reduces biohazard risks and associated containment measures.
Life cycle assessment (LCA) studies comparing traditional and cell-free production methods reveal notable differences in environmental impact categories. Cell-free systems show reduced impacts in global warming potential, eutrophication, and resource depletion metrics. A recent comparative analysis demonstrated that cell-free protein synthesis could reduce greenhouse gas emissions by up to 35% for certain pharmaceutical products when implemented at commercial scale.
Waste stream characterization between the two approaches shows fundamental differences. Traditional methods generate complex biological waste requiring specialized treatment, while cell-free systems produce more chemically defined waste streams that may be easier to process or recycle. This difference translates to approximately 25-30% reduction in waste management costs and environmental burden.
Resource efficiency metrics further highlight cell-free advantages, with raw material utilization rates typically 15-25% higher than traditional methods. This efficiency stems from direct channeling of resources toward product formation rather than cellular maintenance and growth. The simplified supply chains associated with cell-free production also contribute to reduced transportation-related emissions and environmental impacts.
Future sustainability improvements for cell-free systems focus on developing renewable energy integration strategies, implementing circular economy principles for reagent recycling, and optimizing reaction conditions to minimize resource consumption. These advancements could potentially increase the sustainability gap between traditional and cell-free methods by an additional 20-30% over the next decade, positioning cell-free production as an environmentally preferable option for next-generation biopharmaceutical manufacturing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!