Electrochemical Detection Of Hydrogen With Fast Response
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Sensing Technology Background and Objectives
Hydrogen sensing technology has evolved significantly over the past decades, driven by the growing importance of hydrogen as a clean energy carrier and the critical need for safety in hydrogen-based applications. The detection of hydrogen gas is paramount due to its flammability at concentrations between 4% and 75% in air, presenting significant safety hazards in various industrial and energy applications. Early detection systems emerged in the 1960s with basic catalytic sensors, followed by semiconductor-based technologies in the 1980s, and more recently, advanced electrochemical detection methods that offer improved sensitivity and response times.
The evolution of hydrogen sensing technology has been characterized by continuous improvements in detection limits, response times, and reliability. Traditional sensing methods often suffered from slow response rates, cross-sensitivity with other gases, and limited durability in harsh environments. These limitations have driven research toward electrochemical detection methods, which offer promising advantages in terms of response speed, selectivity, and operational stability.
Current technological trends in hydrogen sensing are focused on miniaturization, integration with IoT systems, and development of sensors capable of operating in extreme conditions. The push toward hydrogen economy has accelerated research in this field, with particular emphasis on sensors that can detect hydrogen quickly and accurately at concentrations well below the lower explosive limit of 4%.
The primary objective of electrochemical hydrogen detection technology is to achieve fast response times—ideally within seconds—while maintaining high sensitivity, selectivity, and stability. Fast response is critical in safety applications where early warning of hydrogen leaks can prevent potentially catastrophic incidents. Additionally, these sensors must operate reliably across a wide range of environmental conditions, including varying temperatures, pressures, and humidity levels.
Secondary objectives include reducing power consumption, extending sensor lifetime, minimizing maintenance requirements, and lowering production costs to enable widespread deployment. The development of self-calibrating and self-diagnosing capabilities represents another important goal, particularly for sensors deployed in remote or difficult-to-access locations.
The advancement of electrochemical hydrogen detection technology aligns with broader industry trends toward digitalization and automation, where real-time monitoring and rapid response systems are increasingly essential. As hydrogen continues to gain prominence in the global energy landscape, the demand for fast-response detection technologies is expected to grow substantially, driving further innovation and refinement in this critical field.
The evolution of hydrogen sensing technology has been characterized by continuous improvements in detection limits, response times, and reliability. Traditional sensing methods often suffered from slow response rates, cross-sensitivity with other gases, and limited durability in harsh environments. These limitations have driven research toward electrochemical detection methods, which offer promising advantages in terms of response speed, selectivity, and operational stability.
Current technological trends in hydrogen sensing are focused on miniaturization, integration with IoT systems, and development of sensors capable of operating in extreme conditions. The push toward hydrogen economy has accelerated research in this field, with particular emphasis on sensors that can detect hydrogen quickly and accurately at concentrations well below the lower explosive limit of 4%.
The primary objective of electrochemical hydrogen detection technology is to achieve fast response times—ideally within seconds—while maintaining high sensitivity, selectivity, and stability. Fast response is critical in safety applications where early warning of hydrogen leaks can prevent potentially catastrophic incidents. Additionally, these sensors must operate reliably across a wide range of environmental conditions, including varying temperatures, pressures, and humidity levels.
Secondary objectives include reducing power consumption, extending sensor lifetime, minimizing maintenance requirements, and lowering production costs to enable widespread deployment. The development of self-calibrating and self-diagnosing capabilities represents another important goal, particularly for sensors deployed in remote or difficult-to-access locations.
The advancement of electrochemical hydrogen detection technology aligns with broader industry trends toward digitalization and automation, where real-time monitoring and rapid response systems are increasingly essential. As hydrogen continues to gain prominence in the global energy landscape, the demand for fast-response detection technologies is expected to grow substantially, driving further innovation and refinement in this critical field.
Market Analysis for Rapid Hydrogen Detection Systems
The global market for rapid hydrogen detection systems is experiencing significant growth, driven by the expanding hydrogen economy and increasing safety concerns across various industries. Current market valuation stands at approximately 2.3 billion USD, with projections indicating a compound annual growth rate of 7.8% through 2030. This growth trajectory is primarily fueled by the accelerating adoption of hydrogen as a clean energy carrier and industrial feedstock.
The industrial sector currently represents the largest market segment, accounting for roughly 45% of total demand. Within this segment, petrochemical processing, semiconductor manufacturing, and power generation facilities are the primary consumers of electrochemical hydrogen detection technologies. These industries require fast-response detection systems to prevent catastrophic accidents and ensure operational safety.
The automotive and transportation sector is emerging as the fastest-growing market segment, with hydrogen fuel cell vehicles driving demand for reliable, rapid-response hydrogen sensors. As hydrogen refueling infrastructure expands globally, the need for leak detection systems with electrochemical sensors capable of sub-second response times has become increasingly critical.
Geographically, North America and Europe lead the market with approximately 35% and 30% market share respectively, attributed to stringent safety regulations and substantial investments in hydrogen infrastructure. The Asia-Pacific region, particularly China, Japan, and South Korea, is witnessing the highest growth rate due to aggressive government initiatives promoting hydrogen economies.
Market demand is increasingly focused on miniaturized, portable detection systems with enhanced sensitivity and faster response times. End-users are prioritizing solutions that can detect hydrogen concentrations as low as 10 ppm with response times under 1 second. This trend is driving innovation in electrochemical sensor technologies that combine rapid response with high accuracy and reliability.
Price sensitivity varies significantly across market segments. While industrial applications typically prioritize performance and reliability over cost, emerging applications in consumer markets and small-scale hydrogen installations are creating demand for more cost-effective solutions. The average price point for professional-grade electrochemical hydrogen detectors ranges from 1,500 to 4,000 USD, depending on specifications and capabilities.
Market barriers include high initial costs, technical challenges in achieving both speed and accuracy, and the need for regular calibration and maintenance. However, these challenges are being addressed through ongoing technological advancements in electrode materials, electrolyte formulations, and signal processing algorithms specifically designed for electrochemical hydrogen detection systems.
The industrial sector currently represents the largest market segment, accounting for roughly 45% of total demand. Within this segment, petrochemical processing, semiconductor manufacturing, and power generation facilities are the primary consumers of electrochemical hydrogen detection technologies. These industries require fast-response detection systems to prevent catastrophic accidents and ensure operational safety.
The automotive and transportation sector is emerging as the fastest-growing market segment, with hydrogen fuel cell vehicles driving demand for reliable, rapid-response hydrogen sensors. As hydrogen refueling infrastructure expands globally, the need for leak detection systems with electrochemical sensors capable of sub-second response times has become increasingly critical.
Geographically, North America and Europe lead the market with approximately 35% and 30% market share respectively, attributed to stringent safety regulations and substantial investments in hydrogen infrastructure. The Asia-Pacific region, particularly China, Japan, and South Korea, is witnessing the highest growth rate due to aggressive government initiatives promoting hydrogen economies.
Market demand is increasingly focused on miniaturized, portable detection systems with enhanced sensitivity and faster response times. End-users are prioritizing solutions that can detect hydrogen concentrations as low as 10 ppm with response times under 1 second. This trend is driving innovation in electrochemical sensor technologies that combine rapid response with high accuracy and reliability.
Price sensitivity varies significantly across market segments. While industrial applications typically prioritize performance and reliability over cost, emerging applications in consumer markets and small-scale hydrogen installations are creating demand for more cost-effective solutions. The average price point for professional-grade electrochemical hydrogen detectors ranges from 1,500 to 4,000 USD, depending on specifications and capabilities.
Market barriers include high initial costs, technical challenges in achieving both speed and accuracy, and the need for regular calibration and maintenance. However, these challenges are being addressed through ongoing technological advancements in electrode materials, electrolyte formulations, and signal processing algorithms specifically designed for electrochemical hydrogen detection systems.
Current Electrochemical H2 Sensor Limitations
Despite significant advancements in electrochemical hydrogen sensing technology, current systems face several critical limitations that hinder their widespread adoption in safety-critical applications. Response time remains a primary challenge, with many commercial sensors requiring 10-30 seconds to detect hydrogen concentrations reaching dangerous levels. This delay is particularly problematic in high-risk environments where rapid leak detection is essential for preventing catastrophic events.
Sensitivity issues persist across various electrochemical sensor designs, with detection limits typically ranging from 100-500 ppm, which falls short of requirements for early warning systems in certain applications. The ideal detection threshold for preventive safety measures should be below 100 ppm, a benchmark that many current sensors struggle to achieve consistently.
Cross-sensitivity represents another significant limitation, as most electrochemical hydrogen sensors respond to interfering gases such as carbon monoxide, hydrogen sulfide, and volatile organic compounds. This lack of selectivity leads to false positives and compromises reliability in complex gas environments commonly found in industrial settings.
Long-term stability remains problematic, with sensor drift occurring over time due to electrode poisoning, electrolyte degradation, and reference electrode instability. Many current sensors require recalibration every 3-6 months, which increases maintenance costs and creates potential safety gaps during calibration periods.
Power consumption presents challenges for portable and remote applications, with typical electrochemical hydrogen sensors drawing 50-200 mW during continuous operation. This energy requirement limits deployment in battery-powered devices and wireless sensor networks where energy efficiency is paramount.
Environmental robustness is another critical limitation, as sensor performance often degrades significantly under extreme temperature conditions (below 0°C or above 50°C), high humidity environments, or in the presence of particulate matter. These environmental factors can alter electrode kinetics and membrane permeability, affecting both response time and measurement accuracy.
Manufacturing scalability and cost efficiency remain obstacles to widespread adoption. Current high-performance electrochemical hydrogen sensors typically cost $100-500 per unit, with complex fabrication processes limiting mass production capabilities. This price point restricts deployment in consumer applications and large-scale monitoring networks where hundreds or thousands of sensors might be required.
Miniaturization efforts face challenges related to maintaining performance while reducing form factor, as smaller sensors often sacrifice sensitivity and stability. The integration of these sensors into compact devices or wearable safety equipment requires further technological breakthroughs to overcome these fundamental limitations.
Sensitivity issues persist across various electrochemical sensor designs, with detection limits typically ranging from 100-500 ppm, which falls short of requirements for early warning systems in certain applications. The ideal detection threshold for preventive safety measures should be below 100 ppm, a benchmark that many current sensors struggle to achieve consistently.
Cross-sensitivity represents another significant limitation, as most electrochemical hydrogen sensors respond to interfering gases such as carbon monoxide, hydrogen sulfide, and volatile organic compounds. This lack of selectivity leads to false positives and compromises reliability in complex gas environments commonly found in industrial settings.
Long-term stability remains problematic, with sensor drift occurring over time due to electrode poisoning, electrolyte degradation, and reference electrode instability. Many current sensors require recalibration every 3-6 months, which increases maintenance costs and creates potential safety gaps during calibration periods.
Power consumption presents challenges for portable and remote applications, with typical electrochemical hydrogen sensors drawing 50-200 mW during continuous operation. This energy requirement limits deployment in battery-powered devices and wireless sensor networks where energy efficiency is paramount.
Environmental robustness is another critical limitation, as sensor performance often degrades significantly under extreme temperature conditions (below 0°C or above 50°C), high humidity environments, or in the presence of particulate matter. These environmental factors can alter electrode kinetics and membrane permeability, affecting both response time and measurement accuracy.
Manufacturing scalability and cost efficiency remain obstacles to widespread adoption. Current high-performance electrochemical hydrogen sensors typically cost $100-500 per unit, with complex fabrication processes limiting mass production capabilities. This price point restricts deployment in consumer applications and large-scale monitoring networks where hundreds or thousands of sensors might be required.
Miniaturization efforts face challenges related to maintaining performance while reducing form factor, as smaller sensors often sacrifice sensitivity and stability. The integration of these sensors into compact devices or wearable safety equipment requires further technological breakthroughs to overcome these fundamental limitations.
Fast-Response Electrochemical Detection Methods
01 Electrode materials for improved response time
The choice of electrode materials significantly impacts the response time of electrochemical hydrogen sensors. Materials such as palladium, platinum, and their alloys are commonly used due to their high catalytic activity and hydrogen absorption properties. Nanostructured electrodes and composite materials can further enhance response time by increasing the surface area and improving electron transfer kinetics. These advanced materials enable faster hydrogen detection and more reliable sensor performance in various applications.- Electrode materials for improved response time: Various electrode materials can significantly impact the response time of electrochemical hydrogen sensors. Materials such as palladium, platinum, and their alloys are commonly used due to their catalytic properties and hydrogen affinity. Nanostructured electrodes with high surface area can further enhance response time by providing more active sites for hydrogen detection. Composite materials combining metals with carbon-based materials have also shown improved response characteristics.
- Sensor design and architecture optimization: The physical design and architecture of electrochemical hydrogen sensors play a crucial role in determining response time. Miniaturized sensor designs with reduced diffusion paths allow faster gas access to sensing elements. Thin-film technologies enable the fabrication of sensors with minimal electrode spacing and optimized electrolyte layers. Microelectromechanical systems (MEMS) approaches have been developed to create highly responsive sensor platforms with integrated temperature control and signal processing capabilities.
- Electrolyte composition and properties: The composition and properties of the electrolyte significantly affect hydrogen sensor response time. Solid polymer electrolytes offer advantages over liquid electrolytes by providing stable ionic conductivity while eliminating leakage concerns. Proton-conducting materials with high ionic conductivity facilitate faster electrochemical reactions. Modified electrolytes with additives can enhance proton transfer rates and improve overall sensor performance under various environmental conditions.
- Signal processing and measurement techniques: Advanced signal processing and measurement techniques can effectively reduce the apparent response time of electrochemical hydrogen sensors. Algorithms that analyze the initial rate of signal change can predict final values before equilibrium is reached. Digital filtering techniques help eliminate noise while preserving rapid response characteristics. Impedance-based measurement approaches provide faster detection compared to traditional amperometric methods by analyzing electrochemical interface properties in real-time.
- Environmental factors and operating conditions: Environmental factors and operating conditions significantly impact the response time of electrochemical hydrogen sensors. Temperature compensation mechanisms are essential as reaction kinetics are highly temperature-dependent. Humidity control systems prevent water condensation that can block gas diffusion pathways. Pressure fluctuations can affect diffusion rates and must be accounted for in high-precision applications. Specialized designs for extreme environments incorporate protective membranes and filters that maintain fast response while protecting sensitive components.
02 Sensor design and architecture for rapid detection
The physical design and architecture of electrochemical hydrogen sensors play a crucial role in determining response time. Miniaturized sensor designs with optimized electrode spacing, thin-film configurations, and microfluidic channels can significantly reduce diffusion distances and enhance mass transport. Three-electrode systems with reference, working, and counter electrodes properly arranged can minimize electrical resistance and improve signal stability. These design considerations enable faster hydrogen detection and more reliable performance in safety-critical applications.Expand Specific Solutions03 Electrolyte composition and optimization
The composition and properties of the electrolyte significantly affect the response time of electrochemical hydrogen sensors. Solid polymer electrolytes, ionic liquids, and proton-conducting membranes can facilitate faster ion transport compared to traditional liquid electrolytes. Optimizing electrolyte concentration, pH, and conductivity can reduce internal resistance and improve sensor kinetics. These electrolyte innovations enable more rapid hydrogen detection while maintaining sensitivity and selectivity across varying environmental conditions.Expand Specific Solutions04 Temperature control and environmental compensation
Temperature significantly affects the response time of electrochemical hydrogen sensors. Implementing temperature control systems or compensation algorithms can maintain optimal operating conditions and ensure consistent response times. Some advanced sensors incorporate heating elements to maintain stable temperatures or use temperature sensors for real-time data correction. Environmental factors such as humidity and pressure can also be compensated for through integrated sensing elements and calibration algorithms, ensuring reliable performance across varying conditions.Expand Specific Solutions05 Signal processing and data analysis techniques
Advanced signal processing and data analysis techniques can effectively improve the apparent response time of electrochemical hydrogen sensors. Digital filtering, signal amplification, and noise reduction algorithms can enhance signal quality and allow for earlier detection of hydrogen. Predictive algorithms based on the initial rate of signal change can estimate final values before steady-state is reached. Machine learning approaches can adapt to sensor characteristics over time and compensate for aging effects, maintaining optimal response times throughout the sensor lifecycle.Expand Specific Solutions
Leading Companies in Electrochemical Sensor Industry
The hydrogen electrochemical detection market is in a growth phase, characterized by increasing demand for fast-response sensors across industrial safety, automotive, and energy sectors. The global market is expanding rapidly due to hydrogen's growing role in clean energy transitions. Technologically, the field shows varying maturity levels, with established players like Fraunhofer-Gesellschaft, Panasonic, and Honda Motor developing commercial solutions, while research institutions such as Tohoku University, Delft University of Technology, and China Petroleum & Chemical Corp are advancing next-generation technologies. Companies like Alps Alpine and Aerodyne Research are focusing on miniaturization and integration capabilities, while energy giants like Sinopec are investing in safety-oriented detection systems, creating a competitive landscape balanced between industrial incumbents and specialized technology providers.
Aerodyne Research, Inc.
Technical Solution: Aerodyne Research has developed advanced electrochemical hydrogen sensors utilizing nanostructured palladium-based materials deposited on microelectrode arrays. Their technology employs a proprietary three-electrode configuration with specialized catalytic working electrodes that facilitate rapid hydrogen adsorption and electron transfer. The system incorporates a microfluidic sampling interface that directs gas flow directly to sensing elements, minimizing diffusion barriers. Signal processing algorithms compensate for environmental interferences, allowing detection in complex gas mixtures. Their sensors achieve response times under 2 seconds for concentrations as low as 10 ppm, with a wide dynamic range extending to 4% hydrogen concentration. The technology includes temperature compensation mechanisms and integrated reference electrodes to maintain calibration stability over extended periods.
Strengths: Exceptional response speed (<2 seconds) with high sensitivity across wide concentration ranges. Advanced signal processing enables reliable operation in complex gas mixtures with minimal false positives. Weaknesses: Higher manufacturing costs compared to conventional sensors due to specialized materials and fabrication processes. Requires periodic recalibration in harsh industrial environments.
Honda Motor Co., Ltd.
Technical Solution: Honda has developed a proprietary electrochemical hydrogen detection system specifically designed for automotive safety applications. Their technology employs a multi-layered sensor architecture featuring platinum-doped titanium dioxide thin films deposited on interdigitated gold electrodes. The sensing mechanism utilizes both amperometric and potentiometric measurements simultaneously, with a microcontroller implementing adaptive algorithms that adjust sensitivity based on environmental conditions. Honda's sensors incorporate a hydrophobic membrane that allows hydrogen permeation while blocking water and larger molecules, enabling reliable operation in high-humidity environments. The system achieves response times of approximately 0.8 seconds for hydrogen concentrations above 100 ppm, with a detection limit of 10 ppm. Their technology includes self-diagnostic capabilities that continuously monitor sensor health and performance, triggering alerts when recalibration is needed. Honda has integrated these sensors into their hydrogen fuel cell vehicles, positioning them strategically around hydrogen storage systems and supply lines.
Strengths: Extremely fast response time (<1 second) with robust performance in automotive environments subject to temperature fluctuations and vibration. Comprehensive self-diagnostic capabilities ensure reliable operation with minimal maintenance. Weaknesses: Higher production costs compared to conventional sensors. Requires periodic replacement of sensing elements after extended exposure to certain contaminants found in automotive environments.
Key Patents in Rapid H2 Sensing Technology
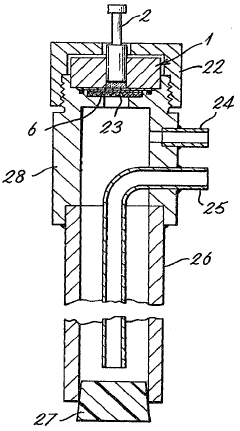
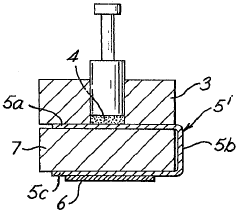
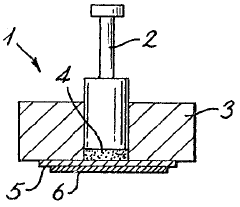
Electrochemical sensor for hydrogen
PatentInactiveCA1288476C
Innovation
- An electrochemical sensor comprising an electronically conductive component for hydrogen dissociation, a solid-state hydrogen cation electrolyte, and a redox reference entity with a mixture of oxidation states, such as Pb(II)/Pb(IV), integrated with a hydrogen-ion conductor like Nafion, protected by a hydrogen-permeable membrane to prevent interference from other gases, allowing accurate voltage measurement.
H2O doped WO3, ultra-fast, high-sensitivity hydrogen sensors
PatentInactiveUS7910373B2
Innovation
- In-situ water doping of tungsten oxide (WO3) thin films during thermal evaporation on a glass substrate, combined with palladium, enhances hydrogen diffusion and charge transfer rates, resulting in faster and more stable hydrogen detection.
Safety Standards for Hydrogen Detection Systems
Safety standards for hydrogen detection systems have evolved significantly to address the unique challenges posed by hydrogen's properties. International standards such as ISO 26142 specifically establish performance requirements for hydrogen detection apparatus designed for stationary applications. These standards mandate that detection systems must respond within seconds to hydrogen concentrations reaching 25% of the lower flammability limit (LFL), which is approximately 1% hydrogen in air. For electrochemical sensors with fast response capabilities, compliance with IEC 60079 series standards is essential, particularly for deployment in hazardous environments where explosive atmospheres may form.
The National Fire Protection Association (NFPA) has developed NFPA 2, which provides comprehensive guidelines for hydrogen technologies and emphasizes detection system requirements. This standard specifies that detection systems must include automatic shutdown mechanisms and alarm systems that activate when hydrogen concentrations exceed predetermined thresholds. Additionally, UL 2075 certification is required for hydrogen gas detection equipment in North America, ensuring devices meet rigorous safety and performance criteria.
European standards, particularly those outlined in the ATEX Directive (2014/34/EU), impose strict requirements on equipment used in potentially explosive atmospheres. For electrochemical hydrogen sensors, this means demonstrating intrinsic safety and reliability under various environmental conditions. The IEC 61779 standard specifically addresses test methods and performance requirements for combustible gas detectors, including those utilizing electrochemical detection principles.
Recent updates to safety standards have increasingly focused on the response time of detection systems. The International Electrotechnical Commission (IEC) now recommends that hydrogen sensors deployed in safety-critical applications should achieve T90 response times (time to reach 90% of final signal) of less than 30 seconds. This requirement is particularly relevant for electrochemical sensors, which must balance rapid response with accuracy and long-term stability.
Calibration and maintenance protocols are also strictly regulated. Standards require regular verification of sensor performance, with calibration intervals typically ranging from 3 to 12 months depending on the application environment. For electrochemical hydrogen sensors, which may experience sensitivity drift over time, more frequent calibration may be necessary to maintain compliance with safety standards.
Cross-sensitivity testing has become a mandatory component of certification processes, as electrochemical sensors may respond to gases other than hydrogen. Standards now require manufacturers to document potential interferents and quantify their impact on measurement accuracy. This is particularly important in industrial settings where multiple gases may be present simultaneously.
The National Fire Protection Association (NFPA) has developed NFPA 2, which provides comprehensive guidelines for hydrogen technologies and emphasizes detection system requirements. This standard specifies that detection systems must include automatic shutdown mechanisms and alarm systems that activate when hydrogen concentrations exceed predetermined thresholds. Additionally, UL 2075 certification is required for hydrogen gas detection equipment in North America, ensuring devices meet rigorous safety and performance criteria.
European standards, particularly those outlined in the ATEX Directive (2014/34/EU), impose strict requirements on equipment used in potentially explosive atmospheres. For electrochemical hydrogen sensors, this means demonstrating intrinsic safety and reliability under various environmental conditions. The IEC 61779 standard specifically addresses test methods and performance requirements for combustible gas detectors, including those utilizing electrochemical detection principles.
Recent updates to safety standards have increasingly focused on the response time of detection systems. The International Electrotechnical Commission (IEC) now recommends that hydrogen sensors deployed in safety-critical applications should achieve T90 response times (time to reach 90% of final signal) of less than 30 seconds. This requirement is particularly relevant for electrochemical sensors, which must balance rapid response with accuracy and long-term stability.
Calibration and maintenance protocols are also strictly regulated. Standards require regular verification of sensor performance, with calibration intervals typically ranging from 3 to 12 months depending on the application environment. For electrochemical hydrogen sensors, which may experience sensitivity drift over time, more frequent calibration may be necessary to maintain compliance with safety standards.
Cross-sensitivity testing has become a mandatory component of certification processes, as electrochemical sensors may respond to gases other than hydrogen. Standards now require manufacturers to document potential interferents and quantify their impact on measurement accuracy. This is particularly important in industrial settings where multiple gases may be present simultaneously.
Material Advancements for Enhanced Sensor Performance
Recent advancements in material science have significantly enhanced the performance of electrochemical hydrogen sensors, particularly in achieving faster response times. Palladium-based materials continue to dominate this field due to their exceptional hydrogen absorption properties. However, innovative modifications to traditional palladium structures have yielded remarkable improvements in sensor responsiveness. Nanostructured palladium, including nanoparticles, nanowires, and nanoporous architectures, demonstrates substantially reduced response times compared to bulk materials, primarily due to increased surface-to-volume ratios and shortened hydrogen diffusion paths.
Palladium alloys represent another frontier in material development, with Pd-Au, Pd-Ag, and Pd-Ni combinations showing enhanced stability and faster kinetics. These alloys strategically modify the electronic structure of palladium, optimizing hydrogen adsorption and desorption processes while mitigating common issues like hydrogen embrittlement that plague pure palladium sensors.
Carbon-based materials have emerged as promising candidates for next-generation hydrogen sensors. Graphene, carbon nanotubes, and their functionalized derivatives exhibit exceptional electrical properties and high surface areas. When decorated with palladium nanoparticles, these carbon nanomaterials demonstrate synergistic effects that dramatically improve response times, sometimes achieving detection in milliseconds rather than seconds.
Metal-organic frameworks (MOFs) represent an exciting development in hydrogen sensing materials. Their highly ordered porous structures can be precisely engineered to facilitate rapid hydrogen diffusion while maintaining selectivity. Palladium-incorporated MOFs combine the benefits of traditional metal catalysts with the advantages of tailored porosity, resulting in sensors with both rapid response and high sensitivity.
Conducting polymers such as polyaniline and polypyrrole have been successfully employed as hydrogen sensing materials, particularly when combined with metal nanoparticles. These composite materials offer flexibility, low-cost production, and room-temperature operation while maintaining competitive response times. Their adaptability to various fabrication techniques makes them particularly attractive for commercial applications.
Recent research has also focused on two-dimensional materials beyond graphene, including transition metal dichalcogenides (TMDs) and MXenes. These materials offer unique electronic properties and abundant active sites for hydrogen interaction. When optimized through defect engineering or heterostructure formation, these 2D materials demonstrate promising response characteristics for hydrogen detection applications.
Palladium alloys represent another frontier in material development, with Pd-Au, Pd-Ag, and Pd-Ni combinations showing enhanced stability and faster kinetics. These alloys strategically modify the electronic structure of palladium, optimizing hydrogen adsorption and desorption processes while mitigating common issues like hydrogen embrittlement that plague pure palladium sensors.
Carbon-based materials have emerged as promising candidates for next-generation hydrogen sensors. Graphene, carbon nanotubes, and their functionalized derivatives exhibit exceptional electrical properties and high surface areas. When decorated with palladium nanoparticles, these carbon nanomaterials demonstrate synergistic effects that dramatically improve response times, sometimes achieving detection in milliseconds rather than seconds.
Metal-organic frameworks (MOFs) represent an exciting development in hydrogen sensing materials. Their highly ordered porous structures can be precisely engineered to facilitate rapid hydrogen diffusion while maintaining selectivity. Palladium-incorporated MOFs combine the benefits of traditional metal catalysts with the advantages of tailored porosity, resulting in sensors with both rapid response and high sensitivity.
Conducting polymers such as polyaniline and polypyrrole have been successfully employed as hydrogen sensing materials, particularly when combined with metal nanoparticles. These composite materials offer flexibility, low-cost production, and room-temperature operation while maintaining competitive response times. Their adaptability to various fabrication techniques makes them particularly attractive for commercial applications.
Recent research has also focused on two-dimensional materials beyond graphene, including transition metal dichalcogenides (TMDs) and MXenes. These materials offer unique electronic properties and abundant active sites for hydrogen interaction. When optimized through defect engineering or heterostructure formation, these 2D materials demonstrate promising response characteristics for hydrogen detection applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!