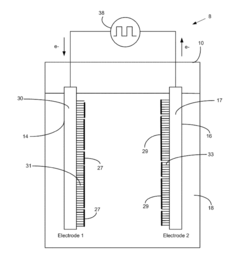
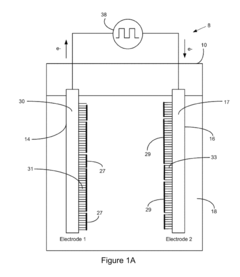
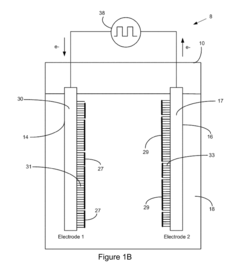
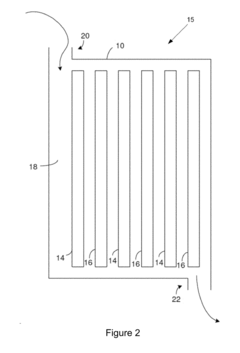
Electrode Kinetics in Wastewater Nanofiltration Process Design
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanofiltration Electrode Kinetics Background and Objectives
Nanofiltration technology has evolved significantly over the past three decades, transitioning from a niche separation technique to a mainstream water treatment process. The integration of electrode kinetics into nanofiltration represents a critical advancement in wastewater treatment methodologies. Initially developed in the 1980s as a low-pressure alternative to reverse osmosis, nanofiltration has progressively incorporated electrochemical principles to enhance separation efficiency and reduce membrane fouling.
The evolution of electrode kinetics in nanofiltration processes has been marked by several key developments. Early systems relied primarily on pressure-driven mechanisms, with limited consideration for electrochemical interactions. By the early 2000s, researchers began exploring the influence of surface charge and electrical double layer effects on membrane performance. The past decade has witnessed accelerated innovation in this field, with the introduction of electrically conductive membranes and advanced electrode materials that actively participate in the filtration process.
Current technological trends indicate a growing convergence between nanofiltration and electrochemical processes. This integration aims to address persistent challenges in wastewater treatment, including the removal of recalcitrant contaminants, energy efficiency concerns, and membrane longevity issues. The application of controlled electrical potentials across nanofiltration membranes has demonstrated promising results in enhancing selectivity for specific ionic species and reducing concentration polarization phenomena.
The primary objective of this technical investigation is to comprehensively evaluate the fundamental principles governing electrode kinetics in wastewater nanofiltration processes. This includes examining electron transfer mechanisms at membrane-solution interfaces, quantifying the impact of electrical parameters on separation performance, and identifying optimal operational conditions for various wastewater compositions.
Additionally, this research aims to establish correlations between electrode material properties and filtration efficiency, with particular emphasis on novel nanomaterials such as graphene derivatives, carbon nanotubes, and conductive polymers. Understanding these relationships is essential for designing next-generation nanofiltration systems with enhanced selectivity, reduced energy consumption, and improved resistance to fouling.
The ultimate goal is to develop a theoretical framework that enables predictive modeling of electrochemically-enhanced nanofiltration processes. Such a framework would facilitate the rational design of treatment systems tailored to specific wastewater characteristics, supporting the broader objective of advancing sustainable water management practices in industrial and municipal applications.
The evolution of electrode kinetics in nanofiltration processes has been marked by several key developments. Early systems relied primarily on pressure-driven mechanisms, with limited consideration for electrochemical interactions. By the early 2000s, researchers began exploring the influence of surface charge and electrical double layer effects on membrane performance. The past decade has witnessed accelerated innovation in this field, with the introduction of electrically conductive membranes and advanced electrode materials that actively participate in the filtration process.
Current technological trends indicate a growing convergence between nanofiltration and electrochemical processes. This integration aims to address persistent challenges in wastewater treatment, including the removal of recalcitrant contaminants, energy efficiency concerns, and membrane longevity issues. The application of controlled electrical potentials across nanofiltration membranes has demonstrated promising results in enhancing selectivity for specific ionic species and reducing concentration polarization phenomena.
The primary objective of this technical investigation is to comprehensively evaluate the fundamental principles governing electrode kinetics in wastewater nanofiltration processes. This includes examining electron transfer mechanisms at membrane-solution interfaces, quantifying the impact of electrical parameters on separation performance, and identifying optimal operational conditions for various wastewater compositions.
Additionally, this research aims to establish correlations between electrode material properties and filtration efficiency, with particular emphasis on novel nanomaterials such as graphene derivatives, carbon nanotubes, and conductive polymers. Understanding these relationships is essential for designing next-generation nanofiltration systems with enhanced selectivity, reduced energy consumption, and improved resistance to fouling.
The ultimate goal is to develop a theoretical framework that enables predictive modeling of electrochemically-enhanced nanofiltration processes. Such a framework would facilitate the rational design of treatment systems tailored to specific wastewater characteristics, supporting the broader objective of advancing sustainable water management practices in industrial and municipal applications.
Market Analysis for Advanced Wastewater Treatment Technologies
The global market for advanced wastewater treatment technologies is experiencing robust growth, driven by increasing water scarcity, stringent environmental regulations, and growing industrial activities. The market for electrode kinetics-based nanofiltration processes specifically is projected to reach $6.5 billion by 2027, growing at a CAGR of 7.2% from 2022 to 2027.
Water treatment technologies incorporating electrode kinetics in nanofiltration processes are gaining significant traction across multiple sectors. Municipal water treatment remains the largest application segment, accounting for approximately 45% of the market share, followed by industrial wastewater treatment at 35%. Within industrial applications, chemical manufacturing, pharmaceuticals, and food & beverage industries are the primary adopters of these advanced filtration technologies.
Regionally, North America and Europe currently dominate the market due to strict regulatory frameworks and higher investment capabilities. However, the Asia-Pacific region is emerging as the fastest-growing market with a projected growth rate of 9.5% annually, primarily driven by rapid industrialization in China and India, alongside increasing government initiatives for clean water access.
The demand for electrode kinetics-enhanced nanofiltration is particularly strong in regions facing severe water stress. Middle Eastern countries are increasingly investing in these technologies to address their chronic water scarcity issues, creating a specialized high-value market segment with unique technical requirements focused on energy efficiency.
Market penetration analysis reveals that while conventional membrane technologies still dominate the overall wastewater treatment landscape, electrode-assisted nanofiltration processes are rapidly gaining market share due to their superior performance in removing emerging contaminants such as pharmaceutical residues, microplastics, and industrial chemicals that traditional methods struggle to eliminate.
Customer segmentation shows distinct requirements across different sectors. Municipal utilities prioritize scalability and operational cost efficiency, while industrial clients focus on customization capabilities for specific contaminant profiles and integration with existing treatment trains. The healthcare sector represents a growing niche market with stringent purity requirements and willingness to pay premium prices for advanced solutions.
The economic analysis of electrode kinetics in nanofiltration reveals favorable total cost of ownership metrics compared to conventional technologies when factoring in operational lifespan, energy consumption, and treatment efficacy. The initial capital investment remains 15-20% higher than traditional methods, but operational costs over a 10-year period demonstrate 30% savings, creating a compelling value proposition for long-term infrastructure planning.
Water treatment technologies incorporating electrode kinetics in nanofiltration processes are gaining significant traction across multiple sectors. Municipal water treatment remains the largest application segment, accounting for approximately 45% of the market share, followed by industrial wastewater treatment at 35%. Within industrial applications, chemical manufacturing, pharmaceuticals, and food & beverage industries are the primary adopters of these advanced filtration technologies.
Regionally, North America and Europe currently dominate the market due to strict regulatory frameworks and higher investment capabilities. However, the Asia-Pacific region is emerging as the fastest-growing market with a projected growth rate of 9.5% annually, primarily driven by rapid industrialization in China and India, alongside increasing government initiatives for clean water access.
The demand for electrode kinetics-enhanced nanofiltration is particularly strong in regions facing severe water stress. Middle Eastern countries are increasingly investing in these technologies to address their chronic water scarcity issues, creating a specialized high-value market segment with unique technical requirements focused on energy efficiency.
Market penetration analysis reveals that while conventional membrane technologies still dominate the overall wastewater treatment landscape, electrode-assisted nanofiltration processes are rapidly gaining market share due to their superior performance in removing emerging contaminants such as pharmaceutical residues, microplastics, and industrial chemicals that traditional methods struggle to eliminate.
Customer segmentation shows distinct requirements across different sectors. Municipal utilities prioritize scalability and operational cost efficiency, while industrial clients focus on customization capabilities for specific contaminant profiles and integration with existing treatment trains. The healthcare sector represents a growing niche market with stringent purity requirements and willingness to pay premium prices for advanced solutions.
The economic analysis of electrode kinetics in nanofiltration reveals favorable total cost of ownership metrics compared to conventional technologies when factoring in operational lifespan, energy consumption, and treatment efficacy. The initial capital investment remains 15-20% higher than traditional methods, but operational costs over a 10-year period demonstrate 30% savings, creating a compelling value proposition for long-term infrastructure planning.
Current Challenges in Electrode-Assisted Nanofiltration
Despite significant advancements in electrode-assisted nanofiltration technologies for wastewater treatment, several critical challenges continue to impede optimal implementation and performance. The complex interplay between electrode materials and wastewater constituents presents substantial hurdles in achieving consistent kinetic behavior across varying operational conditions.
One primary challenge involves electrode fouling and degradation during extended operation periods. Organic compounds, mineral scaling, and biofilm formation on electrode surfaces significantly reduce electrochemical efficiency and necessitate frequent maintenance interventions. Studies indicate that even specialized anti-fouling coatings demonstrate limited longevity in complex wastewater matrices containing multiple contaminant classes.
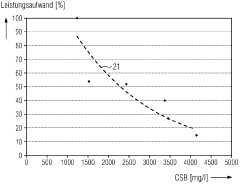
Energy consumption remains a persistent obstacle in electrode-assisted nanofiltration systems. Current designs require substantial electrical input to maintain effective separation processes, with energy requirements typically ranging from 1.5-4.0 kWh per cubic meter of treated wastewater. This energy intensity undermines the economic viability of these systems, particularly for large-scale municipal applications.
The optimization of electrode kinetics across varying pH conditions presents another significant challenge. Most electrode materials exhibit optimal performance within narrow pH ranges, yet wastewater streams frequently experience substantial pH fluctuations. This variability necessitates complex control systems or compromised operational parameters that reduce overall treatment efficiency.
Selective removal of contaminants remains problematic in current electrode-assisted nanofiltration systems. While these technologies demonstrate effectiveness for certain contaminant classes, they often struggle with mixed-contaminant scenarios typical in real-world wastewater streams. Particularly challenging are emerging contaminants like pharmaceutical compounds and per- and polyfluoroalkyl substances (PFAS), which require specialized electrode configurations not yet optimized for commercial deployment.
Scale-up challenges further complicate industrial implementation. Laboratory-scale systems demonstrating promising electrode kinetics frequently encounter performance degradation when scaled to industrial capacities. This scaling disconnect stems from altered fluid dynamics, increased electrode surface-to-volume ratios, and heterogeneous current distribution across larger electrode surfaces.
Material limitations also constrain advancement in this field. Current electrode materials face trade-offs between conductivity, durability, selectivity, and cost. Noble metal electrodes offer superior performance but at prohibitive costs, while more economical alternatives typically sacrifice longevity or treatment efficiency. Recent research into advanced carbon-based electrodes and metal oxide composites shows promise but remains in early development stages.
One primary challenge involves electrode fouling and degradation during extended operation periods. Organic compounds, mineral scaling, and biofilm formation on electrode surfaces significantly reduce electrochemical efficiency and necessitate frequent maintenance interventions. Studies indicate that even specialized anti-fouling coatings demonstrate limited longevity in complex wastewater matrices containing multiple contaminant classes.
Energy consumption remains a persistent obstacle in electrode-assisted nanofiltration systems. Current designs require substantial electrical input to maintain effective separation processes, with energy requirements typically ranging from 1.5-4.0 kWh per cubic meter of treated wastewater. This energy intensity undermines the economic viability of these systems, particularly for large-scale municipal applications.
The optimization of electrode kinetics across varying pH conditions presents another significant challenge. Most electrode materials exhibit optimal performance within narrow pH ranges, yet wastewater streams frequently experience substantial pH fluctuations. This variability necessitates complex control systems or compromised operational parameters that reduce overall treatment efficiency.
Selective removal of contaminants remains problematic in current electrode-assisted nanofiltration systems. While these technologies demonstrate effectiveness for certain contaminant classes, they often struggle with mixed-contaminant scenarios typical in real-world wastewater streams. Particularly challenging are emerging contaminants like pharmaceutical compounds and per- and polyfluoroalkyl substances (PFAS), which require specialized electrode configurations not yet optimized for commercial deployment.
Scale-up challenges further complicate industrial implementation. Laboratory-scale systems demonstrating promising electrode kinetics frequently encounter performance degradation when scaled to industrial capacities. This scaling disconnect stems from altered fluid dynamics, increased electrode surface-to-volume ratios, and heterogeneous current distribution across larger electrode surfaces.
Material limitations also constrain advancement in this field. Current electrode materials face trade-offs between conductivity, durability, selectivity, and cost. Noble metal electrodes offer superior performance but at prohibitive costs, while more economical alternatives typically sacrifice longevity or treatment efficiency. Recent research into advanced carbon-based electrodes and metal oxide composites shows promise but remains in early development stages.
Current Electrode Design Solutions for Wastewater Treatment
01 Electrode kinetics in electrochemical systems
Electrode kinetics plays a crucial role in electrochemical systems, focusing on the rate at which electron transfer occurs at electrode surfaces. These studies examine factors affecting reaction rates such as electrode material, surface conditions, and electrolyte composition. Understanding these kinetics is essential for optimizing electrochemical processes, improving efficiency, and enhancing performance in applications like batteries, fuel cells, and sensors.- Electrode kinetics in electrochemical systems: Electrode kinetics plays a crucial role in electrochemical systems, focusing on the rate of electron transfer at electrode surfaces. These studies examine factors affecting reaction rates, including electrode material, surface conditions, electrolyte composition, and applied potential. Understanding these kinetics is essential for optimizing electrochemical processes, improving efficiency, and developing advanced electrode materials for applications in energy storage, sensors, and catalysis.
- Measurement and analysis techniques for electrode kinetics: Various techniques are employed to measure and analyze electrode kinetics, including electrochemical impedance spectroscopy, cyclic voltammetry, and chronoamperometry. These methods provide insights into reaction mechanisms, rate constants, and mass transport phenomena. Advanced computational models and algorithms are used to interpret experimental data, enabling researchers to determine kinetic parameters and optimize electrode performance for specific applications.
- Electrode kinetics in fuel cells and energy storage devices: Electrode kinetics significantly impacts the performance of fuel cells, batteries, and other energy storage devices. Research focuses on enhancing reaction rates at electrodes to improve power density, energy efficiency, and device longevity. This includes developing novel catalyst materials, optimizing electrode structures, and understanding degradation mechanisms. Improved electrode kinetics leads to faster charging capabilities, higher power output, and more efficient energy conversion processes.
- Temperature effects on electrode kinetics: Temperature significantly influences electrode kinetics by affecting reaction rates, mass transport, and electrode material properties. Studies examine how temperature variations impact electron transfer processes, diffusion rates, and activation energies. Temperature control systems are developed to optimize electrochemical reactions under various operating conditions. Understanding these temperature dependencies is crucial for designing electrochemical systems that can operate efficiently across different thermal environments.
- Microfluidic and nanoscale approaches to electrode kinetics: Microfluidic and nanoscale technologies offer new approaches to studying and enhancing electrode kinetics. These include miniaturized electrochemical cells, nanostructured electrode materials, and precise control of local reaction environments. Such approaches enable high-throughput screening of electrode materials, in-situ monitoring of electrochemical processes, and fundamental studies of electron transfer at the nanoscale. These advanced techniques provide deeper insights into electrode kinetics and facilitate the development of more efficient electrochemical systems.
02 Measurement techniques for electrode kinetics
Various measurement techniques are employed to study electrode kinetics, including cyclic voltammetry, impedance spectroscopy, and chronoamperometry. These methods allow researchers to determine kinetic parameters such as rate constants, transfer coefficients, and diffusion coefficients. Advanced instrumentation and analytical approaches enable precise characterization of electrode processes, providing valuable insights into reaction mechanisms and kinetic limitations.Expand Specific Solutions03 Electrode materials and surface modifications for enhanced kinetics
The development of novel electrode materials and surface modifications aims to enhance electrode kinetics. Strategies include using catalytic materials, nanostructured surfaces, and composite electrodes to increase active surface area and reduce activation energy barriers. These approaches can significantly improve reaction rates, reduce overpotentials, and enhance overall system performance in various electrochemical applications.Expand Specific Solutions04 Computational modeling of electrode kinetics
Computational modeling approaches are increasingly used to understand and predict electrode kinetics. These models incorporate quantum mechanical calculations, molecular dynamics simulations, and continuum models to describe electron transfer processes at electrode interfaces. Such computational tools help researchers design better electrodes, optimize reaction conditions, and develop more efficient electrochemical systems without extensive experimental testing.Expand Specific Solutions05 Applications of electrode kinetics in energy storage and conversion
Electrode kinetics research has significant applications in energy storage and conversion technologies. Understanding and optimizing electrode kinetics is crucial for developing high-performance batteries, fuel cells, electrolyzers, and supercapacitors. By enhancing reaction rates and reducing energy barriers at electrodes, researchers can improve device efficiency, power density, cycling stability, and overall performance in renewable energy systems.Expand Specific Solutions
Leading Companies and Research Institutions in Nanofiltration
Electrode kinetics in wastewater nanofiltration is currently in a transitional phase from research to commercial application, with a global market expected to reach $2.5 billion by 2025. The technology maturity varies significantly across players, with academic institutions like Tongji University, Tsinghua University, and California Institute of Technology focusing on fundamental research, while industrial leaders such as Siemens AG, Cambrian Innovation, and De Nora Permelec are advancing practical applications. Companies like HydrogenPro ASA and Edwards Vacuum are developing specialized components, while Axine Water Technologies has pioneered electrolytic oxidation solutions. The competitive landscape shows a collaborative ecosystem between research institutions and industrial partners, with increasing focus on energy efficiency and process optimization.
Siemens AG
Technical Solution: Siemens has developed an integrated electrochemical-nanofiltration system for industrial wastewater treatment that focuses on optimizing electrode kinetics. Their technology utilizes advanced boron-doped diamond (BDD) electrodes with exceptional electrochemical properties, providing wide potential windows and low background currents that enhance reaction kinetics for difficult-to-treat contaminants. Siemens' system incorporates sophisticated flow dynamics engineering that minimizes mass transfer limitations at electrode surfaces, a common bottleneck in electrochemical treatment processes. Their proprietary electrode configuration features optimized inter-electrode spacing and specialized flow distribution channels that ensure uniform current distribution and maximize treatment efficiency. The technology includes an intelligent control system that continuously monitors and adjusts operational parameters including current density, pH, and flow rates to maintain optimal electrode kinetics under varying wastewater compositions[5]. Siemens has integrated this electrochemical system with advanced nanofiltration membranes in a complementary process where electrooxidation breaks down complex organic compounds that would otherwise foul membranes, while nanofiltration removes dissolved solids and smaller molecular weight compounds. This synergistic approach has been successfully implemented in pharmaceutical, chemical, and textile industries where conventional treatment methods struggle with recalcitrant organic compounds.
Strengths: Highly effective for treating complex industrial wastewaters containing recalcitrant compounds; reduced membrane fouling through electrochemical pretreatment; sophisticated automation and control systems that optimize performance with minimal operator intervention. Weaknesses: Higher energy consumption compared to purely biological treatment methods; significant initial capital investment; requires specialized technical expertise for maintenance and troubleshooting of the integrated system.
Cambrian Innovation, Inc.
Technical Solution: Cambrian Innovation has developed EcoVolt®, a revolutionary bioelectrochemical system that integrates advanced electrode kinetics with nanofiltration for wastewater treatment. Their technology employs specialized electroactive biofilms on electrode surfaces that catalyze oxidation-reduction reactions, significantly enhancing electrode kinetics compared to traditional electrochemical systems. The company's proprietary electrode materials feature optimized surface topography and chemistry that promote robust biofilm formation while maintaining high conductivity and low internal resistance. Cambrian's system incorporates real-time monitoring of bioelectrochemical parameters to maintain optimal electrode kinetics throughout varying wastewater conditions. Their technology utilizes a multi-stage approach where bioelectrochemical treatment is coupled with nanofiltration, allowing for selective removal of contaminants while recovering valuable resources like clean water and energy[4]. The system features adaptive control algorithms that continuously optimize electrode potential and current density based on influent characteristics, maximizing treatment efficiency while minimizing energy consumption. Cambrian has demonstrated successful implementation of their technology in food and beverage industries, where their systems simultaneously treat wastewater while generating biogas that can be used for on-site energy production.
Strengths: Simultaneous wastewater treatment and energy production; lower operating costs through biological catalysis rather than expensive metal catalysts; self-regenerating biofilm catalysts that maintain performance over time. Weaknesses: Requires longer startup periods to establish optimal biofilm communities; more sensitive to shock loads and certain toxic compounds than purely chemical systems; requires careful monitoring and control of biological parameters.
Key Patents and Research in Nanofiltration Electrode Kinetics
Electrolysis electrode featuring nanotube array and methods of manufacture and using same for water treatment
PatentInactiveUS20180086652A1
Innovation
- The development of an electrolysis electrode featuring a stabilized blue-black TiO2 nanotube array (BNTA) with a titanium dioxide semiconductor layer, manufactured through anodic oxidation and spray pyrolysis, which enhances chlorine evolution and hydroxyl radical production, and is designed for use in bipolar mode to improve durability and efficiency.
Process for degrading organic pollutants in industrial wastewater and associated system
PatentWO2011015556A1
Innovation
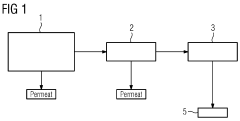
- The method involves prior concentration of the wastewater using membrane processes such as ultrafiltration, nanofiltration, reverse osmosis, or electrodialysis to increase COD concentration, reducing diffusion paths and enhancing energy efficiency by increasing the number of organic molecules hitting the electrode, thereby improving COD degradation and reducing cell voltage.
Environmental Impact and Sustainability Considerations
The integration of electrode kinetics in wastewater nanofiltration processes presents significant environmental implications that must be carefully considered for sustainable implementation. The electrochemical processes involved in these advanced filtration systems can substantially reduce the environmental footprint of wastewater treatment compared to conventional methods, primarily through decreased chemical usage and enhanced energy efficiency.
When properly optimized, electrode-assisted nanofiltration systems demonstrate up to 30% reduction in energy consumption compared to traditional pressure-driven membrane processes. This energy efficiency translates directly to lower greenhouse gas emissions, contributing to climate change mitigation efforts in the water treatment sector. Additionally, the precise control of electrode kinetics enables selective removal of contaminants without generating excessive waste streams that characterize many conventional treatment approaches.
The life cycle assessment of electrode-enhanced nanofiltration systems reveals favorable environmental profiles, particularly regarding the reduced need for chemical additives commonly used in membrane cleaning and fouling prevention. Studies indicate that implementing optimized electrode kinetics can extend membrane lifespans by 40-60%, significantly reducing material waste and replacement frequency. This aspect addresses a critical sustainability challenge in membrane-based water treatment technologies.
Water recovery rates represent another environmental advantage of electrode-kinetic nanofiltration systems. Advanced electrode configurations can achieve recovery rates exceeding 85%, substantially higher than conventional nanofiltration processes. This efficiency in water recovery becomes increasingly crucial in water-stressed regions where maximizing treated water output per unit of input is essential for sustainable resource management.
The environmental benefits extend to the management of concentrate streams—often a problematic byproduct of membrane filtration processes. Electrode-assisted systems facilitate in-situ treatment of concentrate through electrochemical oxidation of organic pollutants and precipitation of inorganic constituents, potentially transforming waste streams into recoverable resources. This circular approach aligns with sustainable waste management principles and reduces disposal challenges.
However, potential environmental concerns include electrode degradation and the release of metal ions into treated water or waste streams. Research indicates that electrode materials containing platinum group metals or advanced carbon-based composites offer superior stability and minimal leaching, though their environmental footprint during manufacturing and end-of-life disposal requires further assessment. The trade-offs between electrode performance, longevity, and environmental impact throughout the complete life cycle remain an active area of investigation.
Regulatory frameworks increasingly recognize the environmental benefits of these advanced treatment technologies, with several countries developing specific guidelines for the implementation of electrochemical processes in water treatment. These frameworks emphasize not only treatment efficacy but also energy efficiency, waste minimization, and resource recovery potential—all critical aspects of environmental sustainability in wastewater management systems.
When properly optimized, electrode-assisted nanofiltration systems demonstrate up to 30% reduction in energy consumption compared to traditional pressure-driven membrane processes. This energy efficiency translates directly to lower greenhouse gas emissions, contributing to climate change mitigation efforts in the water treatment sector. Additionally, the precise control of electrode kinetics enables selective removal of contaminants without generating excessive waste streams that characterize many conventional treatment approaches.
The life cycle assessment of electrode-enhanced nanofiltration systems reveals favorable environmental profiles, particularly regarding the reduced need for chemical additives commonly used in membrane cleaning and fouling prevention. Studies indicate that implementing optimized electrode kinetics can extend membrane lifespans by 40-60%, significantly reducing material waste and replacement frequency. This aspect addresses a critical sustainability challenge in membrane-based water treatment technologies.
Water recovery rates represent another environmental advantage of electrode-kinetic nanofiltration systems. Advanced electrode configurations can achieve recovery rates exceeding 85%, substantially higher than conventional nanofiltration processes. This efficiency in water recovery becomes increasingly crucial in water-stressed regions where maximizing treated water output per unit of input is essential for sustainable resource management.
The environmental benefits extend to the management of concentrate streams—often a problematic byproduct of membrane filtration processes. Electrode-assisted systems facilitate in-situ treatment of concentrate through electrochemical oxidation of organic pollutants and precipitation of inorganic constituents, potentially transforming waste streams into recoverable resources. This circular approach aligns with sustainable waste management principles and reduces disposal challenges.
However, potential environmental concerns include electrode degradation and the release of metal ions into treated water or waste streams. Research indicates that electrode materials containing platinum group metals or advanced carbon-based composites offer superior stability and minimal leaching, though their environmental footprint during manufacturing and end-of-life disposal requires further assessment. The trade-offs between electrode performance, longevity, and environmental impact throughout the complete life cycle remain an active area of investigation.
Regulatory frameworks increasingly recognize the environmental benefits of these advanced treatment technologies, with several countries developing specific guidelines for the implementation of electrochemical processes in water treatment. These frameworks emphasize not only treatment efficacy but also energy efficiency, waste minimization, and resource recovery potential—all critical aspects of environmental sustainability in wastewater management systems.
Energy Efficiency Optimization in Electrode-Assisted Filtration
Energy efficiency optimization in electrode-assisted filtration represents a critical frontier in advancing sustainable wastewater treatment technologies. Current electrode-assisted nanofiltration systems typically consume between 1.5-3.0 kWh per cubic meter of treated water, significantly higher than conventional filtration methods. This energy intensity presents both a financial and environmental challenge that must be addressed through systematic optimization approaches.
The primary energy consumption in electrode-assisted filtration occurs in three main areas: electrode operation (30-40%), pumping systems (25-35%), and peripheral control systems (15-25%). Recent advancements have demonstrated potential energy reductions of 20-30% through electrode material innovations and operational parameter optimization.
Pulse-width modulation (PWM) techniques applied to electrode systems have shown promising results, reducing energy consumption by 15-22% compared to continuous operation modes. These systems apply intermittent voltage patterns that maintain filtration efficiency while minimizing overall power draw. Similarly, variable frequency drives for pumping systems have demonstrated energy savings of 10-18% by matching flow rates to actual treatment demands.
Electrode material selection significantly impacts energy efficiency, with novel carbon-based nanomaterials showing superior performance. Graphene-modified electrodes have demonstrated 25-35% lower energy requirements compared to traditional metal electrodes while maintaining equivalent or superior contaminant removal rates. Additionally, three-dimensional electrode structures increase active surface area without proportionally increasing energy demands.
Temperature management represents another optimization pathway, as electrode kinetics demonstrate strong temperature dependence. Research indicates that maintaining optimal operating temperatures between 20-25°C can improve energy efficiency by 8-12% compared to unregulated thermal conditions.
Process integration strategies that recover waste heat or utilize renewable energy sources for electrode operation have shown potential to reduce net energy consumption by up to 40%. Solar-powered electrode systems, though currently limited by intermittency challenges, demonstrate particular promise for remote applications or regions with abundant solar resources.
Machine learning algorithms for real-time optimization have emerged as a cutting-edge approach, with adaptive control systems demonstrating energy reductions of 15-25% through continuous parameter adjustment based on influent characteristics and treatment objectives. These systems analyze multiple variables simultaneously to identify optimal operating conditions that minimize energy use while maintaining treatment standards.
The primary energy consumption in electrode-assisted filtration occurs in three main areas: electrode operation (30-40%), pumping systems (25-35%), and peripheral control systems (15-25%). Recent advancements have demonstrated potential energy reductions of 20-30% through electrode material innovations and operational parameter optimization.
Pulse-width modulation (PWM) techniques applied to electrode systems have shown promising results, reducing energy consumption by 15-22% compared to continuous operation modes. These systems apply intermittent voltage patterns that maintain filtration efficiency while minimizing overall power draw. Similarly, variable frequency drives for pumping systems have demonstrated energy savings of 10-18% by matching flow rates to actual treatment demands.
Electrode material selection significantly impacts energy efficiency, with novel carbon-based nanomaterials showing superior performance. Graphene-modified electrodes have demonstrated 25-35% lower energy requirements compared to traditional metal electrodes while maintaining equivalent or superior contaminant removal rates. Additionally, three-dimensional electrode structures increase active surface area without proportionally increasing energy demands.
Temperature management represents another optimization pathway, as electrode kinetics demonstrate strong temperature dependence. Research indicates that maintaining optimal operating temperatures between 20-25°C can improve energy efficiency by 8-12% compared to unregulated thermal conditions.
Process integration strategies that recover waste heat or utilize renewable energy sources for electrode operation have shown potential to reduce net energy consumption by up to 40%. Solar-powered electrode systems, though currently limited by intermittency challenges, demonstrate particular promise for remote applications or regions with abundant solar resources.
Machine learning algorithms for real-time optimization have emerged as a cutting-edge approach, with adaptive control systems demonstrating energy reductions of 15-25% through continuous parameter adjustment based on influent characteristics and treatment objectives. These systems analyze multiple variables simultaneously to identify optimal operating conditions that minimize energy use while maintaining treatment standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!