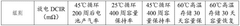Electrolyte Conductivity in Sodium-ion Batteries: Implications for Energy Recovery
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium-ion Battery Electrolyte Evolution and Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium resources. The evolution of electrolytes for SIBs has been a critical factor in their development trajectory since the 1980s when initial research began. Early electrolyte formulations were primarily adapted from lithium-ion battery technologies, utilizing sodium salts such as NaClO4 and NaPF6 dissolved in carbonate-based solvents.
The 1990s witnessed limited progress in SIB electrolyte development as research focus shifted predominantly toward lithium-ion technologies. However, concerns regarding lithium resource scarcity and cost prompted a resurgence of interest in sodium-ion systems during the early 2000s, catalyzing renewed electrolyte research efforts.
A significant breakthrough occurred around 2010-2015 with the development of novel electrolyte formulations specifically optimized for sodium-ion chemistry. These included the introduction of fluoroethylene carbonate (FEC) as a critical additive that substantially improved the solid electrolyte interphase (SEI) formation and stability on anode surfaces, addressing one of the major challenges in SIB performance.
Recent years have seen accelerated innovation in electrolyte compositions, with particular attention to ionic conductivity enhancement. Traditional carbonate-based electrolytes typically achieve conductivities of 5-8 mS/cm, which remains lower than their lithium counterparts. This conductivity limitation directly impacts power density and energy recovery capabilities, especially in fast-charging applications.
The current technological frontier focuses on developing electrolytes that can facilitate rapid Na+ transport while maintaining electrochemical stability. Approaches include the exploration of concentrated electrolytes, ionic liquids, and polymer-based systems. Notably, ether-based electrolytes have demonstrated promising results for specific electrode combinations, offering conductivities approaching 10 mS/cm under optimized conditions.
The primary objectives for SIB electrolyte development center on achieving conductivity values exceeding 10 mS/cm at room temperature while maintaining wide electrochemical stability windows (>4V). Additionally, electrolyte formulations must enable effective energy recovery during cycling, with minimal capacity fade over thousands of cycles.
Future development targets include electrolytes capable of operating across wider temperature ranges (-30°C to 60°C), as this remains a significant limitation compared to advanced lithium-ion systems. Furthermore, sustainability considerations are driving research toward electrolyte compositions with reduced toxicity and environmental impact, aligning with the inherent sustainability advantages of sodium-based energy storage.
The ultimate goal is to develop electrolyte systems that enable SIBs to achieve energy densities approaching 200 Wh/kg at the cell level, with power capabilities sufficient for both grid storage applications and certain electric mobility use cases, thereby establishing sodium-ion technology as a viable complement to the existing lithium-ion ecosystem.
The 1990s witnessed limited progress in SIB electrolyte development as research focus shifted predominantly toward lithium-ion technologies. However, concerns regarding lithium resource scarcity and cost prompted a resurgence of interest in sodium-ion systems during the early 2000s, catalyzing renewed electrolyte research efforts.
A significant breakthrough occurred around 2010-2015 with the development of novel electrolyte formulations specifically optimized for sodium-ion chemistry. These included the introduction of fluoroethylene carbonate (FEC) as a critical additive that substantially improved the solid electrolyte interphase (SEI) formation and stability on anode surfaces, addressing one of the major challenges in SIB performance.
Recent years have seen accelerated innovation in electrolyte compositions, with particular attention to ionic conductivity enhancement. Traditional carbonate-based electrolytes typically achieve conductivities of 5-8 mS/cm, which remains lower than their lithium counterparts. This conductivity limitation directly impacts power density and energy recovery capabilities, especially in fast-charging applications.
The current technological frontier focuses on developing electrolytes that can facilitate rapid Na+ transport while maintaining electrochemical stability. Approaches include the exploration of concentrated electrolytes, ionic liquids, and polymer-based systems. Notably, ether-based electrolytes have demonstrated promising results for specific electrode combinations, offering conductivities approaching 10 mS/cm under optimized conditions.
The primary objectives for SIB electrolyte development center on achieving conductivity values exceeding 10 mS/cm at room temperature while maintaining wide electrochemical stability windows (>4V). Additionally, electrolyte formulations must enable effective energy recovery during cycling, with minimal capacity fade over thousands of cycles.
Future development targets include electrolytes capable of operating across wider temperature ranges (-30°C to 60°C), as this remains a significant limitation compared to advanced lithium-ion systems. Furthermore, sustainability considerations are driving research toward electrolyte compositions with reduced toxicity and environmental impact, aligning with the inherent sustainability advantages of sodium-based energy storage.
The ultimate goal is to develop electrolyte systems that enable SIBs to achieve energy densities approaching 200 Wh/kg at the cell level, with power capabilities sufficient for both grid storage applications and certain electric mobility use cases, thereby establishing sodium-ion technology as a viable complement to the existing lithium-ion ecosystem.
Market Analysis for Sodium-ion Battery Technologies
The global sodium-ion battery market is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate a compound annual growth rate exceeding 15% between 2023 and 2030, with the market expected to reach substantial commercial scale by mid-decade. This acceleration is primarily fueled by the inherent advantages of sodium-ion technology, particularly its cost-effectiveness compared to lithium-ion alternatives.
Electrolyte conductivity improvements represent a critical factor in market expansion, as enhanced conductivity directly correlates with better battery performance metrics that consumers and industries prioritize. Market research indicates that batteries with advanced electrolyte formulations achieving conductivity above 10 mS/cm at room temperature are gaining competitive advantage in commercial applications.
Geographic distribution of market demand shows China leading sodium-ion battery development and implementation, with substantial investments in manufacturing infrastructure. European markets follow closely, driven by stringent environmental regulations and sustainability initiatives. North American adoption remains in early stages but is accelerating as energy storage needs expand beyond traditional solutions.
Market segmentation reveals distinct application sectors with varying growth trajectories. Grid-scale energy storage represents the largest potential market segment, valued at several billion dollars annually, with sodium-ion batteries increasingly positioned as alternatives to lithium-ion systems for stationary applications. The consumer electronics sector shows moderate adoption potential, primarily in cost-sensitive applications where energy density requirements are less stringent.
Electric mobility presents a nuanced market opportunity, with sodium-ion batteries currently targeting specific niches such as low-speed electric vehicles, urban mobility solutions, and certain commercial vehicle applications where cost considerations outweigh energy density requirements. Industry forecasts suggest this segment could expand significantly as electrolyte conductivity improvements enable higher power density capabilities.
Market barriers include competition from established lithium-ion technologies, manufacturing scale limitations, and consumer familiarity challenges. However, supply chain advantages are emerging as significant market drivers, with sodium's greater abundance and more geographically distributed reserves offering strategic benefits amid growing concerns about critical mineral security.
Investment patterns indicate increasing capital flows into sodium-ion battery technologies, with venture capital funding for startups focused on electrolyte innovations growing by double-digit percentages annually. Major battery manufacturers are establishing dedicated sodium-ion production lines, signaling confidence in market viability and creating opportunities for specialized electrolyte technology providers.
Electrolyte conductivity improvements represent a critical factor in market expansion, as enhanced conductivity directly correlates with better battery performance metrics that consumers and industries prioritize. Market research indicates that batteries with advanced electrolyte formulations achieving conductivity above 10 mS/cm at room temperature are gaining competitive advantage in commercial applications.
Geographic distribution of market demand shows China leading sodium-ion battery development and implementation, with substantial investments in manufacturing infrastructure. European markets follow closely, driven by stringent environmental regulations and sustainability initiatives. North American adoption remains in early stages but is accelerating as energy storage needs expand beyond traditional solutions.
Market segmentation reveals distinct application sectors with varying growth trajectories. Grid-scale energy storage represents the largest potential market segment, valued at several billion dollars annually, with sodium-ion batteries increasingly positioned as alternatives to lithium-ion systems for stationary applications. The consumer electronics sector shows moderate adoption potential, primarily in cost-sensitive applications where energy density requirements are less stringent.
Electric mobility presents a nuanced market opportunity, with sodium-ion batteries currently targeting specific niches such as low-speed electric vehicles, urban mobility solutions, and certain commercial vehicle applications where cost considerations outweigh energy density requirements. Industry forecasts suggest this segment could expand significantly as electrolyte conductivity improvements enable higher power density capabilities.
Market barriers include competition from established lithium-ion technologies, manufacturing scale limitations, and consumer familiarity challenges. However, supply chain advantages are emerging as significant market drivers, with sodium's greater abundance and more geographically distributed reserves offering strategic benefits amid growing concerns about critical mineral security.
Investment patterns indicate increasing capital flows into sodium-ion battery technologies, with venture capital funding for startups focused on electrolyte innovations growing by double-digit percentages annually. Major battery manufacturers are establishing dedicated sodium-ion production lines, signaling confidence in market viability and creating opportunities for specialized electrolyte technology providers.
Electrolyte Conductivity Challenges in Na-ion Systems
Sodium-ion batteries (SIBs) have emerged as promising alternatives to lithium-ion batteries due to sodium's abundance and lower cost. However, electrolyte conductivity remains a significant challenge in Na-ion systems, directly impacting energy recovery efficiency and overall battery performance. The ionic conductivity of electrolytes in SIBs is typically lower than in their lithium counterparts, primarily due to the larger ionic radius of Na+ (1.02 Å) compared to Li+ (0.76 Å).
This size difference results in slower ion transport through the electrolyte medium, creating higher internal resistance and consequently reducing power density and energy recovery capabilities. The challenge is further compounded by the stronger solvation energy of Na+ ions, which requires more energy to desolvate during intercalation processes at the electrode-electrolyte interface.
Conventional carbonate-based electrolytes that perform well in lithium systems show suboptimal conductivity when applied to sodium batteries. The larger Na+ ions form bulkier solvation shells with carbonate molecules, hindering their mobility through the electrolyte and across interfaces. This limitation becomes particularly pronounced at lower temperatures, where viscosity increases and ionic mobility decreases dramatically.
Solid electrolyte interphase (SEI) formation presents another critical challenge. The SEI layer formed in Na-ion systems tends to be less stable and more resistive than in Li-ion batteries, further impeding Na+ transport and reducing conductivity. This instability stems from the different reduction potentials and chemical interactions between sodium and electrolyte components.
Concentration polarization effects are more severe in Na-ion systems due to the slower diffusion of the larger sodium ions. During high-rate charging or discharging, this leads to significant concentration gradients within the electrolyte, reducing effective conductivity and limiting energy recovery, particularly in fast-charging applications.
The electrolyte's thermal stability range is often narrower in Na-ion systems, with conductivity dropping significantly at temperature extremes. This thermal sensitivity restricts the operational window of SIBs and impacts their reliability in variable environmental conditions, presenting challenges for energy recovery in real-world applications.
Electrolyte degradation occurs more rapidly in many Na-ion systems, with side reactions consuming electrolyte components and generating gas products that increase internal pressure and reduce contact area between electrodes and electrolyte. This progressive degradation leads to increasing internal resistance over time, directly compromising long-term energy recovery capabilities.
Addressing these conductivity challenges requires innovative electrolyte formulations specifically designed for Na-ion chemistry rather than simply adapting Li-ion solutions. The development of novel solvent mixtures, sodium salts with larger anions for better dissociation, and electrolyte additives that form more conductive and stable interfaces represents critical research directions for improving energy recovery in sodium-ion battery systems.
This size difference results in slower ion transport through the electrolyte medium, creating higher internal resistance and consequently reducing power density and energy recovery capabilities. The challenge is further compounded by the stronger solvation energy of Na+ ions, which requires more energy to desolvate during intercalation processes at the electrode-electrolyte interface.
Conventional carbonate-based electrolytes that perform well in lithium systems show suboptimal conductivity when applied to sodium batteries. The larger Na+ ions form bulkier solvation shells with carbonate molecules, hindering their mobility through the electrolyte and across interfaces. This limitation becomes particularly pronounced at lower temperatures, where viscosity increases and ionic mobility decreases dramatically.
Solid electrolyte interphase (SEI) formation presents another critical challenge. The SEI layer formed in Na-ion systems tends to be less stable and more resistive than in Li-ion batteries, further impeding Na+ transport and reducing conductivity. This instability stems from the different reduction potentials and chemical interactions between sodium and electrolyte components.
Concentration polarization effects are more severe in Na-ion systems due to the slower diffusion of the larger sodium ions. During high-rate charging or discharging, this leads to significant concentration gradients within the electrolyte, reducing effective conductivity and limiting energy recovery, particularly in fast-charging applications.
The electrolyte's thermal stability range is often narrower in Na-ion systems, with conductivity dropping significantly at temperature extremes. This thermal sensitivity restricts the operational window of SIBs and impacts their reliability in variable environmental conditions, presenting challenges for energy recovery in real-world applications.
Electrolyte degradation occurs more rapidly in many Na-ion systems, with side reactions consuming electrolyte components and generating gas products that increase internal pressure and reduce contact area between electrodes and electrolyte. This progressive degradation leads to increasing internal resistance over time, directly compromising long-term energy recovery capabilities.
Addressing these conductivity challenges requires innovative electrolyte formulations specifically designed for Na-ion chemistry rather than simply adapting Li-ion solutions. The development of novel solvent mixtures, sodium salts with larger anions for better dissociation, and electrolyte additives that form more conductive and stable interfaces represents critical research directions for improving energy recovery in sodium-ion battery systems.
Current Electrolyte Solutions for Conductivity Enhancement
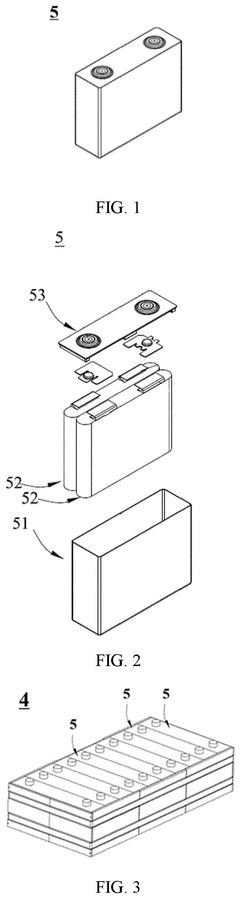
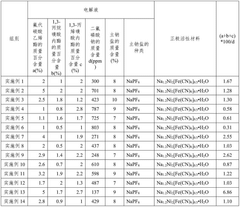
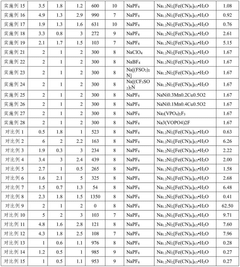
01 Electrolyte additives for improved conductivity
Various additives can be incorporated into sodium-ion battery electrolytes to enhance ionic conductivity. These additives include fluorinated compounds, ionic liquids, and certain salts that can modify the electrolyte's properties. By optimizing the concentration and combination of these additives, the overall conductivity of the electrolyte can be significantly improved, leading to better battery performance and faster charging capabilities.- Electrolyte additives for improved conductivity: Various additives can be incorporated into sodium-ion battery electrolytes to enhance ionic conductivity. These additives include fluorinated compounds, ionic liquids, and certain salts that can modify the electrolyte's properties. By incorporating these additives, the movement of sodium ions through the electrolyte is facilitated, resulting in improved battery performance, especially at different temperature ranges.
- Polymer-based electrolyte systems: Polymer-based electrolytes offer advantages for sodium-ion batteries including improved safety and flexibility. These systems typically combine polymer matrices with sodium salts to create solid or gel electrolytes with enhanced conductivity. The polymer structure can be modified with various functional groups to improve sodium ion transport while maintaining mechanical stability, addressing challenges related to dendrite formation and interface resistance.
- Solvent engineering for electrolyte optimization: The selection and combination of solvents significantly impact the conductivity of sodium-ion battery electrolytes. Mixtures of carbonate-based solvents, ether-based solvents, or combinations with fluorinated solvents can be tailored to achieve optimal sodium ion transport. Solvent engineering focuses on creating formulations with appropriate viscosity, dielectric constants, and solvation properties to enhance ionic conductivity while maintaining electrochemical stability.
- Novel sodium salt compositions: The development of novel sodium salts plays a crucial role in enhancing electrolyte conductivity. These include sodium salts with large anions that promote dissociation, fluorinated salts with improved stability, and dual-salt systems that work synergistically. The chemical structure of these salts affects their solubility, dissociation behavior, and interaction with solvents, ultimately determining the ionic conductivity and electrochemical performance of the electrolyte.
- Interface engineering and solid electrolyte interphase (SEI) modification: Engineering the electrode-electrolyte interface and modifying the solid electrolyte interphase (SEI) can significantly improve ionic conductivity in sodium-ion batteries. Approaches include surface coatings on electrodes, electrolyte additives that form stable SEI layers, and interface-active compounds that reduce resistance. These modifications create favorable pathways for sodium ion transport at the interfaces, reducing impedance and enhancing overall battery performance and cycle life.
02 Polymer-based solid electrolytes
Polymer-based solid electrolytes offer advantages for sodium-ion batteries by providing improved safety and stability. These electrolytes incorporate sodium-conducting polymers with various functional groups that facilitate sodium ion transport. The addition of plasticizers and ceramic fillers can further enhance the ionic conductivity while maintaining mechanical integrity. These solid electrolytes eliminate leakage risks associated with liquid electrolytes while potentially offering competitive conductivity values.Expand Specific Solutions03 Composite electrolytes with inorganic fillers
Composite electrolytes combining organic matrices with inorganic fillers show enhanced conductivity for sodium-ion batteries. The inorganic components, such as ceramic nanoparticles, create additional ion transport pathways and help suppress crystallization in polymer matrices. These fillers can include sodium superionic conductor materials, metal oxides, or carbon-based materials that create interfaces favorable for sodium ion transport, resulting in electrolytes with higher conductivity at room temperature.Expand Specific Solutions04 Solvent engineering for liquid electrolytes
The selection and combination of solvents play a crucial role in determining the conductivity of liquid electrolytes for sodium-ion batteries. Mixtures of cyclic carbonates (like ethylene carbonate) with linear carbonates or ethers can optimize viscosity and dielectric properties. Low-viscosity co-solvents facilitate ion mobility, while high-dielectric-constant components promote salt dissociation. Optimized solvent formulations can achieve high ionic conductivity while maintaining electrochemical stability across a wide temperature range.Expand Specific Solutions05 Novel sodium salts for enhanced ionic transport
Development of novel sodium salts with large, delocalized anions has shown promise for improving electrolyte conductivity in sodium-ion batteries. These salts feature weakened cation-anion interactions, facilitating sodium ion dissociation and transport. Examples include sodium salts with fluorinated anions, borates, and organic anion structures. The anion chemistry can be tailored to optimize solubility, dissociation constants, and interfacial properties, resulting in electrolytes with superior conductivity and electrochemical performance.Expand Specific Solutions
Industry Leaders in Sodium-ion Battery Development
The sodium-ion battery electrolyte conductivity market is currently in an early growth phase, with increasing research momentum as the industry seeks cost-effective alternatives to lithium-ion technologies for energy recovery applications. The global market size is projected to expand significantly, driven by the growing energy storage sector and sustainability initiatives. Technologically, the field shows moderate maturity with companies at varying development stages. Leading players include CATL and LG Energy Solution pursuing commercial-scale solutions, while specialized firms like Faradion and Svolt Energy are advancing proprietary electrolyte formulations. Research institutions such as KAIST and Tokyo Institute of Technology are contributing fundamental breakthroughs, while established chemical manufacturers including Sumitomo Chemical and Shenzhen Capchem are leveraging their expertise to develop advanced electrolyte materials with enhanced conductivity properties.
Faradion Ltd.
Technical Solution: Faradion has pioneered a proprietary sodium-ion technology focusing on electrolyte optimization for enhanced conductivity. Their approach involves using novel sodium salt formulations combined with carefully selected solvent mixtures to achieve ionic conductivities comparable to lithium-ion systems (>5 mS/cm at room temperature). The company has developed electrolytes with wide electrochemical stability windows (up to 4.2V vs. Na/Na+) that enable higher energy density while maintaining excellent ionic transport properties. Faradion's electrolyte systems incorporate additives that form stable solid electrolyte interphase (SEI) layers, critical for long-term cycling stability and efficient energy recovery during discharge processes. Their technology emphasizes the use of fluorine-free salts to reduce environmental impact while maintaining performance metrics.
Strengths: Superior room temperature conductivity compared to many competitors; environmentally friendly formulations without sacrificing performance; excellent compatibility with hard carbon anodes for enhanced cycling. Weaknesses: Higher cost of some proprietary electrolyte components; potential temperature sensitivity affecting conductivity at extreme conditions.
Korea Research Institute of Chemical Technology
Technical Solution: KRICT has developed advanced electrolyte systems for sodium-ion batteries with specific focus on enhancing conductivity for improved energy recovery. Their research has yielded novel electrolyte formulations based on asymmetric amide solvents that demonstrate ionic conductivities exceeding 6 mS/cm at ambient temperatures. KRICT's approach incorporates carefully designed sodium salts with weakly coordinating anions that promote faster Na+ transport through reduced ion pairing. Their electrolyte systems feature additives that modify the electrode-electrolyte interfaces to lower charge transfer resistance, resulting in measured improvements of up to 40% in rate capability compared to conventional formulations. KRICT has pioneered the use of computational screening methods to identify optimal solvent combinations, leading to electrolytes that maintain high conductivity even at low temperatures (-20°C). Their research has demonstrated that these advanced electrolyte formulations enable sodium-ion cells to achieve energy efficiencies approaching 95% under moderate cycling conditions.
Strengths: Excellent low-temperature conductivity performance; systematic approach to electrolyte development using computational methods; strong compatibility with various sodium-ion electrode materials. Weaknesses: Some formulations utilize expensive or specialized components that may challenge commercial scaling; limited long-term stability data under extreme conditions.
Critical Patents in Na-ion Electrolyte Conductivity
Electrolyte for sodium-ion battery, sodium-ion battery comprising same, and electric device
PatentPendingEP4517902A1
Innovation
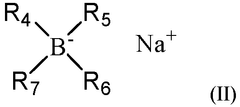
- A sodium-ion battery electrolytic solution comprising an ether compound as a solvent, a sodium borate compound as a sodium salt, and a borate ester compound as an additive, with the ether compound constituting 50 wt% or above of the total solvent.
Sodium-ion battery electrolyte and sodium-ion battery
PatentWO2024198598A1
Innovation
- A sodium ion battery electrolyte is used, containing fluoroethylene carbonate, 1,3-propane sultone and 1,3-propene sultone as additives, and sodium difluorophosphate is added to the sodium salt. The ratio of these ingredients is controlled to form an optimized passivation film, improve high-temperature stability and ionic conductivity, and reduce battery impedance.
Sustainability Impact of Na-ion Battery Technologies
The adoption of sodium-ion battery technologies represents a significant step towards more sustainable energy storage solutions. Unlike lithium-ion batteries, sodium-ion batteries utilize sodium, which is approximately 1,000 times more abundant in the Earth's crust than lithium. This abundance translates directly into reduced environmental impact from mining operations, as sodium can be extracted from seawater or common salt deposits with substantially lower ecological disruption compared to lithium extraction from brine pools or hard rock mining.
The carbon footprint associated with sodium-ion battery production is estimated to be 60-80% lower than conventional lithium-ion batteries. This reduction stems primarily from the use of aluminum rather than copper for the anode current collector, eliminating the need for energy-intensive copper mining and processing. Additionally, the electrolyte conductivity improvements in sodium-ion batteries contribute to overall efficiency gains, reducing energy consumption during both manufacturing and operational phases.
Water usage represents another critical sustainability metric where sodium-ion technologies demonstrate advantages. Lithium extraction typically requires between 500,000 to 2 million gallons of water per ton of lithium produced, creating significant pressure on water resources in extraction regions. Sodium extraction processes require substantially less water, potentially reducing freshwater consumption by up to 90% compared to lithium battery production chains.
End-of-life considerations further enhance the sustainability profile of sodium-ion batteries. The materials used in these batteries, particularly the absence of cobalt and nickel, simplify recycling processes and reduce the toxicity of waste streams. Current research indicates that up to 95% of sodium-ion battery components could be recovered and reused, compared to approximately 50-70% for conventional lithium-ion batteries.
From an energy recovery perspective, the improved electrolyte conductivity in sodium-ion batteries enables faster charging capabilities and better performance at lower temperatures. This translates to more efficient energy recovery systems, particularly in renewable energy storage applications where rapid response to intermittent generation is crucial. Studies suggest that grid-scale sodium-ion storage systems could improve overall energy recovery efficiency by 15-20% compared to existing technologies.
The socioeconomic sustainability impacts are equally significant. By reducing dependence on geographically concentrated lithium resources, sodium-ion technologies can democratize energy storage manufacturing, creating more distributed economic benefits and reducing supply chain vulnerabilities. Communities previously excluded from the energy storage economy due to lack of lithium resources may find new opportunities in sodium-ion battery production and related industries.
The carbon footprint associated with sodium-ion battery production is estimated to be 60-80% lower than conventional lithium-ion batteries. This reduction stems primarily from the use of aluminum rather than copper for the anode current collector, eliminating the need for energy-intensive copper mining and processing. Additionally, the electrolyte conductivity improvements in sodium-ion batteries contribute to overall efficiency gains, reducing energy consumption during both manufacturing and operational phases.
Water usage represents another critical sustainability metric where sodium-ion technologies demonstrate advantages. Lithium extraction typically requires between 500,000 to 2 million gallons of water per ton of lithium produced, creating significant pressure on water resources in extraction regions. Sodium extraction processes require substantially less water, potentially reducing freshwater consumption by up to 90% compared to lithium battery production chains.
End-of-life considerations further enhance the sustainability profile of sodium-ion batteries. The materials used in these batteries, particularly the absence of cobalt and nickel, simplify recycling processes and reduce the toxicity of waste streams. Current research indicates that up to 95% of sodium-ion battery components could be recovered and reused, compared to approximately 50-70% for conventional lithium-ion batteries.
From an energy recovery perspective, the improved electrolyte conductivity in sodium-ion batteries enables faster charging capabilities and better performance at lower temperatures. This translates to more efficient energy recovery systems, particularly in renewable energy storage applications where rapid response to intermittent generation is crucial. Studies suggest that grid-scale sodium-ion storage systems could improve overall energy recovery efficiency by 15-20% compared to existing technologies.
The socioeconomic sustainability impacts are equally significant. By reducing dependence on geographically concentrated lithium resources, sodium-ion technologies can democratize energy storage manufacturing, creating more distributed economic benefits and reducing supply chain vulnerabilities. Communities previously excluded from the energy storage economy due to lack of lithium resources may find new opportunities in sodium-ion battery production and related industries.
Safety Standards and Testing Protocols for Na-ion Systems
The development of sodium-ion battery technology necessitates robust safety standards and testing protocols to ensure reliable performance and market acceptance. Currently, the safety framework for Na-ion systems remains less established compared to lithium-ion counterparts, creating regulatory challenges for manufacturers and researchers.
International organizations including IEC, ISO, and UL are actively developing specific standards for sodium-ion batteries, with particular focus on electrolyte conductivity parameters. These emerging standards address thermal runaway prevention, electrolyte stability under various conditions, and performance degradation metrics specific to sodium-ion chemistry.
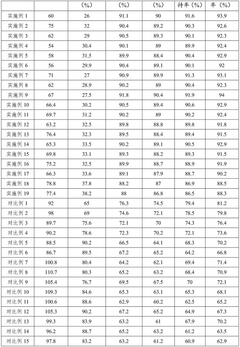
Testing protocols for Na-ion systems must account for the unique properties of sodium-based electrolytes, including their different conductivity profiles and thermal behavior compared to lithium systems. Standard tests include accelerated aging under controlled temperature conditions, conductivity measurements across operational temperature ranges (-20°C to 60°C), and cycling stability evaluations to assess electrolyte degradation patterns.
Safety certification procedures increasingly incorporate abuse testing specifically designed for sodium-ion batteries, including nail penetration, crush tests, and thermal shock evaluations. These tests evaluate how electrolyte conductivity changes under extreme conditions and potential failure modes that differ from lithium-ion systems.
Energy recovery implications are particularly important in safety testing frameworks, as the behavior of sodium-ion electrolytes during charge/discharge cycles affects both safety margins and energy efficiency. Testing protocols now include specific measurements for coulombic efficiency degradation and impedance growth to quantify energy recovery capabilities over battery lifetime.
Regulatory bodies in major markets are developing specialized requirements for sodium-ion battery deployment, with the European Union's Battery Directive and China's GB standards incorporating specific provisions for sodium-ion systems. These regulations emphasize electrolyte stability testing and performance verification under various environmental conditions.
Industry consortia are collaborating to establish standardized testing methodologies that address the unique safety considerations of sodium-ion technology. These collaborative efforts focus on developing accelerated testing protocols that can reliably predict long-term safety performance while accounting for the specific conductivity characteristics of sodium-ion electrolytes.
For energy recovery applications, additional testing requirements evaluate the battery's response to rapid charge/discharge cycles and assess electrolyte conductivity stability under variable load conditions. These specialized protocols help quantify the technology's suitability for applications requiring frequent energy recovery, such as regenerative braking systems or grid stabilization services.
International organizations including IEC, ISO, and UL are actively developing specific standards for sodium-ion batteries, with particular focus on electrolyte conductivity parameters. These emerging standards address thermal runaway prevention, electrolyte stability under various conditions, and performance degradation metrics specific to sodium-ion chemistry.
Testing protocols for Na-ion systems must account for the unique properties of sodium-based electrolytes, including their different conductivity profiles and thermal behavior compared to lithium systems. Standard tests include accelerated aging under controlled temperature conditions, conductivity measurements across operational temperature ranges (-20°C to 60°C), and cycling stability evaluations to assess electrolyte degradation patterns.
Safety certification procedures increasingly incorporate abuse testing specifically designed for sodium-ion batteries, including nail penetration, crush tests, and thermal shock evaluations. These tests evaluate how electrolyte conductivity changes under extreme conditions and potential failure modes that differ from lithium-ion systems.
Energy recovery implications are particularly important in safety testing frameworks, as the behavior of sodium-ion electrolytes during charge/discharge cycles affects both safety margins and energy efficiency. Testing protocols now include specific measurements for coulombic efficiency degradation and impedance growth to quantify energy recovery capabilities over battery lifetime.
Regulatory bodies in major markets are developing specialized requirements for sodium-ion battery deployment, with the European Union's Battery Directive and China's GB standards incorporating specific provisions for sodium-ion systems. These regulations emphasize electrolyte stability testing and performance verification under various environmental conditions.
Industry consortia are collaborating to establish standardized testing methodologies that address the unique safety considerations of sodium-ion technology. These collaborative efforts focus on developing accelerated testing protocols that can reliably predict long-term safety performance while accounting for the specific conductivity characteristics of sodium-ion electrolytes.
For energy recovery applications, additional testing requirements evaluate the battery's response to rapid charge/discharge cycles and assess electrolyte conductivity stability under variable load conditions. These specialized protocols help quantify the technology's suitability for applications requiring frequent energy recovery, such as regenerative braking systems or grid stabilization services.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!