Enhanced Ionic Conductivity in Sodium-ion Batteries: Applications and Impacts
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium-ion Battery Technology Evolution and Objectives
Sodium-ion battery technology has evolved significantly over the past four decades, with its origins dating back to the 1980s when researchers first explored sodium as an alternative to lithium in rechargeable battery systems. The fundamental principle behind sodium-ion batteries mirrors that of lithium-ion batteries, utilizing sodium ions as charge carriers between electrodes. However, the development trajectory has been markedly different, characterized by periods of intense research followed by relative dormancy.
The early 2000s witnessed renewed interest in sodium-ion technology, primarily driven by concerns about lithium resource limitations and geopolitical supply chain vulnerabilities. This resurgence was further catalyzed by breakthroughs in electrode materials, particularly the development of hard carbon anodes and layered oxide cathodes that demonstrated promising sodium storage capabilities.
From 2010 onwards, research momentum accelerated substantially, with significant advancements in electrolyte formulations and electrode architectures specifically designed to enhance ionic conductivity. The period between 2015-2020 marked a transition from academic research to industrial development, with several companies initiating pilot production lines for sodium-ion cells.
Current technological trends focus on addressing the inherent challenges of sodium-ion systems, particularly the larger ionic radius of sodium (1.02Å) compared to lithium (0.76Å), which impacts diffusion kinetics and ultimately affects battery performance. Research efforts are increasingly concentrated on novel electrolyte systems, including solid-state electrolytes, that can facilitate faster sodium ion transport while maintaining structural stability.
The primary objectives of contemporary sodium-ion battery research center on achieving enhanced ionic conductivity through several parallel approaches: developing optimized electrode materials with expanded interlayer spacing to accommodate sodium ions; engineering electrolyte compositions that reduce desolvation energy barriers; and designing electrode-electrolyte interfaces that minimize resistance to ion transfer.
Long-term technological goals include reaching energy densities exceeding 200 Wh/kg at the cell level, cycle life beyond 3000 cycles, and cost reduction to below $80/kWh—metrics that would position sodium-ion batteries as viable alternatives to lithium-ion systems for specific applications, particularly stationary energy storage and low-cost electric mobility solutions.
The evolution trajectory suggests that sodium-ion technology is approaching an inflection point, with fundamental research advances beginning to translate into commercial viability. The next five years are expected to be critical in determining whether enhanced ionic conductivity innovations can bridge the performance gap with lithium-ion systems while capitalizing on sodium's inherent advantages of resource abundance and cost-effectiveness.
The early 2000s witnessed renewed interest in sodium-ion technology, primarily driven by concerns about lithium resource limitations and geopolitical supply chain vulnerabilities. This resurgence was further catalyzed by breakthroughs in electrode materials, particularly the development of hard carbon anodes and layered oxide cathodes that demonstrated promising sodium storage capabilities.
From 2010 onwards, research momentum accelerated substantially, with significant advancements in electrolyte formulations and electrode architectures specifically designed to enhance ionic conductivity. The period between 2015-2020 marked a transition from academic research to industrial development, with several companies initiating pilot production lines for sodium-ion cells.
Current technological trends focus on addressing the inherent challenges of sodium-ion systems, particularly the larger ionic radius of sodium (1.02Å) compared to lithium (0.76Å), which impacts diffusion kinetics and ultimately affects battery performance. Research efforts are increasingly concentrated on novel electrolyte systems, including solid-state electrolytes, that can facilitate faster sodium ion transport while maintaining structural stability.
The primary objectives of contemporary sodium-ion battery research center on achieving enhanced ionic conductivity through several parallel approaches: developing optimized electrode materials with expanded interlayer spacing to accommodate sodium ions; engineering electrolyte compositions that reduce desolvation energy barriers; and designing electrode-electrolyte interfaces that minimize resistance to ion transfer.
Long-term technological goals include reaching energy densities exceeding 200 Wh/kg at the cell level, cycle life beyond 3000 cycles, and cost reduction to below $80/kWh—metrics that would position sodium-ion batteries as viable alternatives to lithium-ion systems for specific applications, particularly stationary energy storage and low-cost electric mobility solutions.
The evolution trajectory suggests that sodium-ion technology is approaching an inflection point, with fundamental research advances beginning to translate into commercial viability. The next five years are expected to be critical in determining whether enhanced ionic conductivity innovations can bridge the performance gap with lithium-ion systems while capitalizing on sodium's inherent advantages of resource abundance and cost-effectiveness.
Market Analysis for Enhanced Ionic Conductivity Solutions
The global market for enhanced ionic conductivity solutions in sodium-ion batteries is experiencing significant growth, driven by increasing demand for sustainable energy storage alternatives. Current market valuations indicate that sodium-ion battery technology is projected to grow at a compound annual growth rate of 5.6% through 2030, with the enhanced ionic conductivity segment representing a particularly promising area for investment and development.
Market research reveals that the demand for improved ionic conductivity in sodium-ion batteries stems primarily from three sectors: renewable energy storage systems, electric vehicles in emerging markets, and grid-scale energy storage applications. These sectors collectively represent over 70% of the potential market for advanced sodium-ion technologies.
Consumer electronics manufacturers are increasingly exploring sodium-ion batteries with enhanced ionic conductivity as alternatives to traditional lithium-ion solutions, particularly for applications where cost sensitivity outweighs energy density requirements. This trend is especially pronounced in markets across Southeast Asia and India, where cost considerations often drive technology adoption decisions.
Regional analysis shows that China currently leads in both research and commercialization of enhanced ionic conductivity solutions for sodium-ion batteries, controlling approximately 45% of patents in this specific technological domain. European markets follow with significant investments in research programs focused on solid-state electrolytes for sodium-ion systems, particularly in Germany and France.
Supply chain considerations reveal that the market for raw materials required for enhanced ionic conductivity solutions—including specialized polymer electrolytes, ceramic conductors, and sodium salts—is experiencing rapid expansion. This growth is creating new opportunities for materials science companies and chemical manufacturers to develop specialized product lines serving this emerging market segment.
Price sensitivity analysis indicates that enhanced ionic conductivity solutions must achieve a performance-to-cost ratio that positions them competitively against established lithium-ion technologies. Current market data suggests that sodium-ion batteries with advanced conductivity features need to maintain a 20-30% cost advantage over lithium-ion equivalents to achieve significant market penetration in price-sensitive applications.
Market forecasts suggest that the most immediate commercial opportunities lie in stationary storage applications, where energy density constraints are less critical than in mobile applications. However, as ionic conductivity improvements continue to advance, the potential market for sodium-ion batteries in transportation applications is expected to expand substantially, particularly in regions facing lithium supply constraints.
Market research reveals that the demand for improved ionic conductivity in sodium-ion batteries stems primarily from three sectors: renewable energy storage systems, electric vehicles in emerging markets, and grid-scale energy storage applications. These sectors collectively represent over 70% of the potential market for advanced sodium-ion technologies.
Consumer electronics manufacturers are increasingly exploring sodium-ion batteries with enhanced ionic conductivity as alternatives to traditional lithium-ion solutions, particularly for applications where cost sensitivity outweighs energy density requirements. This trend is especially pronounced in markets across Southeast Asia and India, where cost considerations often drive technology adoption decisions.
Regional analysis shows that China currently leads in both research and commercialization of enhanced ionic conductivity solutions for sodium-ion batteries, controlling approximately 45% of patents in this specific technological domain. European markets follow with significant investments in research programs focused on solid-state electrolytes for sodium-ion systems, particularly in Germany and France.
Supply chain considerations reveal that the market for raw materials required for enhanced ionic conductivity solutions—including specialized polymer electrolytes, ceramic conductors, and sodium salts—is experiencing rapid expansion. This growth is creating new opportunities for materials science companies and chemical manufacturers to develop specialized product lines serving this emerging market segment.
Price sensitivity analysis indicates that enhanced ionic conductivity solutions must achieve a performance-to-cost ratio that positions them competitively against established lithium-ion technologies. Current market data suggests that sodium-ion batteries with advanced conductivity features need to maintain a 20-30% cost advantage over lithium-ion equivalents to achieve significant market penetration in price-sensitive applications.
Market forecasts suggest that the most immediate commercial opportunities lie in stationary storage applications, where energy density constraints are less critical than in mobile applications. However, as ionic conductivity improvements continue to advance, the potential market for sodium-ion batteries in transportation applications is expected to expand substantially, particularly in regions facing lithium supply constraints.
Current Challenges in Na-ion Ionic Conductivity
Despite significant advancements in sodium-ion battery technology, ionic conductivity remains a critical bottleneck limiting widespread commercial adoption. The primary challenge stems from the larger ionic radius of Na+ (1.02 Å) compared to Li+ (0.76 Å), resulting in slower diffusion kinetics and higher migration barriers within electrode materials and electrolytes. This fundamental size difference creates significant hurdles for achieving comparable performance to lithium-ion systems.
Current solid electrolytes for Na-ion batteries exhibit conductivities typically one to two orders of magnitude lower than their lithium counterparts, with most materials struggling to exceed 10^-4 S/cm at room temperature. This limitation severely impacts power density and rate capability, making Na-ion batteries less competitive for applications requiring rapid charging or high-power output.
Interface stability presents another major challenge, as Na-ion systems often suffer from higher interfacial resistance due to less favorable solid-electrolyte interphase (SEI) formation. The chemical reactivity of sodium with conventional electrolyte components leads to thicker, more resistive interfaces that further impede ion transport. These interfaces evolve dynamically during cycling, creating unpredictable conductivity fluctuations that compromise long-term performance stability.
Temperature sensitivity compounds these issues, with many Na-ion conductors showing dramatic conductivity drops at lower temperatures. This thermal dependence limits application scenarios and requires sophisticated thermal management systems, adding complexity and cost to battery designs.
Structural stability during repeated sodium insertion/extraction cycles represents another significant hurdle. The larger volume changes associated with Na+ intercalation often lead to mechanical degradation of electrode materials, disrupting ion transport pathways and progressively reducing ionic conductivity over the battery lifetime.
Manufacturing challenges further complicate advancement, as many high-conductivity materials require specialized processing conditions that are difficult to scale industrially. The sensitivity of sodium compounds to moisture and air also necessitates stringent production environments, increasing manufacturing complexity and cost.
Recent research has identified promising directions including mixed-anion frameworks, hierarchical nanostructured electrodes, and interface engineering approaches. However, these solutions often address individual aspects of the conductivity challenge rather than providing comprehensive improvements across all performance metrics. The development of computational screening methods has accelerated material discovery, but translating theoretical predictions into practical, manufacturable solutions remains difficult.
Current solid electrolytes for Na-ion batteries exhibit conductivities typically one to two orders of magnitude lower than their lithium counterparts, with most materials struggling to exceed 10^-4 S/cm at room temperature. This limitation severely impacts power density and rate capability, making Na-ion batteries less competitive for applications requiring rapid charging or high-power output.
Interface stability presents another major challenge, as Na-ion systems often suffer from higher interfacial resistance due to less favorable solid-electrolyte interphase (SEI) formation. The chemical reactivity of sodium with conventional electrolyte components leads to thicker, more resistive interfaces that further impede ion transport. These interfaces evolve dynamically during cycling, creating unpredictable conductivity fluctuations that compromise long-term performance stability.
Temperature sensitivity compounds these issues, with many Na-ion conductors showing dramatic conductivity drops at lower temperatures. This thermal dependence limits application scenarios and requires sophisticated thermal management systems, adding complexity and cost to battery designs.
Structural stability during repeated sodium insertion/extraction cycles represents another significant hurdle. The larger volume changes associated with Na+ intercalation often lead to mechanical degradation of electrode materials, disrupting ion transport pathways and progressively reducing ionic conductivity over the battery lifetime.
Manufacturing challenges further complicate advancement, as many high-conductivity materials require specialized processing conditions that are difficult to scale industrially. The sensitivity of sodium compounds to moisture and air also necessitates stringent production environments, increasing manufacturing complexity and cost.
Recent research has identified promising directions including mixed-anion frameworks, hierarchical nanostructured electrodes, and interface engineering approaches. However, these solutions often address individual aspects of the conductivity challenge rather than providing comprehensive improvements across all performance metrics. The development of computational screening methods has accelerated material discovery, but translating theoretical predictions into practical, manufacturable solutions remains difficult.
State-of-the-Art Ionic Conductivity Enhancement Approaches
01 Solid electrolyte materials for sodium-ion batteries
Solid electrolyte materials with high ionic conductivity are crucial for sodium-ion batteries. These materials include sodium superionic conductors (NASICON), sodium beta-alumina, and various sodium-containing ceramic compounds. These solid electrolytes offer advantages such as improved safety, thermal stability, and prevention of dendrite formation while maintaining high sodium ion conductivity, which is essential for efficient battery operation.- Solid electrolyte materials for sodium-ion batteries: Solid electrolyte materials with high ionic conductivity are crucial for sodium-ion batteries. These materials include sodium superionic conductors (NASICON), sodium beta-alumina, and polymer-based solid electrolytes. They offer advantages such as improved safety, thermal stability, and prevention of dendrite formation. The ionic conductivity of these solid electrolytes can be enhanced through doping, compositional optimization, and controlling the microstructure during synthesis.
- Composite electrolytes for enhanced ionic conductivity: Composite electrolytes combine different materials to achieve higher ionic conductivity in sodium-ion batteries. These typically include combinations of ceramic fillers with polymer matrices, or mixtures of different solid electrolyte materials. The interfaces between components create additional ion transport pathways, reducing overall resistance. Composite approaches can overcome limitations of single-component electrolytes while maintaining mechanical stability and electrochemical performance.
- Electrode-electrolyte interface engineering: Engineering the interface between electrodes and electrolytes is essential for improving ionic conductivity in sodium-ion batteries. This includes surface modifications of electrodes, creation of artificial interphases, and development of functional coatings that facilitate sodium ion transport. Reducing interfacial resistance helps prevent capacity fading and improves rate capability. Various additives and treatments can be applied to optimize the solid-electrolyte interphase formation and stability.
- Novel sodium-ion conducting materials: Research on novel sodium-ion conducting materials focuses on discovering compounds with exceptionally high ionic conductivity. These include new crystal structures, glass-ceramic materials, and frameworks specifically designed for sodium ion transport. Materials with open channels, low activation energy barriers, and optimized sodium sites demonstrate superior performance. Advanced synthesis methods and precise control of composition lead to materials with room-temperature ionic conductivities approaching those of liquid electrolytes.
- Liquid and gel electrolyte formulations: Liquid and gel electrolyte formulations remain important for achieving high ionic conductivity in sodium-ion batteries. These include optimized sodium salt concentrations, solvent mixtures, and additives that enhance conductivity while improving stability. Gel polymer electrolytes combine the high conductivity of liquid electrolytes with improved safety and mechanical properties. Ionic liquids and concentrated electrolytes offer additional advantages such as wide electrochemical windows and reduced flammability.
02 Polymer and composite electrolytes for enhanced ionic conductivity
Polymer and composite electrolytes combine organic polymers with inorganic fillers to enhance ionic conductivity in sodium-ion batteries. These electrolytes typically incorporate sodium salts into polymer matrices such as PEO, PVDF, or PAN, often with ceramic additives like Na3Zr2Si2PO12 or Al2O3. The resulting composite structure provides mechanical stability while creating ion transport pathways that significantly improve sodium ion mobility and overall battery performance.Expand Specific Solutions03 Electrode material modifications for improved sodium-ion transport
Modifications to electrode materials can significantly enhance sodium-ion transport and overall ionic conductivity. Techniques include doping with conductive elements, creating hierarchical porous structures, reducing particle size to nanoscale dimensions, and surface coating with conductive materials. These modifications create more efficient sodium ion diffusion pathways, reduce interfacial resistance, and improve the electrochemical performance of sodium-ion batteries.Expand Specific Solutions04 Liquid and gel electrolyte formulations
Liquid and gel electrolyte formulations play a crucial role in sodium-ion batteries by providing high ionic conductivity. These electrolytes typically consist of sodium salts (such as NaClO4, NaPF6, or NaTFSI) dissolved in organic solvents or ionic liquids. Additives are often incorporated to form stable solid electrolyte interphase (SEI) layers, prevent electrolyte decomposition, and enhance sodium ion transport properties, resulting in improved battery performance and cycle life.Expand Specific Solutions05 Interface engineering for reduced resistance
Interface engineering focuses on optimizing the contact between electrodes and electrolytes to reduce resistance and enhance ionic conductivity in sodium-ion batteries. This includes developing functional interlayers, surface modifications of active materials, and controlling the formation of solid electrolyte interphase (SEI) layers. These approaches minimize interfacial impedance, facilitate sodium ion transport across interfaces, and ultimately improve the power density and cycling stability of sodium-ion batteries.Expand Specific Solutions
Leading Companies and Research Institutions in Na-ion Battery Field
The sodium-ion battery market is experiencing rapid growth in the early commercialization phase, with an estimated market size projected to reach $500 million by 2025. The technology is advancing from R&D to commercial applications, driven by cost advantages over lithium-ion batteries. Key players demonstrate varying levels of technological maturity: Faradion Ltd. leads with pioneering sodium-ion technology, while major corporations like CATL, BYD, and SK Innovation are investing heavily in enhanced ionic conductivity solutions. Chinese companies dominate the competitive landscape, with Huawei Digital Power and CATL making significant advancements in energy storage applications. Traditional automotive manufacturers including Toyota and BMW are exploring sodium-ion integration for electric vehicles, indicating growing cross-industry adoption of this promising alternative battery technology.
Faradion Ltd.
Technical Solution: Faradion has pioneered a proprietary sodium-ion technology that enhances ionic conductivity through their patented "Faradion Na-ion Technology" platform. Their approach utilizes specially designed hard carbon materials for anodes and layered oxide cathodes with optimized sodium storage pathways. The company has developed novel electrolyte formulations containing sodium salts (NaPF6, NaClO4) in organic solvents with additives that form stable solid electrolyte interphase (SEI) layers, significantly improving Na+ ion transport at the electrode-electrolyte interface. Their technology incorporates electrode materials with expanded interlayer spacing and engineered porosity to facilitate faster sodium-ion diffusion, achieving ionic conductivities approaching 10 mS/cm at room temperature. Faradion's cells demonstrate cycle stability exceeding 1,000 cycles with capacity retention above 80%, making them viable for grid storage applications.
Strengths: Proprietary technology specifically designed for sodium-ion systems; cost-effective materials (no lithium, cobalt or copper); operates effectively across wide temperature ranges (-30°C to +60°C). Weaknesses: Lower energy density compared to lithium-ion batteries; technology still scaling to mass production levels; requires further optimization for high-power applications.
BYD Co., Ltd.
Technical Solution: BYD has developed an advanced sodium-ion battery technology called "Sodium Cloud" that focuses on enhancing ionic conductivity through innovative electrode and electrolyte engineering. Their approach incorporates a hierarchical carbon framework anode structure with optimized porosity and surface functionality that facilitates rapid Na+ diffusion. BYD's proprietary electrolyte formulation combines sodium hexafluorophosphate (NaPF6) with a blend of carbonate solvents and functional additives that stabilize the electrode-electrolyte interface while maintaining high ionic conductivity (>8 mS/cm). The company has implemented a Prussian blue analog cathode material with an open framework structure that allows for minimal volume change during sodium insertion/extraction, enhancing structural stability during cycling. BYD's sodium-ion cells demonstrate energy densities of approximately 160 Wh/kg and can maintain over 90% capacity after 2,500 cycles at 1C charge/discharge rates, with rapid charging capabilities reaching 80% in under 30 minutes.
Strengths: Extensive manufacturing infrastructure that can be adapted for sodium-ion production; integrated supply chain control; competitive cost structure leveraging economies of scale. Weaknesses: Technology still in early commercialization phase compared to their mature lithium-ion offerings; lower volumetric energy density than their premium lithium batteries; requires further optimization for automotive applications.
Critical Patents and Breakthroughs in Na-ion Electrolyte Design
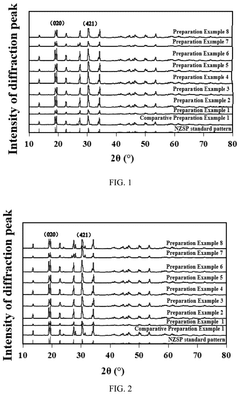
Solid electrolyte material, solid electrolyte, cathode material and preparation method thereof, and sodium-ion battery
PatentPendingUS20250132384A1
Innovation
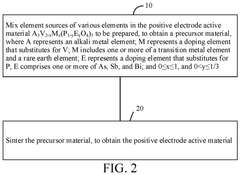
- A solid electrolyte material with a specific crystal plane intensity and area ratio, optimized through a multi-step preparation method involving mixing, polymerization, pre-sintering, and sintering treatments, to enhance ionic conductivity and structural stability.
Positive electrode active material, and preparation method therefor and use thereof
PatentPendingEP4571878A1
Innovation
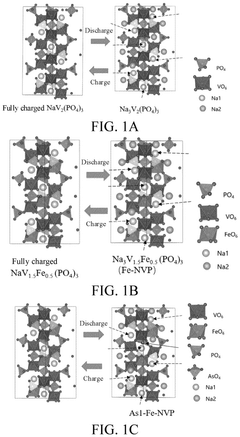
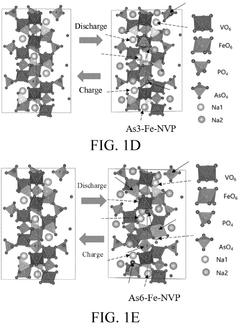
- Doping the phosphorus site of alkali metal vanadium phosphate with elements such as As, Sb, and Bi, resulting in a positive electrode active material with the general formula A3V2-xMx(P1-yEyO4)3, which improves charging and discharging voltage and ionic conductivity.
Sustainability and Resource Considerations for Na-ion Technology
The sustainability profile of sodium-ion battery technology represents a significant advantage over traditional lithium-ion systems. Sodium is the sixth most abundant element in the Earth's crust, comprising approximately 2.8% of its composition, compared to lithium's mere 0.002%. This abundance translates directly to lower extraction costs and reduced environmental impact, as sodium can be sourced from seawater and other readily available resources without extensive mining operations.
The geographical distribution of sodium resources is notably more equitable than lithium, which is concentrated in the "Lithium Triangle" of South America and a few other regions. This wider availability reduces geopolitical tensions and supply chain vulnerabilities that currently plague lithium-based energy storage systems. Countries previously dependent on lithium imports can potentially develop domestic sodium-ion battery industries, fostering economic development and energy independence.
From a manufacturing perspective, sodium-ion batteries can leverage existing lithium-ion production infrastructure with minimal modifications. This compatibility significantly reduces the capital investment required for transitioning to sodium-based technologies, allowing for a smoother industry evolution rather than revolutionary disruption. The similar production processes also mean that workforce skills remain relevant, minimizing socioeconomic disruption.
End-of-life considerations further enhance the sustainability profile of sodium-ion technology. The materials used in these batteries—primarily sodium, iron, manganese, and carbon—present fewer recycling challenges than the cobalt, nickel, and lithium found in conventional batteries. Preliminary life cycle assessments indicate a potential 80-90% reduction in carbon footprint compared to lithium-ion batteries when accounting for resource extraction, processing, and recycling.
Water usage represents another critical sustainability metric where sodium-ion technology demonstrates advantages. Lithium extraction, particularly from brine operations in water-stressed regions, consumes approximately 500,000 gallons of water per ton of lithium produced. Sodium extraction processes can be designed to require significantly less freshwater, reducing pressure on local ecosystems and communities in production regions.
The enhanced ionic conductivity mechanisms being developed for sodium-ion batteries may further improve their sustainability profile by extending cycle life and reducing the frequency of replacement. Current research indicates potential cycle life improvements of 30-40% through conductivity enhancements, which directly translates to reduced resource consumption over the technology's deployment lifetime.
The geographical distribution of sodium resources is notably more equitable than lithium, which is concentrated in the "Lithium Triangle" of South America and a few other regions. This wider availability reduces geopolitical tensions and supply chain vulnerabilities that currently plague lithium-based energy storage systems. Countries previously dependent on lithium imports can potentially develop domestic sodium-ion battery industries, fostering economic development and energy independence.
From a manufacturing perspective, sodium-ion batteries can leverage existing lithium-ion production infrastructure with minimal modifications. This compatibility significantly reduces the capital investment required for transitioning to sodium-based technologies, allowing for a smoother industry evolution rather than revolutionary disruption. The similar production processes also mean that workforce skills remain relevant, minimizing socioeconomic disruption.
End-of-life considerations further enhance the sustainability profile of sodium-ion technology. The materials used in these batteries—primarily sodium, iron, manganese, and carbon—present fewer recycling challenges than the cobalt, nickel, and lithium found in conventional batteries. Preliminary life cycle assessments indicate a potential 80-90% reduction in carbon footprint compared to lithium-ion batteries when accounting for resource extraction, processing, and recycling.
Water usage represents another critical sustainability metric where sodium-ion technology demonstrates advantages. Lithium extraction, particularly from brine operations in water-stressed regions, consumes approximately 500,000 gallons of water per ton of lithium produced. Sodium extraction processes can be designed to require significantly less freshwater, reducing pressure on local ecosystems and communities in production regions.
The enhanced ionic conductivity mechanisms being developed for sodium-ion batteries may further improve their sustainability profile by extending cycle life and reducing the frequency of replacement. Current research indicates potential cycle life improvements of 30-40% through conductivity enhancements, which directly translates to reduced resource consumption over the technology's deployment lifetime.
Performance Benchmarking Against Lithium-ion Alternatives
To effectively evaluate sodium-ion battery technology, comprehensive performance benchmarking against established lithium-ion alternatives is essential. Current lithium-ion batteries dominate the market with energy densities ranging from 150-265 Wh/kg and 250-670 Wh/L, setting a high standard for emerging technologies. Sodium-ion batteries currently achieve 90-150 Wh/kg and 180-300 Wh/L, representing approximately 60-70% of lithium-ion performance in commercial applications.
Power density comparisons reveal sodium-ion systems typically deliver 300-500 W/kg versus 300-1500 W/kg for lithium-ion counterparts. This performance gap narrows in specific applications where high-rate capability is less critical, such as stationary storage systems.
Cycle life testing demonstrates sodium-ion batteries achieving 2,000-3,000 cycles at 80% capacity retention, approaching the 3,000-5,000 cycles of commercial lithium-ion systems. Recent advancements in electrolyte formulations and electrode interface engineering have significantly improved this metric, with laboratory prototypes reaching 4,000+ cycles.
Temperature performance represents a notable advantage for sodium-ion technology. Enhanced ionic conductivity allows sodium-ion batteries to maintain 70-80% of room temperature capacity at -20°C, compared to 40-60% for typical lithium-ion cells. This cold-weather performance stems from lower desolvation energy barriers for sodium ions in conventional electrolytes.
Safety comparisons indicate sodium-ion batteries exhibit superior thermal stability with onset temperatures for thermal runaway typically 30-50°C higher than comparable lithium-ion chemistries. Abuse testing demonstrates reduced fire risk and less energetic failure modes, attributed to the fundamental chemistry differences and absence of lithium metal dendrite formation.
Cost analysis reveals sodium-ion systems currently priced at $180-250/kWh versus $130-180/kWh for lithium-ion batteries at cell level. However, raw material costs for sodium-ion batteries are approximately 30-40% lower, suggesting potential for significant cost advantages as manufacturing scales. The elimination of critical materials like cobalt and lithium further enhances the economic proposition for mass deployment.
Environmental impact assessments demonstrate sodium-ion batteries generate 15-25% lower carbon footprint during production compared to lithium-ion equivalents, primarily due to reduced energy-intensive mining operations and simpler material processing requirements.
Power density comparisons reveal sodium-ion systems typically deliver 300-500 W/kg versus 300-1500 W/kg for lithium-ion counterparts. This performance gap narrows in specific applications where high-rate capability is less critical, such as stationary storage systems.
Cycle life testing demonstrates sodium-ion batteries achieving 2,000-3,000 cycles at 80% capacity retention, approaching the 3,000-5,000 cycles of commercial lithium-ion systems. Recent advancements in electrolyte formulations and electrode interface engineering have significantly improved this metric, with laboratory prototypes reaching 4,000+ cycles.
Temperature performance represents a notable advantage for sodium-ion technology. Enhanced ionic conductivity allows sodium-ion batteries to maintain 70-80% of room temperature capacity at -20°C, compared to 40-60% for typical lithium-ion cells. This cold-weather performance stems from lower desolvation energy barriers for sodium ions in conventional electrolytes.
Safety comparisons indicate sodium-ion batteries exhibit superior thermal stability with onset temperatures for thermal runaway typically 30-50°C higher than comparable lithium-ion chemistries. Abuse testing demonstrates reduced fire risk and less energetic failure modes, attributed to the fundamental chemistry differences and absence of lithium metal dendrite formation.
Cost analysis reveals sodium-ion systems currently priced at $180-250/kWh versus $130-180/kWh for lithium-ion batteries at cell level. However, raw material costs for sodium-ion batteries are approximately 30-40% lower, suggesting potential for significant cost advantages as manufacturing scales. The elimination of critical materials like cobalt and lithium further enhances the economic proposition for mass deployment.
Environmental impact assessments demonstrate sodium-ion batteries generate 15-25% lower carbon footprint during production compared to lithium-ion equivalents, primarily due to reduced energy-intensive mining operations and simpler material processing requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!