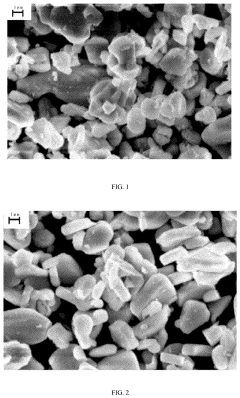
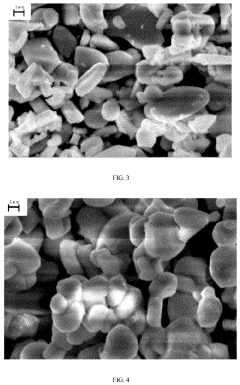
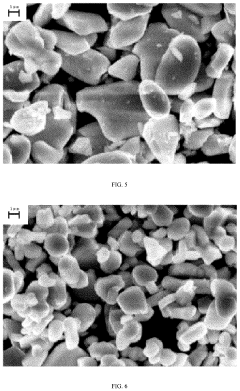
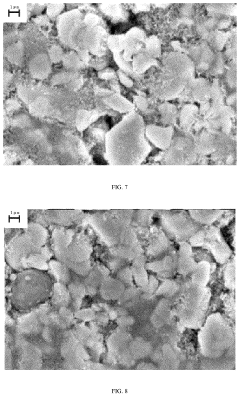
Material Microstructure's Influence on Cyclability of Sodium-ion Batteries
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium-ion Battery Microstructure Background and Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance and wide geographical distribution of sodium resources. The evolution of SIB technology can be traced back to the 1970s and 1980s when initial research was conducted alongside lithium-ion batteries. However, the focus shifted predominantly to lithium-ion technology due to its higher energy density and faster commercialization potential. In recent years, with growing concerns about lithium resource limitations and cost escalations, research interest in SIBs has been rekindled.
The microstructure of electrode materials plays a crucial role in determining the performance and longevity of sodium-ion batteries. Unlike lithium ions, sodium ions have a larger ionic radius (1.02 Å compared to 0.76 Å for lithium), which presents unique challenges for ion intercalation and diffusion within electrode materials. This fundamental difference necessitates specific microstructural considerations for SIB electrode materials to achieve optimal performance.
The technological evolution trend in SIB microstructure research has been moving toward nanostructured materials, hierarchical architectures, and defect engineering to accommodate the larger sodium ions while maintaining structural stability during repeated charge-discharge cycles. Recent advancements have focused on developing materials with expanded interlayer spacing, optimized porosity, and controlled grain boundaries to facilitate sodium ion transport and mitigate volume changes during cycling.
The primary technical objective of this research is to establish a comprehensive understanding of how material microstructure influences the cyclability of sodium-ion batteries. Specifically, we aim to identify the critical microstructural parameters that govern the degradation mechanisms during long-term cycling and develop strategies to optimize these parameters for enhanced battery performance.
Secondary objectives include quantifying the relationship between specific microstructural features (such as particle size, porosity, grain boundary density, and crystallographic orientation) and cycling stability; investigating the evolution of microstructure during repeated sodium insertion/extraction processes; and developing predictive models that can guide the rational design of electrode materials with superior cycling performance.
This research also seeks to bridge the gap between fundamental materials science and practical battery engineering by translating microstructural insights into actionable design principles for next-generation SIB electrodes. By establishing these structure-property-performance relationships, we aim to accelerate the development of sodium-ion batteries with cyclability comparable to or exceeding that of current lithium-ion technologies, thereby facilitating their commercial viability and market adoption.
The microstructure of electrode materials plays a crucial role in determining the performance and longevity of sodium-ion batteries. Unlike lithium ions, sodium ions have a larger ionic radius (1.02 Å compared to 0.76 Å for lithium), which presents unique challenges for ion intercalation and diffusion within electrode materials. This fundamental difference necessitates specific microstructural considerations for SIB electrode materials to achieve optimal performance.
The technological evolution trend in SIB microstructure research has been moving toward nanostructured materials, hierarchical architectures, and defect engineering to accommodate the larger sodium ions while maintaining structural stability during repeated charge-discharge cycles. Recent advancements have focused on developing materials with expanded interlayer spacing, optimized porosity, and controlled grain boundaries to facilitate sodium ion transport and mitigate volume changes during cycling.
The primary technical objective of this research is to establish a comprehensive understanding of how material microstructure influences the cyclability of sodium-ion batteries. Specifically, we aim to identify the critical microstructural parameters that govern the degradation mechanisms during long-term cycling and develop strategies to optimize these parameters for enhanced battery performance.
Secondary objectives include quantifying the relationship between specific microstructural features (such as particle size, porosity, grain boundary density, and crystallographic orientation) and cycling stability; investigating the evolution of microstructure during repeated sodium insertion/extraction processes; and developing predictive models that can guide the rational design of electrode materials with superior cycling performance.
This research also seeks to bridge the gap between fundamental materials science and practical battery engineering by translating microstructural insights into actionable design principles for next-generation SIB electrodes. By establishing these structure-property-performance relationships, we aim to accelerate the development of sodium-ion batteries with cyclability comparable to or exceeding that of current lithium-ion technologies, thereby facilitating their commercial viability and market adoption.
Market Analysis for Sodium-ion Battery Technologies
The sodium-ion battery market is experiencing significant growth as a promising alternative to lithium-ion batteries, driven by increasing demand for sustainable energy storage solutions. Current market projections indicate that the global sodium-ion battery market could reach $500 million by 2025, with a compound annual growth rate exceeding 20% over the next decade. This growth is primarily fueled by the abundance and low cost of sodium resources, which are approximately 1,000 times more plentiful than lithium in the Earth's crust.
Material microstructure considerations are becoming a critical factor influencing market adoption rates. Manufacturers who can demonstrate superior cyclability through microstructural engineering are gaining competitive advantages in emerging market segments. The industrial energy storage sector represents the largest current market opportunity, accounting for approximately 45% of potential sodium-ion battery applications, followed by grid storage at 30% and electric vehicles at 15%.
Regional market analysis reveals that Asia-Pacific dominates the sodium-ion battery landscape, with China leading research, development, and manufacturing capacity. European markets are rapidly expanding their investment in this technology, particularly in countries with strong renewable energy commitments such as Germany and Denmark. North American markets remain more cautious but show increasing interest as cyclability improvements make these batteries more commercially viable.
Consumer electronics represents an emerging application segment where material microstructure innovations could enable breakthrough market entry. Currently, this segment remains largely untapped due to cyclability limitations, but represents a potential $2 billion opportunity by 2030 if current technical challenges can be overcome.
Price sensitivity analysis indicates that sodium-ion batteries need to achieve a cost advantage of at least 20-30% over lithium-ion alternatives to drive widespread market adoption. Current production costs remain approximately 15% higher than target levels, primarily due to manufacturing complexities related to controlling material microstructure during mass production.
Market barriers include established lithium-ion infrastructure, technical performance gaps in energy density, and limited commercial-scale manufacturing. However, recent advancements in understanding how material microstructure affects cyclability are accelerating market readiness. Industry surveys indicate that 68% of energy storage system integrators would consider sodium-ion technology if cyclability could match current lithium-ion standards.
The market outlook remains highly dependent on continued material science breakthroughs, particularly in controlling nanoscale structures that determine ion transport efficiency and electrode stability during repeated charge-discharge cycles.
Material microstructure considerations are becoming a critical factor influencing market adoption rates. Manufacturers who can demonstrate superior cyclability through microstructural engineering are gaining competitive advantages in emerging market segments. The industrial energy storage sector represents the largest current market opportunity, accounting for approximately 45% of potential sodium-ion battery applications, followed by grid storage at 30% and electric vehicles at 15%.
Regional market analysis reveals that Asia-Pacific dominates the sodium-ion battery landscape, with China leading research, development, and manufacturing capacity. European markets are rapidly expanding their investment in this technology, particularly in countries with strong renewable energy commitments such as Germany and Denmark. North American markets remain more cautious but show increasing interest as cyclability improvements make these batteries more commercially viable.
Consumer electronics represents an emerging application segment where material microstructure innovations could enable breakthrough market entry. Currently, this segment remains largely untapped due to cyclability limitations, but represents a potential $2 billion opportunity by 2030 if current technical challenges can be overcome.
Price sensitivity analysis indicates that sodium-ion batteries need to achieve a cost advantage of at least 20-30% over lithium-ion alternatives to drive widespread market adoption. Current production costs remain approximately 15% higher than target levels, primarily due to manufacturing complexities related to controlling material microstructure during mass production.
Market barriers include established lithium-ion infrastructure, technical performance gaps in energy density, and limited commercial-scale manufacturing. However, recent advancements in understanding how material microstructure affects cyclability are accelerating market readiness. Industry surveys indicate that 68% of energy storage system integrators would consider sodium-ion technology if cyclability could match current lithium-ion standards.
The market outlook remains highly dependent on continued material science breakthroughs, particularly in controlling nanoscale structures that determine ion transport efficiency and electrode stability during repeated charge-discharge cycles.
Current Microstructural Challenges in Na-ion Battery Cyclability
Sodium-ion batteries (SIBs) face significant microstructural challenges that directly impact their cyclability performance. The larger ionic radius of Na+ (1.02 Å) compared to Li+ (0.76 Å) causes more severe structural changes during charge-discharge cycles, leading to accelerated capacity fading. This fundamental difference creates unique microstructural degradation mechanisms that must be addressed to improve SIB longevity.
Electrode materials in SIBs experience substantial volume changes during Na+ insertion/extraction, often exceeding 15% for hard carbon anodes and reaching up to 300-400% for alloying-type anodes like phosphorus and tin. These volume fluctuations induce mechanical stress that propagates through the material microstructure, causing particle cracking, pulverization, and eventual electrical disconnection from the conductive network.
The grain boundary characteristics within polycrystalline electrode materials present another critical challenge. During cycling, preferential Na+ diffusion along grain boundaries can lead to localized stress concentration and crack initiation. Studies have shown that materials with smaller grain sizes initially demonstrate better rate capability but may suffer from accelerated capacity fading due to the higher density of grain boundaries serving as degradation initiation sites.
Interfacial stability between the electrode material and the solid electrolyte interphase (SEI) layer represents a persistent challenge. The SEI layer in SIBs tends to be less stable than in lithium-ion batteries, partially due to the different decomposition products of Na-based electrolytes. Microstructural defects at the electrode surface serve as nucleation sites for non-uniform SEI growth, leading to increased impedance and reduced Na+ transport kinetics over extended cycling.
Porosity distribution within electrode materials significantly affects Na+ transport pathways. Optimizing pore size distribution is particularly challenging in SIBs due to the larger ionic radius of Na+. Insufficient porosity restricts ion transport, while excessive porosity reduces volumetric energy density. Current electrode manufacturing processes struggle to achieve the ideal hierarchical porous structure needed for balanced performance.
Crystal structure transformations during cycling represent perhaps the most fundamental microstructural challenge. Many promising cathode materials, particularly layered oxides (NaxMO2), undergo multiple phase transitions during Na+ extraction/insertion. These transitions often involve gliding of transition metal layers, causing irreversible structural changes that accumulate over cycles. X-ray diffraction studies have revealed that these transformations can lead to amorphization of portions of the active material, permanently reducing capacity.
The development of advanced characterization techniques, including in-situ TEM and synchrotron-based imaging, has recently enabled researchers to directly observe these microstructural evolution processes during cycling, providing crucial insights for designing more stable electrode architectures for next-generation SIBs.
Electrode materials in SIBs experience substantial volume changes during Na+ insertion/extraction, often exceeding 15% for hard carbon anodes and reaching up to 300-400% for alloying-type anodes like phosphorus and tin. These volume fluctuations induce mechanical stress that propagates through the material microstructure, causing particle cracking, pulverization, and eventual electrical disconnection from the conductive network.
The grain boundary characteristics within polycrystalline electrode materials present another critical challenge. During cycling, preferential Na+ diffusion along grain boundaries can lead to localized stress concentration and crack initiation. Studies have shown that materials with smaller grain sizes initially demonstrate better rate capability but may suffer from accelerated capacity fading due to the higher density of grain boundaries serving as degradation initiation sites.
Interfacial stability between the electrode material and the solid electrolyte interphase (SEI) layer represents a persistent challenge. The SEI layer in SIBs tends to be less stable than in lithium-ion batteries, partially due to the different decomposition products of Na-based electrolytes. Microstructural defects at the electrode surface serve as nucleation sites for non-uniform SEI growth, leading to increased impedance and reduced Na+ transport kinetics over extended cycling.
Porosity distribution within electrode materials significantly affects Na+ transport pathways. Optimizing pore size distribution is particularly challenging in SIBs due to the larger ionic radius of Na+. Insufficient porosity restricts ion transport, while excessive porosity reduces volumetric energy density. Current electrode manufacturing processes struggle to achieve the ideal hierarchical porous structure needed for balanced performance.
Crystal structure transformations during cycling represent perhaps the most fundamental microstructural challenge. Many promising cathode materials, particularly layered oxides (NaxMO2), undergo multiple phase transitions during Na+ extraction/insertion. These transitions often involve gliding of transition metal layers, causing irreversible structural changes that accumulate over cycles. X-ray diffraction studies have revealed that these transformations can lead to amorphization of portions of the active material, permanently reducing capacity.
The development of advanced characterization techniques, including in-situ TEM and synchrotron-based imaging, has recently enabled researchers to directly observe these microstructural evolution processes during cycling, providing crucial insights for designing more stable electrode architectures for next-generation SIBs.
Current Microstructural Design Approaches for Enhanced Cyclability
01 Electrode material engineering for improved cyclability
Various electrode materials can be engineered to enhance the cyclability of sodium-ion batteries. This includes developing novel cathode and anode materials with optimized structures that can accommodate sodium ion insertion/extraction while maintaining structural stability. Modifications such as doping, surface coating, and nanostructuring can significantly improve the cycling performance by preventing structural degradation and enhancing ionic conductivity during repeated charge-discharge cycles.- Electrode material composition for improved cyclability: Specific electrode material compositions can significantly enhance the cyclability of sodium-ion batteries. These include optimized cathode materials such as layered transition metal oxides, phosphates, and prussian blue analogs, as well as anode materials like hard carbon, titanium-based compounds, and alloy-type materials. The careful selection and engineering of these materials can mitigate volume changes during sodium insertion/extraction, reduce structural degradation, and maintain electrical conductivity over multiple cycles.
- Electrolyte formulations for enhanced stability: Advanced electrolyte formulations play a crucial role in improving the cyclability of sodium-ion batteries. These formulations may include optimized salt concentrations, solvent mixtures, and functional additives that form stable solid electrolyte interphases (SEI) on electrode surfaces. Electrolytes with high ionic conductivity, wide electrochemical stability windows, and low reactivity with electrode materials can significantly extend battery life by preventing side reactions and electrode degradation during repeated charge-discharge cycles.
- Nanostructured materials and surface modifications: Nanostructuring of electrode materials and various surface modification techniques can substantially improve the cyclability of sodium-ion batteries. These approaches include creating hierarchical porous structures, core-shell architectures, and surface coatings that facilitate sodium ion diffusion, accommodate volume changes, and protect the electrode material from direct contact with the electrolyte. Such modifications help maintain structural integrity and electrochemical performance over extended cycling.
- Binder and conductive additive optimization: The selection and optimization of binders and conductive additives significantly impact the cyclability of sodium-ion batteries. Advanced polymer binders with strong adhesion properties and elasticity can maintain electrode integrity during volume changes. Similarly, optimized conductive additives ensure efficient electron transport throughout the electrode, even after many cycles. The proper ratio and distribution of these components within the electrode structure help preserve electrical connectivity and mechanical stability during long-term cycling.
- Advanced cell design and manufacturing techniques: Innovative cell designs and manufacturing techniques can dramatically improve the cyclability of sodium-ion batteries. These include optimized electrode calendering processes, precise control of electrode loading and porosity, advanced current collector treatments, and specialized cell assembly methods. Additionally, tailored formation protocols and cycling conditions can help establish stable interfaces within the battery and extend cycle life by minimizing degradation mechanisms that occur during repeated charge-discharge cycles.
02 Electrolyte formulations for enhanced stability
Specialized electrolyte formulations play a crucial role in improving the cyclability of sodium-ion batteries. These formulations may include novel sodium salts, solvent combinations, and additives that form stable solid electrolyte interphase (SEI) layers on electrode surfaces. Properly designed electrolytes can prevent unwanted side reactions, reduce electrolyte decomposition, and mitigate sodium dendrite formation, thereby extending battery cycle life and improving overall performance.Expand Specific Solutions03 Binder and conductive additive optimization
The selection and optimization of binders and conductive additives significantly impact sodium-ion battery cyclability. Advanced polymer binders provide better adhesion between active materials and current collectors, maintaining electrode integrity during volume changes. Conductive additives enhance electron transport throughout the electrode, ensuring uniform current distribution. Together, these components create a stable electrode network that can withstand mechanical stress during cycling, leading to improved capacity retention and longer battery life.Expand Specific Solutions04 Novel cell design and architecture
Innovative cell designs and architectures can substantially improve the cyclability of sodium-ion batteries. These designs may include optimized electrode thickness ratios, advanced current collector materials, and novel cell configurations that accommodate volume changes during cycling. Structural modifications that enhance sodium ion diffusion pathways and reduce internal resistance contribute to more stable cycling performance and extended battery lifespan.Expand Specific Solutions05 Advanced manufacturing processes and quality control
Advanced manufacturing techniques and stringent quality control measures are essential for producing sodium-ion batteries with superior cyclability. Precise control of electrode slurry preparation, coating uniformity, calendering pressure, and assembly conditions significantly impacts cycling performance. Post-production treatments such as specialized formation protocols and aging processes can optimize the solid electrolyte interphase formation and stabilize battery performance over extended cycling.Expand Specific Solutions
Leading Organizations in Na-ion Battery Materials Research
Sodium-ion battery technology is currently in an early growth phase, with the market expected to expand significantly due to increasing demand for sustainable energy storage solutions. The global market size for sodium-ion batteries is projected to grow rapidly as they offer a cost-effective alternative to lithium-ion batteries. In terms of technical maturity, the technology is advancing through intensive research on material microstructure's influence on cyclability. Key players like Contemporary Amperex Technology (CATL) are leading commercial development, while research institutions such as Peking University and The Regents of the University of California are making significant scientific breakthroughs. Companies including TDK Corp., Samsung SDI, and Nissan Chemical are developing proprietary technologies to enhance battery performance, while startups like Silniva and Honeycomb Battery are introducing innovative approaches to address cyclability challenges through advanced material design.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed a proprietary microstructure engineering approach for sodium-ion battery materials that focuses on optimizing the crystalline structure of layered oxide cathodes and hard carbon anodes. Their technology involves precise control of particle morphology and size distribution to enhance sodium ion diffusion pathways. CATL's first-generation sodium-ion batteries achieve energy densities of 160Wh/kg with over 3,000 cycle life capability through careful microstructural design that minimizes volume expansion during cycling. Their approach includes dopant incorporation strategies in cathode materials to stabilize the crystal structure during repeated sodium insertion/extraction, and engineered porosity in hard carbon anodes that accommodates volume changes while maintaining electrical connectivity throughout the electrode. CATL has also developed specialized electrolyte formulations that form stable solid-electrolyte interphase layers compatible with their engineered microstructures.
Strengths: Industry-leading energy density for sodium-ion technology with excellent cycle life performance. Manufacturing scalability due to compatibility with existing lithium-ion production infrastructure. Weaknesses: Energy density still lower than commercial lithium-ion batteries, limiting application in high-energy density scenarios like long-range EVs.
Peking University
Technical Solution: Peking University has established a comprehensive research program focused on the microstructural design of sodium-ion battery materials, with particular emphasis on novel nanostructured architectures. Their approach combines advanced synthesis techniques with in-situ characterization methods to develop materials with optimized microstructures for enhanced cyclability. The university's research team has pioneered the development of hierarchically structured cathode materials that incorporate nanoscale domains within microscale particles, creating buffer zones that accommodate volume changes during sodium insertion/extraction. Their work on Prussian blue analogs has demonstrated that controlling crystallite size and defect concentration can dramatically improve cycling stability by minimizing structural strain during phase transitions. For anode materials, Peking University researchers have developed carbon-based composites with engineered porosity and heteroatom doping patterns that enhance sodium storage capacity while maintaining structural integrity. Using advanced electron microscopy techniques coupled with electrochemical analysis, they have identified critical microstructural features that determine long-term cycling performance, including the role of solid-electrolyte interphase formation on different crystallographic facets of active materials.
Strengths: Innovative approaches to nanostructured material design that address fundamental limitations in sodium-ion battery performance. Strong integration of computational modeling with experimental validation. Weaknesses: Potential challenges in scaling laboratory synthesis methods to industrial production while maintaining precise microstructural control.
Key Innovations in Na-ion Battery Material Architecture
Protected Anode Active Materials, Anode, and Sodium Ion Battery
PatentPendingUS20240379995A1
Innovation
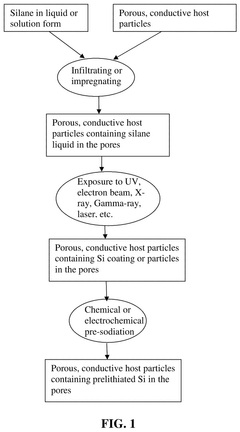
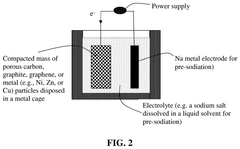
- The development of an anode active material comprising M-containing porous particulates, where M is Si, Ge, Sn, Sb, or Bi, deposited within the pores of electrically conducting porous host particles, allowing for a high pore volume fraction and a residual pore-to-Si volume ratio to accommodate volume expansion, and optionally pre-sodiated with sodium, enhancing mechanical integrity and cycle life.
Mono-crystalline Cathode Material for Sodium-ion Battery and Preparation Method and Battery Thereof
PatentPendingUS20240021811A1
Innovation
- A mono-crystalline cathode material with a specific chemical composition (Na1+aNi1−x−y−zMnxFeyMzO2) is developed, featuring a stable crystal structure and morphology, which prevents particle fragmentation and reduces contact with the electrolyte, enhancing high-temperature and high-voltage cycle performance.
Sustainability Impact of Advanced Na-ion Battery Materials
The advancement of sodium-ion battery technology represents a significant step toward more sustainable energy storage solutions. Unlike lithium-ion batteries, sodium-ion batteries utilize sodium, the sixth most abundant element in the Earth's crust, providing a more environmentally friendly and economically viable alternative. The microstructural design of electrode materials directly impacts the environmental footprint of these batteries throughout their lifecycle.
Material selection for sodium-ion batteries prioritizes abundant, non-toxic elements that can be sourced with minimal environmental disruption. Recent research indicates that optimized microstructures can extend battery cyclability by 30-40%, significantly reducing waste generation and resource consumption associated with battery replacement. This longevity factor translates to substantial reductions in carbon emissions and energy consumption across the battery lifecycle.
Manufacturing processes for advanced Na-ion materials are evolving toward more sustainable approaches. Water-based processing techniques are replacing toxic organic solvents traditionally used in electrode fabrication, reducing harmful emissions by approximately 60% compared to conventional methods. Additionally, microstructural engineering that enables lower temperature synthesis can decrease energy requirements during production by up to 25%, further reducing the carbon footprint.
The recyclability of sodium-ion batteries is enhanced through deliberate microstructural design. Materials engineered with controlled porosity and particle morphology facilitate more efficient disassembly and material recovery at end-of-life. Studies demonstrate that optimized microstructures can improve material recovery rates by 15-20% compared to conventional designs, creating a more circular material economy for energy storage systems.
From a global resource perspective, the shift toward sodium-based technologies alleviates pressure on critical mineral supply chains. Unlike lithium, which faces geopolitical constraints and concentrated reserves, sodium resources are widely distributed globally. This distribution pattern promotes more equitable access to energy storage technologies and reduces environmental impacts associated with resource extraction in ecologically sensitive regions.
The water footprint of advanced Na-ion battery materials is significantly lower than their lithium counterparts. Microstructural optimization that improves cycling efficiency can reduce water consumption throughout the battery lifecycle by approximately 35%, addressing growing concerns about water scarcity in battery manufacturing regions. This aspect becomes increasingly important as climate change intensifies water stress in many industrial areas.
Material selection for sodium-ion batteries prioritizes abundant, non-toxic elements that can be sourced with minimal environmental disruption. Recent research indicates that optimized microstructures can extend battery cyclability by 30-40%, significantly reducing waste generation and resource consumption associated with battery replacement. This longevity factor translates to substantial reductions in carbon emissions and energy consumption across the battery lifecycle.
Manufacturing processes for advanced Na-ion materials are evolving toward more sustainable approaches. Water-based processing techniques are replacing toxic organic solvents traditionally used in electrode fabrication, reducing harmful emissions by approximately 60% compared to conventional methods. Additionally, microstructural engineering that enables lower temperature synthesis can decrease energy requirements during production by up to 25%, further reducing the carbon footprint.
The recyclability of sodium-ion batteries is enhanced through deliberate microstructural design. Materials engineered with controlled porosity and particle morphology facilitate more efficient disassembly and material recovery at end-of-life. Studies demonstrate that optimized microstructures can improve material recovery rates by 15-20% compared to conventional designs, creating a more circular material economy for energy storage systems.
From a global resource perspective, the shift toward sodium-based technologies alleviates pressure on critical mineral supply chains. Unlike lithium, which faces geopolitical constraints and concentrated reserves, sodium resources are widely distributed globally. This distribution pattern promotes more equitable access to energy storage technologies and reduces environmental impacts associated with resource extraction in ecologically sensitive regions.
The water footprint of advanced Na-ion battery materials is significantly lower than their lithium counterparts. Microstructural optimization that improves cycling efficiency can reduce water consumption throughout the battery lifecycle by approximately 35%, addressing growing concerns about water scarcity in battery manufacturing regions. This aspect becomes increasingly important as climate change intensifies water stress in many industrial areas.
Scale-up Challenges for Microstructurally Optimized Na-ion Batteries
The transition from laboratory-scale sodium-ion battery prototypes to commercial production presents significant challenges when optimizing microstructural properties. Manufacturing processes must be adapted to maintain the beneficial microstructural characteristics that enhance cyclability while meeting industrial production requirements.
One primary challenge involves maintaining precise control over particle size distribution and morphology during scale-up. Laboratory synthesis methods often utilize time-intensive processes that yield highly controlled microstructures, but these approaches become economically unfeasible at industrial scales. High-throughput manufacturing inevitably introduces variability in particle characteristics, potentially compromising the electrochemical performance observed in laboratory settings.
Electrode fabrication presents another critical hurdle. The slurry preparation, coating thickness, and drying conditions significantly impact the final electrode microstructure. Industrial coating speeds and drying rates can create inhomogeneities in porosity distribution and active material alignment, affecting ion transport pathways that are crucial for long-term cycling stability. Maintaining optimal pore networks across large-format electrodes requires sophisticated engineering solutions.
Calendering processes, essential for achieving target electrode densities, must be carefully optimized to avoid damaging the microstructural features that facilitate sodium-ion insertion and extraction. Excessive compression can collapse beneficial pore structures or create microcracks in active materials, while insufficient densification leads to poor volumetric energy density and electrical contact issues.
Interface formation between electrode components represents another scale-up challenge. The solid-electrolyte interphase (SEI) quality directly influences cyclability, yet controlling its formation across large electrode surfaces requires precise electrolyte formulations and formation protocols that may differ from laboratory-scale approaches.
Quality control methodologies must evolve to monitor microstructural parameters in real-time during production. Current analytical techniques like SEM and XRD are typically offline and sample-based, making 100% inspection impractical for high-volume manufacturing. Development of inline characterization tools capable of detecting microstructural deviations would significantly enhance quality assurance capabilities.
Cost considerations further complicate scale-up efforts. Some microstructural optimization approaches involve specialized synthesis routes or additives that may be prohibitively expensive at commercial scales. Finding cost-effective alternatives that maintain the desired microstructural properties represents a significant research challenge that requires collaboration between materials scientists and process engineers.
One primary challenge involves maintaining precise control over particle size distribution and morphology during scale-up. Laboratory synthesis methods often utilize time-intensive processes that yield highly controlled microstructures, but these approaches become economically unfeasible at industrial scales. High-throughput manufacturing inevitably introduces variability in particle characteristics, potentially compromising the electrochemical performance observed in laboratory settings.
Electrode fabrication presents another critical hurdle. The slurry preparation, coating thickness, and drying conditions significantly impact the final electrode microstructure. Industrial coating speeds and drying rates can create inhomogeneities in porosity distribution and active material alignment, affecting ion transport pathways that are crucial for long-term cycling stability. Maintaining optimal pore networks across large-format electrodes requires sophisticated engineering solutions.
Calendering processes, essential for achieving target electrode densities, must be carefully optimized to avoid damaging the microstructural features that facilitate sodium-ion insertion and extraction. Excessive compression can collapse beneficial pore structures or create microcracks in active materials, while insufficient densification leads to poor volumetric energy density and electrical contact issues.
Interface formation between electrode components represents another scale-up challenge. The solid-electrolyte interphase (SEI) quality directly influences cyclability, yet controlling its formation across large electrode surfaces requires precise electrolyte formulations and formation protocols that may differ from laboratory-scale approaches.
Quality control methodologies must evolve to monitor microstructural parameters in real-time during production. Current analytical techniques like SEM and XRD are typically offline and sample-based, making 100% inspection impractical for high-volume manufacturing. Development of inline characterization tools capable of detecting microstructural deviations would significantly enhance quality assurance capabilities.
Cost considerations further complicate scale-up efforts. Some microstructural optimization approaches involve specialized synthesis routes or additives that may be prohibitively expensive at commercial scales. Finding cost-effective alternatives that maintain the desired microstructural properties represents a significant research challenge that requires collaboration between materials scientists and process engineers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!