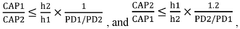Mass Transfer Challenges in Sodium-ion Batteries: Solutions and Improvements
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium-ion Battery Technology Background and Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries (LIBs) in recent years, primarily driven by concerns over lithium resource scarcity and rising costs. The development of SIBs can be traced back to the 1970s and 1980s, when researchers first began exploring sodium intercalation chemistry. However, the rapid commercialization of LIBs in the 1990s shifted focus away from sodium-based systems until the early 2010s, when renewed interest emerged due to sustainability considerations.
The fundamental operating principles of SIBs mirror those of LIBs, involving the shuttling of sodium ions between cathode and anode materials during charge-discharge cycles. However, sodium ions present unique challenges due to their larger ionic radius (1.02Å compared to lithium's 0.76Å), which significantly impacts mass transfer kinetics within battery components. This size difference fundamentally alters the intercalation chemistry, electrode material selection, and electrolyte requirements.
Current technological evolution in SIBs is focused on addressing these mass transfer limitations through innovative material design and engineering approaches. Research trends indicate growing emphasis on hierarchical electrode structures, advanced electrolyte formulations, and interface engineering to facilitate more efficient sodium ion transport. The field is witnessing a transition from fundamental research to practical application development, with increasing industry participation.
The primary technical objectives in this domain include enhancing sodium ion diffusion coefficients within electrode materials, optimizing electrolyte conductivity, and developing electrode architectures that can accommodate the volume changes associated with sodium insertion/extraction. Specifically, researchers aim to achieve energy densities exceeding 200 Wh/kg at the cell level, cycling stability beyond 2000 cycles, and rate capabilities comparable to commercial LIBs.
Another critical objective is cost reduction, with targets set at approximately 30-40% lower than equivalent LIB systems. This cost advantage, coupled with the abundance of sodium resources (sodium is the sixth most abundant element in Earth's crust), positions SIBs as particularly suitable for stationary energy storage applications where energy density constraints are less stringent than in portable electronics or electric vehicles.
The technology roadmap for SIBs envisions gradual market penetration beginning with grid-scale storage applications, followed by expansion into electric bicycles, low-speed electric vehicles, and eventually mainstream electric transportation. This progression aligns with projected improvements in energy density and cycle life as mass transfer challenges are systematically addressed through continued research and development efforts.
The fundamental operating principles of SIBs mirror those of LIBs, involving the shuttling of sodium ions between cathode and anode materials during charge-discharge cycles. However, sodium ions present unique challenges due to their larger ionic radius (1.02Å compared to lithium's 0.76Å), which significantly impacts mass transfer kinetics within battery components. This size difference fundamentally alters the intercalation chemistry, electrode material selection, and electrolyte requirements.
Current technological evolution in SIBs is focused on addressing these mass transfer limitations through innovative material design and engineering approaches. Research trends indicate growing emphasis on hierarchical electrode structures, advanced electrolyte formulations, and interface engineering to facilitate more efficient sodium ion transport. The field is witnessing a transition from fundamental research to practical application development, with increasing industry participation.
The primary technical objectives in this domain include enhancing sodium ion diffusion coefficients within electrode materials, optimizing electrolyte conductivity, and developing electrode architectures that can accommodate the volume changes associated with sodium insertion/extraction. Specifically, researchers aim to achieve energy densities exceeding 200 Wh/kg at the cell level, cycling stability beyond 2000 cycles, and rate capabilities comparable to commercial LIBs.
Another critical objective is cost reduction, with targets set at approximately 30-40% lower than equivalent LIB systems. This cost advantage, coupled with the abundance of sodium resources (sodium is the sixth most abundant element in Earth's crust), positions SIBs as particularly suitable for stationary energy storage applications where energy density constraints are less stringent than in portable electronics or electric vehicles.
The technology roadmap for SIBs envisions gradual market penetration beginning with grid-scale storage applications, followed by expansion into electric bicycles, low-speed electric vehicles, and eventually mainstream electric transportation. This progression aligns with projected improvements in energy density and cycle life as mass transfer challenges are systematically addressed through continued research and development efforts.
Market Analysis for Sodium-ion Energy Storage Solutions
The sodium-ion battery market is experiencing significant growth as a promising alternative to lithium-ion technologies, driven by increasing demand for sustainable and cost-effective energy storage solutions. Current market projections indicate that the global sodium-ion battery market could reach $500 million by 2025, with a compound annual growth rate exceeding 20% over the next decade. This growth trajectory is supported by the abundant nature of sodium resources, which are approximately 1,000 times more plentiful than lithium in the Earth's crust.
The market segmentation for sodium-ion batteries reveals diverse application potential. Grid-scale energy storage represents the largest current market segment, accounting for approximately 45% of demand, as utilities seek economical solutions for renewable energy integration. The electric vehicle sector, though currently limited in sodium-ion adoption, shows promising growth potential, particularly in markets sensitive to raw material costs and in applications where energy density requirements are less stringent, such as urban delivery vehicles and public transportation.
Consumer electronics constitutes another emerging market segment, particularly for applications where cost considerations outweigh the need for maximum energy density. Industrial applications, including backup power systems and material handling equipment, represent approximately 15% of the current market share.
Geographically, China leads the sodium-ion battery market development, with substantial government investment and research initiatives. The European market is growing rapidly due to stringent environmental regulations and renewable energy targets, while North America shows increasing interest driven by energy security concerns and sustainability goals.
Key market drivers include raw material economics, with sodium-based cathode materials costing 30-40% less than their lithium counterparts. Environmental considerations also play a significant role, as sodium extraction has a lower ecological footprint compared to lithium mining operations. Additionally, supply chain security concerns regarding critical battery materials are accelerating interest in sodium-ion technology as a strategic alternative.
Market barriers primarily relate to performance limitations, particularly the lower energy density of sodium-ion batteries compared to lithium-ion counterparts. Current sodium-ion technologies achieve approximately 160 Wh/kg versus 250+ Wh/kg for advanced lithium-ion cells. Manufacturing scale remains limited, with production capacity at less than 5% of lithium-ion battery manufacturing, resulting in higher unit costs despite lower raw material expenses.
Customer adoption patterns indicate that price-sensitive markets and applications with moderate energy density requirements are the early adopters of sodium-ion technology. The total addressable market is expanding as performance improvements address mass transfer challenges, with potential to capture 8-10% of the global battery market by 2030.
The market segmentation for sodium-ion batteries reveals diverse application potential. Grid-scale energy storage represents the largest current market segment, accounting for approximately 45% of demand, as utilities seek economical solutions for renewable energy integration. The electric vehicle sector, though currently limited in sodium-ion adoption, shows promising growth potential, particularly in markets sensitive to raw material costs and in applications where energy density requirements are less stringent, such as urban delivery vehicles and public transportation.
Consumer electronics constitutes another emerging market segment, particularly for applications where cost considerations outweigh the need for maximum energy density. Industrial applications, including backup power systems and material handling equipment, represent approximately 15% of the current market share.
Geographically, China leads the sodium-ion battery market development, with substantial government investment and research initiatives. The European market is growing rapidly due to stringent environmental regulations and renewable energy targets, while North America shows increasing interest driven by energy security concerns and sustainability goals.
Key market drivers include raw material economics, with sodium-based cathode materials costing 30-40% less than their lithium counterparts. Environmental considerations also play a significant role, as sodium extraction has a lower ecological footprint compared to lithium mining operations. Additionally, supply chain security concerns regarding critical battery materials are accelerating interest in sodium-ion technology as a strategic alternative.
Market barriers primarily relate to performance limitations, particularly the lower energy density of sodium-ion batteries compared to lithium-ion counterparts. Current sodium-ion technologies achieve approximately 160 Wh/kg versus 250+ Wh/kg for advanced lithium-ion cells. Manufacturing scale remains limited, with production capacity at less than 5% of lithium-ion battery manufacturing, resulting in higher unit costs despite lower raw material expenses.
Customer adoption patterns indicate that price-sensitive markets and applications with moderate energy density requirements are the early adopters of sodium-ion technology. The total addressable market is expanding as performance improvements address mass transfer challenges, with potential to capture 8-10% of the global battery market by 2030.
Mass Transfer Challenges and Technical Barriers
Sodium-ion batteries (SIBs) face significant mass transfer challenges that currently limit their widespread commercial adoption despite their promising cost and sustainability advantages over lithium-ion batteries. The primary technical barrier stems from the larger ionic radius of Na+ (1.02 Å) compared to Li+ (0.76 Å), which fundamentally affects ion diffusion kinetics throughout the battery system. This size difference creates substantial obstacles for sodium ion transport within electrode materials, across interfaces, and through the electrolyte.
In electrode materials, the larger sodium ions cause increased structural strain during intercalation and de-intercalation processes, leading to mechanical degradation and capacity fading over multiple charge-discharge cycles. Hard carbon anodes, while promising for SIBs, suffer from limited sodium storage sites and sluggish diffusion pathways that restrict rate capability. Cathode materials face similar challenges, with many conventional structures unable to accommodate sodium ions efficiently without significant lattice distortion.
The solid-electrolyte interphase (SEI) formation in SIBs presents another critical barrier. Unlike the relatively stable SEI in lithium-ion batteries, sodium-based SEI layers tend to be more unstable and permeable, leading to continuous electrolyte decomposition and impedance growth. This instability is particularly problematic at high current densities, where the mass transfer limitations become more pronounced and can lead to dendrite formation and potential safety hazards.
Electrolyte systems for SIBs face the dual challenge of ensuring adequate ionic conductivity while maintaining compatibility with electrode materials. Conventional carbonate-based electrolytes often demonstrate lower Na+ conductivity compared to Li+ conductivity in analogous systems. Additionally, the solvation/desolvation energetics of sodium ions differ significantly from lithium, affecting the charge transfer resistance at electrode-electrolyte interfaces.
Temperature sensitivity represents another technical barrier, with sodium-ion diffusion coefficients showing greater variation across operating temperature ranges compared to lithium systems. This creates challenges for applications requiring performance consistency across diverse environmental conditions, particularly in cold climates where mass transfer limitations become more severe.
The separator materials in SIBs must also be optimized specifically for sodium ion transport, as materials designed for lithium systems may not provide optimal porosity, tortuosity, and wettability characteristics for the larger sodium ions. This often results in concentration polarization during high-rate operation, further limiting battery performance.
These mass transfer challenges collectively contribute to lower energy density, reduced power capability, and shorter cycle life in current sodium-ion battery technologies compared to their lithium-ion counterparts, representing the primary technical barriers that must be overcome to enable widespread commercialization.
In electrode materials, the larger sodium ions cause increased structural strain during intercalation and de-intercalation processes, leading to mechanical degradation and capacity fading over multiple charge-discharge cycles. Hard carbon anodes, while promising for SIBs, suffer from limited sodium storage sites and sluggish diffusion pathways that restrict rate capability. Cathode materials face similar challenges, with many conventional structures unable to accommodate sodium ions efficiently without significant lattice distortion.
The solid-electrolyte interphase (SEI) formation in SIBs presents another critical barrier. Unlike the relatively stable SEI in lithium-ion batteries, sodium-based SEI layers tend to be more unstable and permeable, leading to continuous electrolyte decomposition and impedance growth. This instability is particularly problematic at high current densities, where the mass transfer limitations become more pronounced and can lead to dendrite formation and potential safety hazards.
Electrolyte systems for SIBs face the dual challenge of ensuring adequate ionic conductivity while maintaining compatibility with electrode materials. Conventional carbonate-based electrolytes often demonstrate lower Na+ conductivity compared to Li+ conductivity in analogous systems. Additionally, the solvation/desolvation energetics of sodium ions differ significantly from lithium, affecting the charge transfer resistance at electrode-electrolyte interfaces.
Temperature sensitivity represents another technical barrier, with sodium-ion diffusion coefficients showing greater variation across operating temperature ranges compared to lithium systems. This creates challenges for applications requiring performance consistency across diverse environmental conditions, particularly in cold climates where mass transfer limitations become more severe.
The separator materials in SIBs must also be optimized specifically for sodium ion transport, as materials designed for lithium systems may not provide optimal porosity, tortuosity, and wettability characteristics for the larger sodium ions. This often results in concentration polarization during high-rate operation, further limiting battery performance.
These mass transfer challenges collectively contribute to lower energy density, reduced power capability, and shorter cycle life in current sodium-ion battery technologies compared to their lithium-ion counterparts, representing the primary technical barriers that must be overcome to enable widespread commercialization.
Current Mass Transfer Enhancement Strategies
01 Electrode material design for enhanced mass transfer
Specialized electrode materials can significantly improve mass transfer in sodium-ion batteries. These materials include nanostructured composites, porous frameworks, and layered materials that provide shorter diffusion paths for sodium ions. The optimized structure allows for faster ion transport, reduced diffusion resistance, and improved rate capability. Advanced electrode designs also help mitigate volume changes during cycling, leading to better structural stability and longer battery life.- Electrode material design for enhanced mass transfer: Specialized electrode materials can significantly improve mass transfer in sodium-ion batteries. These materials include nanostructured composites, porous frameworks, and layered materials that provide shorter diffusion paths for sodium ions. The optimized structure allows for faster ion transport, reduced internal resistance, and improved rate capability. Advanced electrode designs also incorporate conductive additives to enhance electron transfer alongside ion movement.
- Electrolyte formulations for improved ionic conductivity: Novel electrolyte compositions can enhance sodium ion mobility and mass transfer within batteries. These formulations include optimized salt concentrations, solvent mixtures, and additives that reduce viscosity and improve ion dissociation. Some electrolytes incorporate ionic liquids or polymer-based systems that maintain high conductivity while improving safety. Advanced electrolyte designs also focus on forming stable solid-electrolyte interfaces that facilitate consistent ion transfer without degradation over multiple cycles.
- Structural modifications for enhanced ion diffusion: Structural engineering of battery components can significantly improve mass transfer properties. This includes creating hierarchical pore structures, introducing defects or vacancies that serve as ion transport channels, and designing interfaces that facilitate ion movement. Some approaches involve doping materials with specific elements to modify the crystal structure and create favorable pathways for sodium ion migration. These modifications can reduce diffusion barriers and enhance the overall kinetics of the electrochemical reactions.
- Advanced separator technologies: Innovative separator designs can significantly impact mass transfer in sodium-ion batteries. These include thin, highly porous membranes with optimized tortuosity for efficient ion transport while maintaining mechanical integrity. Some separators incorporate functional coatings or are made from composite materials that enhance wettability and ion conductivity. Advanced separators also feature modified surface properties that reduce interfacial resistance and prevent dendrite formation, thereby maintaining consistent mass transfer throughout battery operation.
- Temperature and pressure control systems: Environmental control mechanisms can optimize mass transfer in sodium-ion batteries. These systems regulate operating temperature and pressure to maintain ideal conditions for ion mobility and reaction kinetics. Some designs incorporate thermal management solutions that prevent localized hotspots and ensure uniform ion transport throughout the cell. Advanced systems may include pressure-modulation techniques that optimize the physical contact between battery components, reducing interfacial resistance and enhancing mass transfer efficiency during charging and discharging cycles.
02 Electrolyte formulations for improved ionic conductivity
Novel electrolyte compositions can enhance mass transfer in sodium-ion batteries by improving ionic conductivity and interfacial properties. These formulations include optimized salt concentrations, solvent mixtures, and additives that facilitate sodium ion transport. Advanced electrolytes can form stable solid-electrolyte interphases, reduce viscosity, and enhance wettability of electrode materials. These improvements lead to faster charge-discharge rates and better overall battery performance at various operating temperatures.Expand Specific Solutions03 Interface engineering for reduced mass transfer resistance
Interface engineering techniques can minimize resistance to mass transfer at electrode-electrolyte interfaces in sodium-ion batteries. These approaches include surface coatings, functional interlayers, and interface modification treatments that improve ion transport across boundaries. By optimizing the interface properties, these methods reduce charge transfer resistance, prevent unwanted side reactions, and enhance the kinetics of sodium ion insertion/extraction processes, resulting in improved power density and cycling stability.Expand Specific Solutions04 Structural design for enhanced sodium ion diffusion
Advanced structural designs can facilitate sodium ion diffusion throughout battery components. These include hierarchical porous structures, 3D interconnected networks, and optimized particle morphologies that create efficient ion transport pathways. Such designs minimize diffusion distances, provide abundant active sites, and accommodate volume changes during cycling. The enhanced structural features lead to improved rate performance, better utilization of active materials, and reduced concentration polarization during high-rate operation.Expand Specific Solutions05 Advanced characterization and modeling of mass transfer phenomena
Sophisticated characterization techniques and computational modeling approaches enable deeper understanding and optimization of mass transfer processes in sodium-ion batteries. These methods include in-situ/operando measurements, advanced imaging techniques, and multi-scale simulation models that reveal ion transport mechanisms at various length and time scales. By providing insights into diffusion pathways, rate-limiting steps, and degradation mechanisms, these approaches guide the rational design of battery components for enhanced mass transfer properties and overall performance.Expand Specific Solutions
Leading Companies and Research Institutions
The sodium-ion battery market is currently in an early growth phase, characterized by increasing R&D investments and emerging commercial applications. The global market size is projected to expand significantly as mass transfer challenges are addressed, with estimates suggesting multi-billion dollar potential by 2030. Technologically, companies are at varying maturity levels: established players like Faradion, Shenzhen Zhenhua New Material, and SVOLT Energy have achieved pilot production, while newer entrants such as Nayuan New Material and Trina Energy Storage are developing proprietary solutions. Academic institutions including MIT, Cornell, and Central South University are advancing fundamental research. The competitive landscape features both battery manufacturers and chemical companies like Sumitomo Chemical and OCI, indicating a diversifying ecosystem focused on overcoming electrolyte stability and electrode interface challenges.
Faradion Ltd.
Technical Solution: Faradion has developed proprietary sodium-ion technology addressing mass transfer challenges through advanced electrode design and electrolyte formulations. Their approach focuses on optimizing the sodium-ion diffusion kinetics by engineering layered oxide cathode materials with expanded interlayer spacing, facilitating faster Na+ ion transport. The company has pioneered a "hard carbon" anode structure with optimized porosity and surface area that enhances sodium ion intercalation rates. Their electrolyte systems incorporate fluoroethylene carbonate (FEC) additives that form stable solid electrolyte interphase (SEI) layers, reducing interfacial resistance. Faradion's cells demonstrate improved rate capability with capacity retention of over 80% after 1000 cycles at 1C rates, significantly outperforming early sodium-ion battery designs. Their technology has been validated in commercial-sized pouch cells with energy densities approaching 140-160 Wh/kg.
Strengths: Proprietary hard carbon anode technology enables faster charging rates and better low-temperature performance than competitors. Their electrolyte formulations create more stable SEI layers, reducing capacity fade. Weaknesses: Energy density still lags behind advanced lithium-ion systems, and manufacturing scale remains limited compared to established battery technologies.
Svolt Energy Technology Co., Ltd.
Technical Solution: Svolt has developed an innovative approach to sodium-ion battery mass transfer challenges through their proprietary "sodium-ion highway" electrode architecture. This design features hierarchical porous structures in both cathode and anode materials that create optimized ion transport pathways. Their cathode materials utilize Prussian white analogues (Na₂FeFe(CN)₆) with controlled vacancies and water content, achieving sodium diffusion coefficients up to 10⁻¹⁰ cm²/s, significantly higher than conventional materials. For anodes, Svolt employs a composite structure combining hard carbon with pre-sodiated materials that reduces the initial irreversible capacity loss common in sodium-ion systems. Their electrolyte formulation incorporates sodium bis(fluorosulfonyl)imide (NaFSI) salt in diglyme solvent, which demonstrates superior ionic conductivity (>7 mS/cm) and wider electrochemical stability windows compared to conventional electrolytes. Svolt's cells demonstrate exceptional rate capability, retaining over 70% capacity at 5C discharge rates and showing minimal capacity degradation over 2000 cycles at moderate rates.
Strengths: Their hierarchical electrode architecture significantly improves sodium-ion diffusion kinetics, enabling faster charging capabilities. The proprietary electrolyte formulation provides excellent low-temperature performance down to -20°C. Weaknesses: The Prussian white cathode materials face challenges with moisture sensitivity during manufacturing, requiring stringent production controls. The technology also faces cost barriers for the specialized salts used in their advanced electrolyte systems.
Key Patents and Innovations in Na-ion Mass Transfer
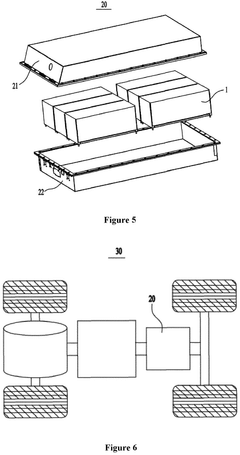
Sodium-ion battery, battery module, battery pack and electrical device
PatentPendingUS20250006927A1
Innovation
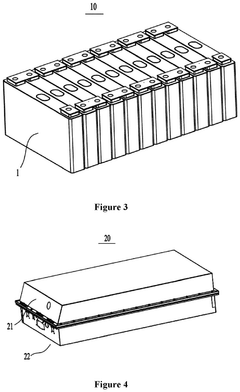
- Adjusting the porosity and compaction density of positive and negative electrode active material layers and separators to ensure optimal ion migration, with specific porosity ratios and compaction density ratios that allow for efficient deintercalation and intercalation of sodium ions, thereby improving charging and discharging performance.
Sodium-ion battery, battery module, battery pack and electric device
PatentPendingEP4451405A1
Innovation
- Adjusting the porosity and compaction density of positive and negative electrode active material layers and separators to ensure efficient sodium ion migration, with specific porosity ratios and compaction density ranges that facilitate complete intercalation and deintercalation, thereby improving charging and discharging performance.
Material Science Advancements for Na-ion Batteries
Material science advancements have been pivotal in addressing the mass transfer challenges in sodium-ion batteries. Recent developments in electrode materials have significantly improved ionic conductivity and structural stability during charge-discharge cycles. Researchers have focused on optimizing particle morphology and size distribution to shorten diffusion paths for sodium ions, resulting in enhanced rate capability and cycling performance.
Carbon-based materials have emerged as promising candidates for sodium-ion battery anodes due to their excellent electrical conductivity and structural flexibility. Hard carbon, derived from sustainable biomass sources, demonstrates superior sodium storage capacity compared to graphite used in lithium-ion batteries. The disordered structure of hard carbon provides abundant active sites for sodium ion intercalation, facilitating faster mass transfer kinetics.
For cathode materials, layered transition metal oxides (NaxMO2, where M represents metals like Fe, Mn, Co, Ni) have been extensively studied. Recent breakthroughs in crystal engineering have led to expanded interlayer spacing, which accommodates the larger sodium ions and enables more efficient ion transport. Prussian blue analogs represent another promising cathode material family, offering an open framework structure that facilitates rapid sodium ion diffusion.
Electrolyte formulations have also advanced significantly, with new sodium salt compositions and solvent systems designed specifically for sodium-ion chemistry. Fluorinated compounds and ether-based solvents have demonstrated improved ionic conductivity and interfacial stability. Additionally, the development of solid-state electrolytes for sodium-ion batteries has gained momentum, with ceramic and polymer-based systems showing promising results in mitigating dendrite formation and enhancing safety.
Nanostructured materials represent a frontier in addressing mass transfer limitations. Hierarchical porous structures, nanofibers, and 2D materials like MXenes provide shortened diffusion pathways and increased surface area for sodium ion storage. These nanoarchitectures effectively alleviate the volume expansion issues during cycling, maintaining structural integrity and electrochemical performance over extended periods.
Surface modification techniques, including atomic layer deposition and functional coating layers, have been employed to stabilize the electrode-electrolyte interface. These approaches minimize parasitic reactions and form stable solid electrolyte interphase layers, which are crucial for facilitating consistent sodium ion transport while preventing capacity fade during long-term cycling.
Carbon-based materials have emerged as promising candidates for sodium-ion battery anodes due to their excellent electrical conductivity and structural flexibility. Hard carbon, derived from sustainable biomass sources, demonstrates superior sodium storage capacity compared to graphite used in lithium-ion batteries. The disordered structure of hard carbon provides abundant active sites for sodium ion intercalation, facilitating faster mass transfer kinetics.
For cathode materials, layered transition metal oxides (NaxMO2, where M represents metals like Fe, Mn, Co, Ni) have been extensively studied. Recent breakthroughs in crystal engineering have led to expanded interlayer spacing, which accommodates the larger sodium ions and enables more efficient ion transport. Prussian blue analogs represent another promising cathode material family, offering an open framework structure that facilitates rapid sodium ion diffusion.
Electrolyte formulations have also advanced significantly, with new sodium salt compositions and solvent systems designed specifically for sodium-ion chemistry. Fluorinated compounds and ether-based solvents have demonstrated improved ionic conductivity and interfacial stability. Additionally, the development of solid-state electrolytes for sodium-ion batteries has gained momentum, with ceramic and polymer-based systems showing promising results in mitigating dendrite formation and enhancing safety.
Nanostructured materials represent a frontier in addressing mass transfer limitations. Hierarchical porous structures, nanofibers, and 2D materials like MXenes provide shortened diffusion pathways and increased surface area for sodium ion storage. These nanoarchitectures effectively alleviate the volume expansion issues during cycling, maintaining structural integrity and electrochemical performance over extended periods.
Surface modification techniques, including atomic layer deposition and functional coating layers, have been employed to stabilize the electrode-electrolyte interface. These approaches minimize parasitic reactions and form stable solid electrolyte interphase layers, which are crucial for facilitating consistent sodium ion transport while preventing capacity fade during long-term cycling.
Sustainability and Cost Analysis of Na-ion Technology
Sodium-ion battery technology presents a compelling case for sustainability and cost-effectiveness compared to traditional lithium-ion batteries. The abundance of sodium resources is a primary advantage, with sodium being the sixth most abundant element in the Earth's crust, approximately 1000 times more plentiful than lithium. This natural abundance translates directly into lower raw material costs, with sodium carbonate priced at approximately $300/ton compared to lithium carbonate at $20,000/ton (as of 2023).
From an environmental perspective, sodium-ion batteries offer significant advantages throughout their lifecycle. The extraction of sodium compounds generally requires less water and energy than lithium extraction, particularly avoiding the intensive brine evaporation processes used in lithium production. This results in a substantially lower carbon footprint during the manufacturing phase, with some studies indicating up to 30% reduction in greenhouse gas emissions compared to equivalent lithium-ion production.
The manufacturing process for sodium-ion batteries can largely utilize existing lithium-ion production infrastructure with minimal modifications, avoiding the need for entirely new manufacturing facilities. This adaptability significantly reduces the capital expenditure required for transitioning to sodium-ion technology, making it an economically viable alternative for battery manufacturers seeking to diversify their product offerings.
End-of-life considerations further enhance the sustainability profile of sodium-ion batteries. The absence of cobalt and nickel in many sodium-ion chemistries eliminates concerns related to these critical materials, which face supply constraints and ethical mining issues. Recycling processes for sodium-ion batteries are potentially simpler and more cost-effective due to the reduced presence of precious metals, though specialized recycling infrastructure will need development as the technology scales.
Economic modeling suggests that at mass production scales, sodium-ion batteries could achieve costs below $80/kWh, compared to the current $100-120/kWh for lithium-ion technologies. This cost advantage becomes particularly significant in applications where energy density requirements are moderate, such as stationary storage and certain electric vehicle segments.
The total cost of ownership analysis reveals additional benefits, including potentially longer cycle life in certain formulations and better performance at lower temperatures, reducing the need for thermal management systems. These operational advantages, combined with lower initial costs, position sodium-ion technology as an economically attractive solution for grid storage applications and entry-level electric mobility.
From an environmental perspective, sodium-ion batteries offer significant advantages throughout their lifecycle. The extraction of sodium compounds generally requires less water and energy than lithium extraction, particularly avoiding the intensive brine evaporation processes used in lithium production. This results in a substantially lower carbon footprint during the manufacturing phase, with some studies indicating up to 30% reduction in greenhouse gas emissions compared to equivalent lithium-ion production.
The manufacturing process for sodium-ion batteries can largely utilize existing lithium-ion production infrastructure with minimal modifications, avoiding the need for entirely new manufacturing facilities. This adaptability significantly reduces the capital expenditure required for transitioning to sodium-ion technology, making it an economically viable alternative for battery manufacturers seeking to diversify their product offerings.
End-of-life considerations further enhance the sustainability profile of sodium-ion batteries. The absence of cobalt and nickel in many sodium-ion chemistries eliminates concerns related to these critical materials, which face supply constraints and ethical mining issues. Recycling processes for sodium-ion batteries are potentially simpler and more cost-effective due to the reduced presence of precious metals, though specialized recycling infrastructure will need development as the technology scales.
Economic modeling suggests that at mass production scales, sodium-ion batteries could achieve costs below $80/kWh, compared to the current $100-120/kWh for lithium-ion technologies. This cost advantage becomes particularly significant in applications where energy density requirements are moderate, such as stationary storage and certain electric vehicle segments.
The total cost of ownership analysis reveals additional benefits, including potentially longer cycle life in certain formulations and better performance at lower temperatures, reducing the need for thermal management systems. These operational advantages, combined with lower initial costs, position sodium-ion technology as an economically attractive solution for grid storage applications and entry-level electric mobility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!