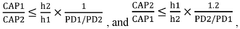Limits of Porosity in Sodium-ion Batteries for Mobile Electronics
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium-ion Battery Porosity Background and Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries (LIBs) due to the abundance and wide geographical distribution of sodium resources. The development of SIBs can be traced back to the 1970s, but significant research momentum has only been gained in the past decade as concerns about lithium supply constraints have intensified. The evolution of SIB technology has been marked by continuous improvements in electrode materials, electrolytes, and battery architecture, with porosity control becoming increasingly recognized as a critical factor in performance optimization.
Porosity in battery electrodes refers to the void spaces within the electrode structure that facilitate ion transport and electrolyte penetration. Historically, porosity has been viewed primarily as a necessary compromise between energy density and power capability. However, recent research has revealed that porosity characteristics—including pore size distribution, connectivity, and tortuosity—play far more complex roles in determining battery performance metrics such as capacity retention, rate capability, and cycle life.
For mobile electronics applications, the technical objectives related to SIB porosity optimization are multifaceted. Primary goals include achieving energy densities comparable to commercial LIBs (>250 Wh/kg), improving fast-charging capabilities without compromising safety, and extending cycle life to over 1000 cycles with minimal capacity degradation. These objectives necessitate a delicate balance in porosity design, as excessive porosity reduces volumetric energy density while insufficient porosity limits ion transport and rate performance.
Current technological trends indicate a shift toward engineered porosity rather than incidental void spaces. Advanced manufacturing techniques such as freeze-casting, template-directed synthesis, and 3D printing are enabling more precise control over pore architecture. Simultaneously, computational modeling approaches are evolving to better predict the relationship between electrode microstructure and electrochemical performance, allowing for more rational design of porous electrodes.
The fundamental challenge lies in understanding the limits of porosity manipulation in sodium-ion systems. Unlike lithium ions, sodium ions have larger ionic radii (1.02Å vs. 0.76Å), which affects diffusion kinetics and places different constraints on optimal pore structures. Additionally, the mechanical stresses associated with sodium insertion/extraction can lead to more significant volume changes, requiring porosity designs that accommodate structural evolution during cycling.
Our technical objectives therefore focus on determining the theoretical and practical limits of porosity optimization in SIBs for mobile electronics, identifying the critical porosity parameters that most significantly impact performance metrics, and developing design principles that can guide the next generation of high-performance sodium-ion batteries with optimized porous structures.
Porosity in battery electrodes refers to the void spaces within the electrode structure that facilitate ion transport and electrolyte penetration. Historically, porosity has been viewed primarily as a necessary compromise between energy density and power capability. However, recent research has revealed that porosity characteristics—including pore size distribution, connectivity, and tortuosity—play far more complex roles in determining battery performance metrics such as capacity retention, rate capability, and cycle life.
For mobile electronics applications, the technical objectives related to SIB porosity optimization are multifaceted. Primary goals include achieving energy densities comparable to commercial LIBs (>250 Wh/kg), improving fast-charging capabilities without compromising safety, and extending cycle life to over 1000 cycles with minimal capacity degradation. These objectives necessitate a delicate balance in porosity design, as excessive porosity reduces volumetric energy density while insufficient porosity limits ion transport and rate performance.
Current technological trends indicate a shift toward engineered porosity rather than incidental void spaces. Advanced manufacturing techniques such as freeze-casting, template-directed synthesis, and 3D printing are enabling more precise control over pore architecture. Simultaneously, computational modeling approaches are evolving to better predict the relationship between electrode microstructure and electrochemical performance, allowing for more rational design of porous electrodes.
The fundamental challenge lies in understanding the limits of porosity manipulation in sodium-ion systems. Unlike lithium ions, sodium ions have larger ionic radii (1.02Å vs. 0.76Å), which affects diffusion kinetics and places different constraints on optimal pore structures. Additionally, the mechanical stresses associated with sodium insertion/extraction can lead to more significant volume changes, requiring porosity designs that accommodate structural evolution during cycling.
Our technical objectives therefore focus on determining the theoretical and practical limits of porosity optimization in SIBs for mobile electronics, identifying the critical porosity parameters that most significantly impact performance metrics, and developing design principles that can guide the next generation of high-performance sodium-ion batteries with optimized porous structures.
Market Analysis for Na-ion Batteries in Mobile Electronics
The sodium-ion battery market for mobile electronics is experiencing significant growth as manufacturers seek alternatives to traditional lithium-ion technologies. Current market projections indicate that the global sodium-ion battery market could reach $500 million by 2025, with a compound annual growth rate of approximately 18-20% over the next five years. This growth is primarily driven by increasing demand for sustainable and cost-effective energy storage solutions in consumer electronics.
Mobile electronics represent a particularly promising segment for sodium-ion battery adoption. The smartphone market alone, with annual shipments exceeding 1.3 billion units, presents a substantial opportunity for sodium-ion battery integration. Additionally, wearable devices, tablets, and portable gaming consoles collectively account for hundreds of millions of units shipped annually, further expanding the potential market.
Consumer demand patterns indicate growing interest in devices with longer battery life, faster charging capabilities, and improved sustainability profiles. Market surveys show that approximately 65% of consumers consider battery performance a critical factor in purchasing decisions for mobile devices, while 42% express willingness to pay a premium for devices with improved environmental credentials.
The cost advantage of sodium-ion batteries is particularly relevant for mid-range and entry-level mobile electronics. With sodium resources being approximately 1,000 times more abundant than lithium globally, raw material costs are significantly lower. Manufacturing costs for sodium-ion cells are estimated to be 20-30% lower than comparable lithium-ion cells, potentially enabling more affordable devices or improved profit margins for manufacturers.
Regional market analysis reveals varying adoption potentials. Asia-Pacific currently leads in both production capacity and market demand, with China investing heavily in sodium-ion technology development. European markets show strong interest driven by sustainability regulations, while North American adoption is expected to accelerate as the technology matures and performance improves.
Competitive analysis indicates that several major battery manufacturers and electronics companies are actively developing sodium-ion solutions for mobile applications. These include established players diversifying from lithium-ion production as well as startups focused exclusively on sodium-ion technology. Strategic partnerships between battery developers and device manufacturers are becoming increasingly common as the technology approaches commercial viability.
Market barriers primarily relate to performance limitations, particularly energy density constraints imposed by porosity challenges. Consumer expectations set by lithium-ion performance create adoption hurdles that sodium-ion technology must overcome through continued innovation in electrode materials and battery architecture.
Mobile electronics represent a particularly promising segment for sodium-ion battery adoption. The smartphone market alone, with annual shipments exceeding 1.3 billion units, presents a substantial opportunity for sodium-ion battery integration. Additionally, wearable devices, tablets, and portable gaming consoles collectively account for hundreds of millions of units shipped annually, further expanding the potential market.
Consumer demand patterns indicate growing interest in devices with longer battery life, faster charging capabilities, and improved sustainability profiles. Market surveys show that approximately 65% of consumers consider battery performance a critical factor in purchasing decisions for mobile devices, while 42% express willingness to pay a premium for devices with improved environmental credentials.
The cost advantage of sodium-ion batteries is particularly relevant for mid-range and entry-level mobile electronics. With sodium resources being approximately 1,000 times more abundant than lithium globally, raw material costs are significantly lower. Manufacturing costs for sodium-ion cells are estimated to be 20-30% lower than comparable lithium-ion cells, potentially enabling more affordable devices or improved profit margins for manufacturers.
Regional market analysis reveals varying adoption potentials. Asia-Pacific currently leads in both production capacity and market demand, with China investing heavily in sodium-ion technology development. European markets show strong interest driven by sustainability regulations, while North American adoption is expected to accelerate as the technology matures and performance improves.
Competitive analysis indicates that several major battery manufacturers and electronics companies are actively developing sodium-ion solutions for mobile applications. These include established players diversifying from lithium-ion production as well as startups focused exclusively on sodium-ion technology. Strategic partnerships between battery developers and device manufacturers are becoming increasingly common as the technology approaches commercial viability.
Market barriers primarily relate to performance limitations, particularly energy density constraints imposed by porosity challenges. Consumer expectations set by lithium-ion performance create adoption hurdles that sodium-ion technology must overcome through continued innovation in electrode materials and battery architecture.
Current Porosity Limitations and Technical Challenges
The current porosity limitations in sodium-ion batteries (SIBs) for mobile electronics present significant technical challenges that impede their widespread commercial adoption. Despite the theoretical advantages of sodium as an abundant and cost-effective alternative to lithium, the practical implementation of high-porosity electrodes in SIBs faces several critical constraints.
Electrode porosity in SIBs is currently limited to approximately 30-40%, significantly lower than the optimal range of 45-55% that would maximize both energy density and power capability. This limitation stems primarily from the larger ionic radius of Na+ (1.02Å) compared to Li+ (0.76Å), which creates fundamental challenges in ion transport dynamics through porous structures. The larger sodium ions experience greater steric hindrance and stronger electrostatic interactions with the host materials, resulting in slower diffusion kinetics.
Material stability presents another major challenge, as high-porosity electrodes in SIBs often suffer from accelerated structural degradation. The repeated insertion and extraction of the larger sodium ions create more significant volume changes (up to 15-20% in many materials) compared to lithium-ion systems. This expansion-contraction cycle leads to mechanical stress that can collapse pore structures over multiple charge-discharge cycles, reducing the effective porosity and battery lifespan.
The electrolyte-electrode interface in high-porosity SIB systems exhibits problematic behavior, with excessive solid electrolyte interphase (SEI) formation that can block pores and increase internal resistance. Current electrolyte formulations designed for sodium systems show 30-40% higher reactivity with electrode surfaces compared to their lithium counterparts, resulting in thicker and less stable SEI layers that progressively reduce effective porosity.
Manufacturing challenges further complicate the situation, as conventional electrode fabrication techniques struggle to maintain consistent pore size distribution when adapted for sodium-ion chemistry. The standard slurry-casting methods typically result in 15-25% variation in pore size across the electrode, compared to 5-10% in optimized lithium-ion manufacturing processes.
Temperature sensitivity represents an additional limitation, with high-porosity SIB electrodes showing dramatic performance degradation outside the narrow 15-35°C operating window. This thermal sensitivity is particularly problematic for mobile electronics applications, which may experience varied environmental conditions during use.
The combined effect of these limitations results in sodium-ion batteries with energy densities currently capped at approximately 120-150 Wh/kg at the cell level, significantly below the 200-260 Wh/kg achieved in commercial lithium-ion cells. This energy density gap, largely attributable to porosity-related challenges, remains one of the primary barriers to SIB adoption in mobile electronics where space and weight constraints are critical considerations.
Electrode porosity in SIBs is currently limited to approximately 30-40%, significantly lower than the optimal range of 45-55% that would maximize both energy density and power capability. This limitation stems primarily from the larger ionic radius of Na+ (1.02Å) compared to Li+ (0.76Å), which creates fundamental challenges in ion transport dynamics through porous structures. The larger sodium ions experience greater steric hindrance and stronger electrostatic interactions with the host materials, resulting in slower diffusion kinetics.
Material stability presents another major challenge, as high-porosity electrodes in SIBs often suffer from accelerated structural degradation. The repeated insertion and extraction of the larger sodium ions create more significant volume changes (up to 15-20% in many materials) compared to lithium-ion systems. This expansion-contraction cycle leads to mechanical stress that can collapse pore structures over multiple charge-discharge cycles, reducing the effective porosity and battery lifespan.
The electrolyte-electrode interface in high-porosity SIB systems exhibits problematic behavior, with excessive solid electrolyte interphase (SEI) formation that can block pores and increase internal resistance. Current electrolyte formulations designed for sodium systems show 30-40% higher reactivity with electrode surfaces compared to their lithium counterparts, resulting in thicker and less stable SEI layers that progressively reduce effective porosity.
Manufacturing challenges further complicate the situation, as conventional electrode fabrication techniques struggle to maintain consistent pore size distribution when adapted for sodium-ion chemistry. The standard slurry-casting methods typically result in 15-25% variation in pore size across the electrode, compared to 5-10% in optimized lithium-ion manufacturing processes.
Temperature sensitivity represents an additional limitation, with high-porosity SIB electrodes showing dramatic performance degradation outside the narrow 15-35°C operating window. This thermal sensitivity is particularly problematic for mobile electronics applications, which may experience varied environmental conditions during use.
The combined effect of these limitations results in sodium-ion batteries with energy densities currently capped at approximately 120-150 Wh/kg at the cell level, significantly below the 200-260 Wh/kg achieved in commercial lithium-ion cells. This energy density gap, largely attributable to porosity-related challenges, remains one of the primary barriers to SIB adoption in mobile electronics where space and weight constraints are critical considerations.
Current Approaches to Porosity Optimization
01 Porous electrode materials for sodium-ion batteries
Porous electrode materials enhance sodium-ion battery performance by providing increased surface area for ion transport and storage. These materials facilitate faster sodium ion diffusion, improve capacity, and enhance cycling stability. Various manufacturing techniques can create controlled porosity in electrode materials, including templating methods and chemical etching processes, resulting in optimized pore size distribution for efficient battery operation.- Porous electrode materials for sodium-ion batteries: Porous electrode materials can significantly enhance the performance of sodium-ion batteries by providing larger surface areas for sodium ion insertion/extraction. These materials facilitate faster ion diffusion and electron transport, leading to improved capacity and rate capability. The controlled porosity in electrode materials also helps accommodate volume changes during cycling, resulting in better structural stability and longer cycle life.
- Hard carbon with optimized porosity for sodium storage: Hard carbon materials with tailored porosity structures are particularly effective for sodium-ion batteries. The micropores and mesopores in hard carbon provide abundant sodium storage sites while maintaining structural integrity during charge-discharge cycles. By controlling the carbonization process and precursor materials, the porosity characteristics can be optimized to enhance sodium ion adsorption and diffusion kinetics, resulting in improved capacity retention and cycling stability.
- Porous composite materials with conductive networks: Composite materials combining porous structures with conductive networks offer enhanced performance for sodium-ion batteries. These materials typically incorporate carbon-based components (such as graphene or carbon nanotubes) with metal oxides or other active materials. The porous architecture provides channels for electrolyte penetration and ion transport, while the conductive network ensures efficient electron transfer throughout the electrode. This synergistic combination results in improved rate capability and cycling performance.
- Hierarchical porous structures for enhanced ion transport: Hierarchical porous structures featuring interconnected macro-, meso-, and micropores offer significant advantages for sodium-ion batteries. These multi-scale porous architectures provide optimized ion transport pathways at different length scales. Macropores facilitate rapid electrolyte infiltration, mesopores enable efficient ion diffusion, and micropores provide abundant active sites for sodium storage. This hierarchical design minimizes diffusion limitations and enhances the utilization of active materials, particularly at high charge-discharge rates.
- Porosity control through template-assisted synthesis methods: Template-assisted synthesis methods enable precise control over the porosity characteristics of electrode materials for sodium-ion batteries. These approaches utilize sacrificial templates (such as silica spheres, metal-organic frameworks, or polymer templates) that are removed after material formation, leaving behind well-defined porous structures. The resulting materials exhibit tailored pore size distributions, pore volumes, and specific surface areas that can be optimized for sodium ion storage. This controlled porosity engineering leads to enhanced electrochemical performance and battery lifespan.
02 Hard carbon with optimized porosity for sodium storage
Hard carbon materials with tailored porosity structures serve as effective anode materials for sodium-ion batteries. The microporous and mesoporous structure of hard carbon facilitates sodium ion insertion and extraction, improving capacity and rate capability. By controlling carbonization conditions and precursor selection, the porosity characteristics can be optimized to enhance sodium storage properties while maintaining structural stability during charge-discharge cycles.Expand Specific Solutions03 Porous composite materials for sodium-ion battery electrodes
Composite materials combining multiple components with controlled porosity offer enhanced performance for sodium-ion battery electrodes. These composites typically incorporate carbon matrices with embedded active materials, creating hierarchical porous structures that facilitate both ion and electron transport. The synergistic effect between components improves capacity, cycling stability, and rate capability compared to single-component materials, while the porous architecture accommodates volume changes during cycling.Expand Specific Solutions04 Porosity control in sodium-ion battery separators and electrolytes
The porosity of separators and solid electrolytes significantly impacts sodium-ion battery performance. Optimized pore structures in separators ensure efficient ion transport while maintaining mechanical integrity and preventing short circuits. For solid electrolytes, controlled porosity facilitates sodium ion migration through interconnected channels. Advanced manufacturing techniques allow precise control of pore size distribution, tortuosity, and connectivity, resulting in improved ionic conductivity and battery safety.Expand Specific Solutions05 Porosity engineering for improved sodium-ion diffusion kinetics
Strategic engineering of porosity in battery components enhances sodium-ion diffusion kinetics, addressing a key limitation of sodium-ion batteries. Techniques such as hierarchical pore design, gradient porosity structures, and directional pore channels optimize ion transport pathways. These approaches reduce diffusion distances, minimize transport resistance, and improve rate performance. Additionally, porosity engineering helps accommodate the larger size of sodium ions compared to lithium ions, enabling faster charging and discharging capabilities.Expand Specific Solutions
Leading Companies and Research Institutions in Na-ion Technology
The sodium-ion battery market for mobile electronics is currently in an early growth phase, characterized by increasing research activity but limited commercial deployment. The market size remains modest compared to lithium-ion technologies, though projections indicate significant expansion potential as porosity limitations are addressed. Technologically, companies are at varying maturity levels: established players like CATL, LG Chem, and Panasonic are leveraging their battery expertise to develop sodium-ion solutions, while specialized firms like Faradion and Nexeon are pioneering innovative approaches to overcome porosity constraints. Academic institutions including MIT, University of Maryland, and Delft University are contributing fundamental research to address material science challenges. The competitive landscape features both traditional battery manufacturers and emerging startups focused on novel electrode materials and cell architectures to maximize energy density while managing the inherent porosity limitations of sodium-ion technology.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have pioneered groundbreaking work on the fundamental science of porosity in sodium-ion battery materials. Their approach focuses on multi-scale porosity engineering, from atomic to microscopic levels, to optimize sodium-ion transport while maintaining mechanical integrity. MIT's research has established quantitative relationships between pore structure characteristics (size distribution, connectivity, tortuosity) and electrochemical performance metrics critical for mobile electronics applications. Their work with advanced characterization techniques, including in-situ TEM and synchrotron-based X-ray tomography, has revealed how sodium-ion transport mechanisms differ from lithium-ion systems, particularly regarding the impact of porosity. The team has developed novel hierarchical carbon materials with tailored porosity that demonstrate superior rate capability while maintaining high capacity. Their computational models accurately predict the optimal porosity ranges (32-38%) for specific mobile electronics use cases, balancing energy density, power capability, and cycle life requirements. MIT's research also addresses manufacturing considerations, developing scalable techniques to precisely control porosity during electrode fabrication.
Strengths: Cutting-edge fundamental understanding of sodium-ion transport in porous media, providing scientific foundations for industrial development. Their multi-disciplinary approach integrates materials science, electrochemistry, and advanced manufacturing. Weaknesses: As an academic institution, MIT faces challenges in scaling technologies to commercial production, and their research, while scientifically rigorous, may require significant engineering development before implementation in consumer products.
Faradion Ltd.
Technical Solution: Faradion has pioneered advanced sodium-ion battery technology focusing specifically on porosity optimization. Their proprietary hard carbon anode materials feature controlled porosity structures that enhance sodium-ion intercalation while maintaining structural integrity during cycling. The company has developed a hierarchical pore structure design with both micropores (<2nm) and mesopores (2-50nm) that facilitates faster ion transport while maximizing active material loading. Their research demonstrates that an optimal porosity range of 35-45% in cathode materials strikes the balance between energy density and rate capability. Faradion's layered oxide cathodes incorporate engineered void spaces that accommodate the larger sodium-ion radius (compared to lithium), addressing one of the fundamental challenges in sodium-ion technology. Their batteries achieve energy densities approaching 160 Wh/kg at the cell level, making them increasingly viable for mobile electronics applications.
Strengths: Superior cycle stability compared to competitors, with over 2000 cycles demonstrated at 80% capacity retention. Their technology operates effectively across wider temperature ranges (-20°C to 60°C) than many sodium-ion alternatives. Weaknesses: Still faces energy density limitations compared to advanced lithium-ion cells, and the higher porosity requirements increase overall battery volume, challenging integration into compact mobile devices.
Key Patents and Research on Porosity Control Mechanisms
Sodium-ion battery, battery module, battery pack and electrical device
PatentPendingUS20250006927A1
Innovation
- Adjusting the porosity and compaction density of positive and negative electrode active material layers and separators to ensure optimal ion migration, with specific porosity ratios and compaction density ratios that allow for efficient deintercalation and intercalation of sodium ions, thereby improving charging and discharging performance.
Sodium-ion battery, battery module, battery pack and electric device
PatentPendingEP4451405A1
Innovation
- Adjusting the porosity and compaction density of positive and negative electrode active material layers and separators to ensure efficient sodium ion migration, with specific porosity ratios and compaction density ranges that facilitate complete intercalation and deintercalation, thereby improving charging and discharging performance.
Sustainability and Resource Considerations
The sustainability profile of sodium-ion batteries represents a significant advantage over lithium-ion alternatives, particularly when considering resource availability and environmental impact. Sodium is approximately 1,000 times more abundant than lithium in the Earth's crust, with vast reserves distributed more equitably across global regions. This abundance translates to lower extraction costs and reduced geopolitical supply risks, making sodium-ion technology particularly attractive for large-scale applications.
When examining porosity limitations in sodium-ion batteries for mobile electronics, sustainability considerations become increasingly important. Higher porosity electrode designs, while beneficial for ion transport, require additional manufacturing processes and often involve more complex binder systems. These additional steps can increase the environmental footprint of production unless carefully optimized. Conversely, the extended cycle life potentially enabled by optimized porosity structures could significantly reduce battery replacement frequency and associated waste.
Material selection for porous electrodes presents another sustainability dimension. Current high-performance sodium-ion batteries often utilize hard carbon derived from renewable biomass sources, offering a lower carbon footprint compared to synthetic graphite used in lithium-ion batteries. However, some advanced porous electrode structures require specialized materials or coatings that may introduce rare elements or energy-intensive processing steps, potentially offsetting some sustainability advantages.
Water consumption during manufacturing represents a critical resource consideration, particularly for highly porous electrode structures that often require aqueous processing and extensive washing steps. Closed-loop water recycling systems and waterless manufacturing techniques are being developed specifically for sodium-ion battery production to address this challenge, though implementation remains limited in current manufacturing facilities.
End-of-life management for sodium-ion batteries with varying porosity structures presents both challenges and opportunities. The absence of cobalt and nickel in many sodium-ion chemistries simplifies recycling processes compared to lithium-ion batteries. However, highly engineered porous structures may complicate material separation and recovery. Emerging recycling technologies specifically designed for sodium-ion batteries show promise for recovering up to 95% of electrode materials while maintaining the economic viability of recycling operations.
Carbon footprint analyses indicate that sodium-ion batteries with optimized porosity can achieve 60-80% lower greenhouse gas emissions during production compared to equivalent lithium-ion batteries, primarily due to less energy-intensive material extraction and processing. This advantage becomes particularly significant when considering the full lifecycle environmental impact of mobile electronic devices.
When examining porosity limitations in sodium-ion batteries for mobile electronics, sustainability considerations become increasingly important. Higher porosity electrode designs, while beneficial for ion transport, require additional manufacturing processes and often involve more complex binder systems. These additional steps can increase the environmental footprint of production unless carefully optimized. Conversely, the extended cycle life potentially enabled by optimized porosity structures could significantly reduce battery replacement frequency and associated waste.
Material selection for porous electrodes presents another sustainability dimension. Current high-performance sodium-ion batteries often utilize hard carbon derived from renewable biomass sources, offering a lower carbon footprint compared to synthetic graphite used in lithium-ion batteries. However, some advanced porous electrode structures require specialized materials or coatings that may introduce rare elements or energy-intensive processing steps, potentially offsetting some sustainability advantages.
Water consumption during manufacturing represents a critical resource consideration, particularly for highly porous electrode structures that often require aqueous processing and extensive washing steps. Closed-loop water recycling systems and waterless manufacturing techniques are being developed specifically for sodium-ion battery production to address this challenge, though implementation remains limited in current manufacturing facilities.
End-of-life management for sodium-ion batteries with varying porosity structures presents both challenges and opportunities. The absence of cobalt and nickel in many sodium-ion chemistries simplifies recycling processes compared to lithium-ion batteries. However, highly engineered porous structures may complicate material separation and recovery. Emerging recycling technologies specifically designed for sodium-ion batteries show promise for recovering up to 95% of electrode materials while maintaining the economic viability of recycling operations.
Carbon footprint analyses indicate that sodium-ion batteries with optimized porosity can achieve 60-80% lower greenhouse gas emissions during production compared to equivalent lithium-ion batteries, primarily due to less energy-intensive material extraction and processing. This advantage becomes particularly significant when considering the full lifecycle environmental impact of mobile electronic devices.
Manufacturing Scalability and Cost Analysis
The scalability of sodium-ion battery manufacturing processes presents both opportunities and challenges for mass production in mobile electronics applications. Current manufacturing infrastructure for lithium-ion batteries can be adapted for sodium-ion production with moderate modifications, particularly in electrode preparation and cell assembly stages. However, the specific requirements for controlling porosity in sodium-ion battery electrodes necessitate specialized equipment and process parameters that differ from traditional lithium-ion manufacturing lines.
Cost analysis reveals that sodium-ion batteries with optimized porosity structures could achieve 15-20% lower production costs compared to lithium-ion counterparts, primarily due to the abundance and lower cost of sodium raw materials. Nevertheless, the initial capital expenditure for establishing dedicated manufacturing facilities with precise porosity control capabilities remains a significant barrier to entry for many manufacturers.
The relationship between porosity control and manufacturing yield is particularly critical. Production data indicates that maintaining consistent porosity levels across large electrode batches remains challenging, with current processes showing 8-12% variation in porosity parameters. This variability directly impacts performance consistency and increases quality control costs, offsetting some of the raw material cost advantages.
From a technical perspective, the scalable production of high-porosity electrodes requires innovations in slurry formulation and coating technologies. Conventional slot-die coating methods struggle to maintain uniform pore distribution when scaled to industrial production speeds of 30-50 m/min. Alternative approaches such as freeze-casting and template-assisted techniques show promise in laboratory settings but face significant hurdles in scaling to commercial production volumes.
Energy consumption during manufacturing represents another important cost factor. The drying and calendering processes for controlling electrode porosity are particularly energy-intensive, accounting for approximately 25% of the total energy consumption in cell production. Innovations in low-temperature drying technologies and more efficient calendering processes could significantly reduce both environmental impact and production costs.
Supply chain considerations also affect manufacturing scalability. While sodium compounds are geographically more distributed than lithium resources, the specialized binders and electrolyte additives required for optimized porosity control in sodium-ion batteries currently have limited supplier networks. This supply constraint could potentially create bottlenecks as production scales, necessitating strategic supplier development initiatives by manufacturers entering this space.
Cost analysis reveals that sodium-ion batteries with optimized porosity structures could achieve 15-20% lower production costs compared to lithium-ion counterparts, primarily due to the abundance and lower cost of sodium raw materials. Nevertheless, the initial capital expenditure for establishing dedicated manufacturing facilities with precise porosity control capabilities remains a significant barrier to entry for many manufacturers.
The relationship between porosity control and manufacturing yield is particularly critical. Production data indicates that maintaining consistent porosity levels across large electrode batches remains challenging, with current processes showing 8-12% variation in porosity parameters. This variability directly impacts performance consistency and increases quality control costs, offsetting some of the raw material cost advantages.
From a technical perspective, the scalable production of high-porosity electrodes requires innovations in slurry formulation and coating technologies. Conventional slot-die coating methods struggle to maintain uniform pore distribution when scaled to industrial production speeds of 30-50 m/min. Alternative approaches such as freeze-casting and template-assisted techniques show promise in laboratory settings but face significant hurdles in scaling to commercial production volumes.
Energy consumption during manufacturing represents another important cost factor. The drying and calendering processes for controlling electrode porosity are particularly energy-intensive, accounting for approximately 25% of the total energy consumption in cell production. Innovations in low-temperature drying technologies and more efficient calendering processes could significantly reduce both environmental impact and production costs.
Supply chain considerations also affect manufacturing scalability. While sodium compounds are geographically more distributed than lithium resources, the specialized binders and electrolyte additives required for optimized porosity control in sodium-ion batteries currently have limited supplier networks. This supply constraint could potentially create bottlenecks as production scales, necessitating strategic supplier development initiatives by manufacturers entering this space.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!