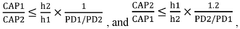Enhancing Mass Transfer in Sodium-ion Batteries for Improved Capacity
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium-ion Battery Technology Evolution and Objectives
Sodium-ion battery technology has evolved significantly since its conceptualization in the 1980s, emerging as a promising alternative to lithium-ion batteries due to sodium's abundant availability and lower cost. The initial research phase focused primarily on understanding sodium intercalation mechanisms, with limited practical applications due to performance constraints. By the early 2000s, research momentum accelerated with the development of hard carbon anodes and layered oxide cathodes, establishing foundational materials for modern sodium-ion batteries.
The 2010s marked a pivotal era with breakthrough innovations in electrode materials, including Prussian blue analogs, polyanionic compounds, and advanced carbon-based materials. These developments addressed critical challenges in sodium storage capacity and cycling stability. Concurrently, electrolyte formulations evolved from simple sodium salts to complex systems incorporating additives for enhanced interfacial stability and ionic conductivity.
Recent technological advancements have focused on nanostructured materials and composite electrodes to facilitate sodium-ion transport and mitigate volume expansion issues. The integration of computational modeling and high-throughput screening methodologies has accelerated material discovery, enabling rational design of electrode architectures with optimized mass transfer properties.
Current research objectives center on enhancing mass transfer kinetics within sodium-ion batteries to overcome capacity limitations. This involves developing electrode materials with optimized porosity, reduced diffusion pathways, and improved electronic conductivity. Specific goals include achieving energy densities exceeding 200 Wh/kg at the cell level, cycle life beyond 2000 cycles, and rate capabilities supporting fast charging within 30 minutes.
Another critical objective is addressing the fundamental challenges of sodium-ion transport at interfaces. This includes engineering stable solid-electrolyte interphases (SEI) that facilitate efficient ion transfer while preventing parasitic reactions. Research aims to understand and control the formation mechanisms of these interfacial layers to enhance overall battery performance.
Long-term technological goals extend to developing all-solid-state sodium-ion batteries with superior safety profiles and energy densities. This requires innovative solid electrolytes with high ionic conductivity at ambient temperatures and stable interfaces with electrode materials. Additionally, there is growing emphasis on sustainable manufacturing processes and end-of-life recycling strategies to establish sodium-ion batteries as an environmentally responsible energy storage solution.
The evolution trajectory suggests that mass transfer enhancement will remain a central focus, with interdisciplinary approaches combining materials science, electrochemistry, and engineering principles to overcome current limitations and realize the full potential of sodium-ion battery technology.
The 2010s marked a pivotal era with breakthrough innovations in electrode materials, including Prussian blue analogs, polyanionic compounds, and advanced carbon-based materials. These developments addressed critical challenges in sodium storage capacity and cycling stability. Concurrently, electrolyte formulations evolved from simple sodium salts to complex systems incorporating additives for enhanced interfacial stability and ionic conductivity.
Recent technological advancements have focused on nanostructured materials and composite electrodes to facilitate sodium-ion transport and mitigate volume expansion issues. The integration of computational modeling and high-throughput screening methodologies has accelerated material discovery, enabling rational design of electrode architectures with optimized mass transfer properties.
Current research objectives center on enhancing mass transfer kinetics within sodium-ion batteries to overcome capacity limitations. This involves developing electrode materials with optimized porosity, reduced diffusion pathways, and improved electronic conductivity. Specific goals include achieving energy densities exceeding 200 Wh/kg at the cell level, cycle life beyond 2000 cycles, and rate capabilities supporting fast charging within 30 minutes.
Another critical objective is addressing the fundamental challenges of sodium-ion transport at interfaces. This includes engineering stable solid-electrolyte interphases (SEI) that facilitate efficient ion transfer while preventing parasitic reactions. Research aims to understand and control the formation mechanisms of these interfacial layers to enhance overall battery performance.
Long-term technological goals extend to developing all-solid-state sodium-ion batteries with superior safety profiles and energy densities. This requires innovative solid electrolytes with high ionic conductivity at ambient temperatures and stable interfaces with electrode materials. Additionally, there is growing emphasis on sustainable manufacturing processes and end-of-life recycling strategies to establish sodium-ion batteries as an environmentally responsible energy storage solution.
The evolution trajectory suggests that mass transfer enhancement will remain a central focus, with interdisciplinary approaches combining materials science, electrochemistry, and engineering principles to overcome current limitations and realize the full potential of sodium-ion battery technology.
Market Analysis for Sodium-ion Energy Storage Solutions
The global sodium-ion battery market is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate a compound annual growth rate exceeding 15% between 2023 and 2030, with the market expected to reach substantial commercial scale by mid-decade. This acceleration is primarily fueled by the inherent advantages of sodium-ion technology, particularly its cost-effectiveness compared to lithium-ion alternatives.
The cost advantage stems from sodium's greater natural abundance, with sodium resources approximately 1,000 times more plentiful than lithium in the Earth's crust. This abundance translates to lower raw material costs, with sodium carbonate priced at roughly one-third the cost of lithium carbonate. The economic benefits extend beyond material costs to include simplified supply chains and reduced geopolitical dependencies, as sodium resources are more evenly distributed globally than lithium deposits.
Market segmentation reveals distinct application sectors for sodium-ion batteries. The stationary energy storage sector represents the largest immediate opportunity, particularly for grid-scale applications where energy density constraints are less critical than cost considerations. The electric vehicle segment presents a growing secondary market, especially for entry-level vehicles and urban mobility solutions where moderate energy density is acceptable.
Regional market analysis shows China leading sodium-ion battery development and commercialization, with companies like CATL and BYD making substantial investments. European markets follow, driven by stringent sustainability regulations and circular economy initiatives. North America shows increasing interest, particularly for grid stabilization applications in renewable energy integration.
Consumer electronics represents an emerging application segment, particularly for devices where cost sensitivity outweighs extreme miniaturization requirements. Industrial applications, including backup power systems and material handling equipment, constitute another growing segment where sodium-ion technology's safety advantages and operational temperature range prove beneficial.
Market barriers include the technology's current energy density limitations compared to advanced lithium-ion chemistries, manufacturing scale constraints, and limited commercial deployment history. However, these barriers are progressively diminishing as research advances in mass transfer enhancement techniques directly address energy density limitations.
The competitive landscape features established battery manufacturers expanding into sodium-ion technology alongside specialized startups focused exclusively on sodium-based solutions. This market structure is driving both innovation and commercialization pathways, with increasing venture capital investment flowing into the sector.
The cost advantage stems from sodium's greater natural abundance, with sodium resources approximately 1,000 times more plentiful than lithium in the Earth's crust. This abundance translates to lower raw material costs, with sodium carbonate priced at roughly one-third the cost of lithium carbonate. The economic benefits extend beyond material costs to include simplified supply chains and reduced geopolitical dependencies, as sodium resources are more evenly distributed globally than lithium deposits.
Market segmentation reveals distinct application sectors for sodium-ion batteries. The stationary energy storage sector represents the largest immediate opportunity, particularly for grid-scale applications where energy density constraints are less critical than cost considerations. The electric vehicle segment presents a growing secondary market, especially for entry-level vehicles and urban mobility solutions where moderate energy density is acceptable.
Regional market analysis shows China leading sodium-ion battery development and commercialization, with companies like CATL and BYD making substantial investments. European markets follow, driven by stringent sustainability regulations and circular economy initiatives. North America shows increasing interest, particularly for grid stabilization applications in renewable energy integration.
Consumer electronics represents an emerging application segment, particularly for devices where cost sensitivity outweighs extreme miniaturization requirements. Industrial applications, including backup power systems and material handling equipment, constitute another growing segment where sodium-ion technology's safety advantages and operational temperature range prove beneficial.
Market barriers include the technology's current energy density limitations compared to advanced lithium-ion chemistries, manufacturing scale constraints, and limited commercial deployment history. However, these barriers are progressively diminishing as research advances in mass transfer enhancement techniques directly address energy density limitations.
The competitive landscape features established battery manufacturers expanding into sodium-ion technology alongside specialized startups focused exclusively on sodium-based solutions. This market structure is driving both innovation and commercialization pathways, with increasing venture capital investment flowing into the sector.
Mass Transfer Challenges in Na-ion Battery Systems
Mass transfer limitations represent one of the most significant challenges in sodium-ion battery (SIB) systems, directly impacting their capacity, rate capability, and cycle life. Unlike their lithium-ion counterparts, sodium ions possess a larger ionic radius (1.02Å vs. 0.76Å for Li+), resulting in slower diffusion kinetics through electrode materials and electrolytes. This fundamental difference creates inherent mass transfer bottlenecks that must be addressed to realize the full potential of SIB technology.
The mass transfer process in SIB systems can be categorized into several critical pathways: ion transport through the electrolyte, ion diffusion across the solid-electrolyte interphase (SEI), and ion intercalation/deintercalation within the electrode materials. Each pathway presents unique challenges that collectively limit battery performance.
In the electrolyte phase, sodium ion transport is hindered by higher viscosity and lower ionic conductivity compared to lithium-based systems. Conventional carbonate-based electrolytes optimized for lithium-ion batteries often demonstrate suboptimal performance when applied to sodium systems. The larger solvation shell of sodium ions increases the effective migration radius, further reducing mobility through the electrolyte medium.
The solid-electrolyte interphase in SIBs exhibits distinct characteristics from those in lithium-ion batteries. The SEI formed in sodium systems tends to be less stable and more resistive, creating a significant barrier to ion transport at the electrode-electrolyte interface. This interface resistance contributes substantially to overall cell impedance and limits the rate capability of sodium-ion batteries.
Within electrode materials, sodium ion diffusion faces severe limitations due to structural constraints. Many promising electrode materials exhibit narrow ion diffusion channels that become restrictive for the larger sodium ions. This is particularly problematic in layered oxide cathodes and hard carbon anodes, where ion insertion/extraction processes are rate-determining steps during battery operation.
The mass transfer challenges are further exacerbated during fast charging conditions, where concentration gradients become more pronounced. These gradients lead to localized depletion of sodium ions near electrode surfaces, resulting in capacity underutilization and potential degradation mechanisms such as sodium plating and structural distortion of active materials.
Temperature dependence of mass transfer processes adds another layer of complexity. At lower temperatures, the aforementioned limitations become more severe, dramatically reducing usable capacity and power capability. This temperature sensitivity presents significant challenges for applications requiring operation across wide temperature ranges.
The mass transfer process in SIB systems can be categorized into several critical pathways: ion transport through the electrolyte, ion diffusion across the solid-electrolyte interphase (SEI), and ion intercalation/deintercalation within the electrode materials. Each pathway presents unique challenges that collectively limit battery performance.
In the electrolyte phase, sodium ion transport is hindered by higher viscosity and lower ionic conductivity compared to lithium-based systems. Conventional carbonate-based electrolytes optimized for lithium-ion batteries often demonstrate suboptimal performance when applied to sodium systems. The larger solvation shell of sodium ions increases the effective migration radius, further reducing mobility through the electrolyte medium.
The solid-electrolyte interphase in SIBs exhibits distinct characteristics from those in lithium-ion batteries. The SEI formed in sodium systems tends to be less stable and more resistive, creating a significant barrier to ion transport at the electrode-electrolyte interface. This interface resistance contributes substantially to overall cell impedance and limits the rate capability of sodium-ion batteries.
Within electrode materials, sodium ion diffusion faces severe limitations due to structural constraints. Many promising electrode materials exhibit narrow ion diffusion channels that become restrictive for the larger sodium ions. This is particularly problematic in layered oxide cathodes and hard carbon anodes, where ion insertion/extraction processes are rate-determining steps during battery operation.
The mass transfer challenges are further exacerbated during fast charging conditions, where concentration gradients become more pronounced. These gradients lead to localized depletion of sodium ions near electrode surfaces, resulting in capacity underutilization and potential degradation mechanisms such as sodium plating and structural distortion of active materials.
Temperature dependence of mass transfer processes adds another layer of complexity. At lower temperatures, the aforementioned limitations become more severe, dramatically reducing usable capacity and power capability. This temperature sensitivity presents significant challenges for applications requiring operation across wide temperature ranges.
Current Mass Transfer Enhancement Strategies
01 Electrode material design for enhanced mass transfer
Specialized electrode materials can be designed to facilitate improved mass transfer in sodium-ion batteries. These materials often feature optimized structures such as porous architectures, nanostructured components, or hierarchical designs that provide shorter diffusion paths for sodium ions. By engineering electrode materials with high surface area and accessible channels, the mass transfer kinetics can be significantly enhanced, leading to improved battery performance, faster charging capabilities, and higher power density.- Electrode material design for enhanced mass transfer: Innovative electrode materials can significantly improve mass transfer in sodium-ion batteries. These designs focus on optimizing the structure and composition of electrode materials to facilitate sodium ion movement. Approaches include developing porous structures, nanostructured materials, and composite electrodes that provide shorter diffusion paths and more active sites for sodium ions, resulting in improved battery performance and cycling stability.
- Electrolyte formulations for improved ionic conductivity: Advanced electrolyte formulations play a crucial role in enhancing mass transfer in sodium-ion batteries. These formulations include novel liquid electrolytes, solid-state electrolytes, and hybrid systems that facilitate faster sodium ion transport between electrodes. Additives and solvent combinations are designed to optimize the electrolyte-electrode interface, reduce interfacial resistance, and improve the overall ionic conductivity, leading to better battery performance.
- Interface engineering for reduced mass transfer resistance: Interface engineering techniques focus on modifying the electrode-electrolyte interface to reduce mass transfer resistance in sodium-ion batteries. These approaches include surface coatings, functional interlayers, and interface modification strategies that improve the wetting properties, reduce side reactions, and facilitate smoother ion transport across interfaces. By optimizing these interfaces, the overall mass transfer efficiency and battery performance can be significantly enhanced.
- Structural design of battery components for efficient mass transport: The structural design of battery components significantly impacts mass transfer in sodium-ion batteries. This includes innovative cell architectures, electrode configurations, and separator designs that minimize diffusion distances and optimize ion transport pathways. Three-dimensional structures, gradient porosity designs, and advanced manufacturing techniques are employed to create battery components that facilitate more efficient mass transport throughout the battery system.
- Temperature and pressure control systems for mass transfer optimization: Temperature and pressure control systems are critical for optimizing mass transfer in sodium-ion batteries. These systems regulate operating conditions to enhance ion mobility, reduce viscosity of electrolytes, and maintain optimal reaction kinetics. Advanced thermal management solutions, pressure regulation mechanisms, and smart battery management systems work together to create ideal conditions for efficient mass transfer, extending battery life and improving performance across various operating environments.
02 Electrolyte formulations for improved ionic conductivity
Advanced electrolyte formulations play a crucial role in sodium-ion battery mass transfer. These formulations may include optimized salt concentrations, solvent mixtures, or additives that enhance sodium ion mobility. Novel electrolytes can reduce the formation of resistive interfaces, facilitate faster ion transport across electrode-electrolyte boundaries, and maintain stability during cycling. The development of electrolytes with high ionic conductivity and low viscosity directly impacts the mass transfer efficiency in sodium-ion batteries.Expand Specific Solutions03 Interface engineering for reduced mass transfer resistance
Engineering the interfaces between electrode materials and electrolytes can significantly reduce mass transfer resistance in sodium-ion batteries. This approach involves surface modifications, protective coatings, or functional interlayers that facilitate sodium ion transport while minimizing unwanted side reactions. By controlling the solid-electrolyte interphase formation and stability, these engineering strategies can enhance the rate capability and cycling performance of sodium-ion batteries through improved mass transfer at critical interfaces.Expand Specific Solutions04 Structural design of battery components for optimized mass transfer
The structural design of battery components, including electrode architecture, separator properties, and cell configuration, significantly impacts mass transfer in sodium-ion batteries. Innovations in this area include gradient structures, 3D electrode designs, and advanced separator technologies that facilitate efficient sodium ion movement throughout the battery. By optimizing the physical arrangement and connectivity of battery components, mass transfer limitations can be overcome, resulting in improved energy density and rate performance.Expand Specific Solutions05 Advanced characterization and modeling of mass transfer phenomena
Advanced characterization techniques and computational modeling approaches are essential for understanding and optimizing mass transfer in sodium-ion batteries. These methods include in-situ/operando measurements, multi-scale modeling, and machine learning algorithms that can reveal the dynamics of sodium ion transport under various conditions. By gaining deeper insights into mass transfer mechanisms and bottlenecks, researchers can develop more effective strategies to enhance battery performance, predict degradation pathways, and design next-generation sodium-ion battery systems.Expand Specific Solutions
Leading Companies and Research Institutions in Na-ion Battery Field
The sodium-ion battery market for enhanced mass transfer is currently in an early growth phase, characterized by increasing R&D investments and emerging commercial applications. The global market size is projected to expand significantly, driven by the need for sustainable energy storage alternatives to lithium-ion batteries. Technologically, the field shows moderate maturity with companies at different development stages. Leading players include CATL, which has pioneered commercial sodium-ion batteries, BYD and Faradion (acquired by Reliance) focusing on advanced electrode materials, and research institutions like MIT and Nanjing University contributing fundamental innovations. Shenzhen Capchem and GEM are advancing electrolyte formulations, while Trina Energy Storage and Liyang HiNa are developing integrated storage solutions with improved mass transfer characteristics.
Faradion Ltd.
Technical Solution: Faradion has pioneered a proprietary sodium-ion technology that focuses on enhancing mass transfer through advanced electrode design and electrolyte formulations. Their approach involves using layered oxide cathodes with optimized particle morphology and controlled porosity to facilitate sodium ion diffusion. The company has developed a unique "rapid insertion" framework where they engineer electrode materials with expanded interlayer spacing specifically tailored for the larger ionic radius of sodium compared to lithium. This allows for faster ion transport within the electrode structure. Additionally, Faradion employs specialized carbon-coated hard carbon anodes with hierarchical pore structures that significantly improve sodium ion accessibility to active sites. Their electrolyte systems incorporate fluoroethylene carbonate (FEC) additives that form stable solid electrolyte interphase (SEI) layers, reducing interfacial resistance and enhancing overall mass transfer kinetics.
Strengths: Faradion's technology enables sodium-ion batteries with energy densities approaching 160 Wh/kg, making them competitive with some lithium-ion chemistries while using more abundant materials. Their solutions operate effectively across wider temperature ranges (-30°C to 60°C) than many competitors. Weaknesses: The technology still faces challenges with long-term cycling stability compared to mature lithium-ion systems, and the power density remains lower than high-performance lithium-ion batteries.
Liyang HiNa Battery Technology Co., Ltd.
Technical Solution: HiNa Battery has developed a specialized sodium-ion battery technology platform called "Na-Fast" that directly addresses mass transfer limitations through innovative material design and cell engineering. Their approach centers on a proprietary layered oxide cathode material (Na₂/₃Fe₁/₃Mn₂/₃O₂) with optimized crystal structure that provides wide sodium ion diffusion channels. HiNa's cathode particles feature a unique hierarchical structure with primary nanoparticles assembled into microspheres, creating shortened diffusion paths while maintaining high tap density. For anodes, HiNa employs a hard carbon material with engineered porosity and surface functionality that enhances sodium ion adsorption kinetics and reduces interfacial resistance. A key innovation in their technology is the "dual-phase" electrolyte system that combines conventional liquid electrolytes with a sodium-conducting polymer phase at the electrode interfaces, creating preferential ion transport pathways. HiNa has also implemented advanced electrode manufacturing techniques that precisely control electrode microstructure, including porosity distribution and tortuosity, to optimize mass transport throughout the cell. Their sodium-ion cells demonstrate exceptional rate capability, achieving 80% capacity retention at 2C discharge rates and the ability to charge to 80% capacity in under 30 minutes.
Strengths: HiNa's technology offers excellent cycling stability with over 3,000 cycles demonstrated at moderate depths of discharge, significantly outperforming many competing sodium-ion solutions. Their batteries maintain consistent performance across a wide temperature range (-20°C to 60°C). Weaknesses: The current energy density (approximately 145 Wh/kg) remains lower than commercial lithium-ion batteries, and their manufacturing scale is still limited compared to major battery producers.
Critical Patents and Innovations in Na-ion Mass Transfer
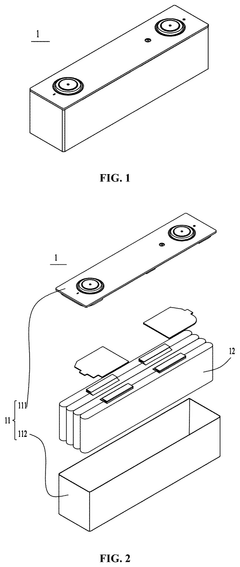
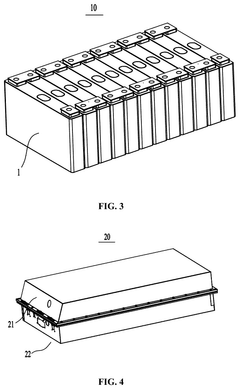
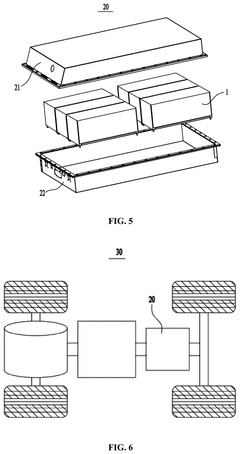
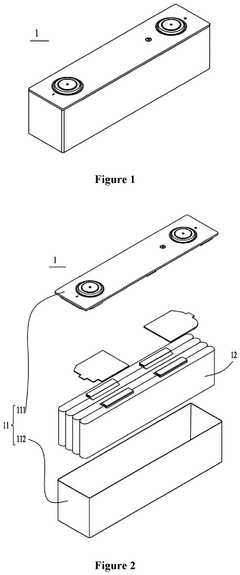
Sodium-ion battery, battery module, battery pack and electrical device
PatentPendingUS20250006927A1
Innovation
- Adjusting the porosity and compaction density of positive and negative electrode active material layers and separators to ensure optimal ion migration, with specific porosity ratios and compaction density ratios that allow for efficient deintercalation and intercalation of sodium ions, thereby improving charging and discharging performance.
Sodium-ion battery, battery module, battery pack and electric device
PatentPendingEP4451405A1
Innovation
- Adjusting the porosity and compaction density of positive and negative electrode active material layers and separators to ensure efficient sodium ion migration, with specific porosity ratios and compaction density ranges that facilitate complete intercalation and deintercalation, thereby improving charging and discharging performance.
Material Science Advancements for Na-ion Electrodes
Recent advancements in material science have significantly contributed to overcoming the challenges associated with sodium-ion battery electrodes. The development of novel electrode materials with optimized structures has been pivotal in enhancing mass transfer capabilities, directly addressing capacity limitations in Na-ion batteries. These innovations focus primarily on increasing ionic conductivity, reducing diffusion pathways, and improving structural stability during charge-discharge cycles.
Nanostructured materials represent a breakthrough in electrode design, offering increased surface area and shortened diffusion paths for sodium ions. Carbon-based materials, particularly hard carbons derived from biomass precursors, have demonstrated exceptional sodium storage capabilities due to their disordered structure and abundant defect sites. These materials provide numerous active sites for sodium ion adsorption while maintaining structural integrity during repeated cycling.
Layered transition metal oxides (NaxMO2, where M represents metals like Mn, Fe, Co) have emerged as promising cathode materials, with researchers focusing on optimizing interlayer spacing to facilitate sodium ion intercalation. Phosphate-based compounds, particularly NASICON-type structures (Na Super Ionic CONductor), exhibit three-dimensional frameworks that enable superior ionic conductivity and structural stability, addressing key limitations in mass transfer.
Surface modification techniques, including atomic layer deposition and functional coating applications, have proven effective in stabilizing electrode-electrolyte interfaces. These modifications create protective layers that prevent unwanted side reactions while maintaining efficient ion transport pathways. Additionally, dopant incorporation strategies have been employed to engineer electronic structures and create defects that enhance sodium ion diffusion kinetics.
Composite electrode formulations combining different materials have shown synergistic effects in improving both electronic and ionic conductivity. For instance, integrating conductive carbon networks with active materials has significantly enhanced electron transport while maintaining efficient sodium ion diffusion. These composite approaches effectively address the multifaceted challenges of mass transfer in sodium-ion systems.
Advanced characterization techniques, including in-situ X-ray diffraction and transmission electron microscopy, have been instrumental in understanding structural evolution during cycling. These insights have guided the rational design of electrode materials with optimized morphologies and compositions specifically tailored for sodium-ion storage. Computational modeling approaches have further accelerated material discovery by predicting promising candidates with favorable sodium diffusion properties before experimental validation.
Nanostructured materials represent a breakthrough in electrode design, offering increased surface area and shortened diffusion paths for sodium ions. Carbon-based materials, particularly hard carbons derived from biomass precursors, have demonstrated exceptional sodium storage capabilities due to their disordered structure and abundant defect sites. These materials provide numerous active sites for sodium ion adsorption while maintaining structural integrity during repeated cycling.
Layered transition metal oxides (NaxMO2, where M represents metals like Mn, Fe, Co) have emerged as promising cathode materials, with researchers focusing on optimizing interlayer spacing to facilitate sodium ion intercalation. Phosphate-based compounds, particularly NASICON-type structures (Na Super Ionic CONductor), exhibit three-dimensional frameworks that enable superior ionic conductivity and structural stability, addressing key limitations in mass transfer.
Surface modification techniques, including atomic layer deposition and functional coating applications, have proven effective in stabilizing electrode-electrolyte interfaces. These modifications create protective layers that prevent unwanted side reactions while maintaining efficient ion transport pathways. Additionally, dopant incorporation strategies have been employed to engineer electronic structures and create defects that enhance sodium ion diffusion kinetics.
Composite electrode formulations combining different materials have shown synergistic effects in improving both electronic and ionic conductivity. For instance, integrating conductive carbon networks with active materials has significantly enhanced electron transport while maintaining efficient sodium ion diffusion. These composite approaches effectively address the multifaceted challenges of mass transfer in sodium-ion systems.
Advanced characterization techniques, including in-situ X-ray diffraction and transmission electron microscopy, have been instrumental in understanding structural evolution during cycling. These insights have guided the rational design of electrode materials with optimized morphologies and compositions specifically tailored for sodium-ion storage. Computational modeling approaches have further accelerated material discovery by predicting promising candidates with favorable sodium diffusion properties before experimental validation.
Sustainability and Cost Analysis of Na-ion Technology
Sustainability and Cost Analysis of Na-ion Technology represents a critical dimension in evaluating the viability of sodium-ion batteries as an alternative to lithium-ion technologies. From an environmental perspective, sodium-ion batteries offer significant advantages due to the abundant nature of sodium resources. Unlike lithium, sodium is the sixth most abundant element in the Earth's crust, with reserves widely distributed across the globe, reducing geopolitical supply risks and environmental impacts associated with resource extraction.
The carbon footprint of Na-ion battery production is estimated to be 20-30% lower than that of conventional Li-ion batteries, primarily due to less energy-intensive mining and processing requirements. Life cycle assessments indicate that Na-ion technology could reduce greenhouse gas emissions by approximately 60-90 kg CO2-eq per kWh of battery capacity compared to lithium-based alternatives, particularly when considering end-to-end production chains.
From an economic standpoint, raw material costs for Na-ion batteries present a compelling case. Current market analyses suggest that sodium carbonate costs approximately $300-400 per ton, whereas lithium carbonate prices have fluctuated between $5,000-15,000 per ton in recent years. This translates to potential material cost savings of 30-40% for cathode materials, which represent a significant portion of battery production expenses.
Manufacturing infrastructure compatibility further enhances the cost-effectiveness of Na-ion technology. Existing Li-ion production facilities can be adapted for Na-ion battery manufacturing with relatively minor modifications, estimated to require only 15-20% of the capital investment needed for new dedicated facilities. This adaptability significantly reduces barriers to market entry and accelerates commercial deployment timelines.
End-of-life considerations also favor Na-ion technology, with recycling processes potentially simpler and less hazardous than those for lithium-based systems. Recovery rates for key materials from Na-ion batteries are projected to reach 80-90%, compared to 50-70% for conventional Li-ion batteries, creating additional value in the circular economy framework.
The total cost of ownership analysis reveals that despite currently lower energy densities, Na-ion batteries could achieve cost parity with Li-ion technologies in specific applications by 2025, with projected costs of $70-80 per kWh compared to $90-100 per kWh for lithium-ion systems. This economic advantage becomes particularly pronounced in stationary storage applications where energy density constraints are less critical than in mobile applications.
The carbon footprint of Na-ion battery production is estimated to be 20-30% lower than that of conventional Li-ion batteries, primarily due to less energy-intensive mining and processing requirements. Life cycle assessments indicate that Na-ion technology could reduce greenhouse gas emissions by approximately 60-90 kg CO2-eq per kWh of battery capacity compared to lithium-based alternatives, particularly when considering end-to-end production chains.
From an economic standpoint, raw material costs for Na-ion batteries present a compelling case. Current market analyses suggest that sodium carbonate costs approximately $300-400 per ton, whereas lithium carbonate prices have fluctuated between $5,000-15,000 per ton in recent years. This translates to potential material cost savings of 30-40% for cathode materials, which represent a significant portion of battery production expenses.
Manufacturing infrastructure compatibility further enhances the cost-effectiveness of Na-ion technology. Existing Li-ion production facilities can be adapted for Na-ion battery manufacturing with relatively minor modifications, estimated to require only 15-20% of the capital investment needed for new dedicated facilities. This adaptability significantly reduces barriers to market entry and accelerates commercial deployment timelines.
End-of-life considerations also favor Na-ion technology, with recycling processes potentially simpler and less hazardous than those for lithium-based systems. Recovery rates for key materials from Na-ion batteries are projected to reach 80-90%, compared to 50-70% for conventional Li-ion batteries, creating additional value in the circular economy framework.
The total cost of ownership analysis reveals that despite currently lower energy densities, Na-ion batteries could achieve cost parity with Li-ion technologies in specific applications by 2025, with projected costs of $70-80 per kWh compared to $90-100 per kWh for lithium-ion systems. This economic advantage becomes particularly pronounced in stationary storage applications where energy density constraints are less critical than in mobile applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!