Material Microstructure Changes in Sodium-ion Batteries: Market Impacts
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-ion Battery Microstructure Evolution and Research Objectives
Sodium-ion battery technology has evolved significantly over the past decade, emerging as a promising alternative to lithium-ion batteries. The microstructural changes in sodium-ion battery materials represent a critical area of research that directly impacts performance, durability, and commercial viability. Historically, the development of these batteries has been hindered by challenges related to sodium's larger ionic radius compared to lithium, which affects intercalation dynamics and structural stability during charge-discharge cycles.
The evolution of sodium-ion battery technology can be traced back to the 1980s, but significant advancements have only materialized in the last decade. Research has intensified as concerns about lithium supply constraints and geopolitical factors affecting lithium availability have grown. This renewed interest has driven innovations in electrode materials, electrolytes, and battery architecture designed specifically to accommodate sodium's unique properties.
Current technological trends indicate a shift toward developing advanced cathode materials with optimized crystal structures that can better accommodate sodium ion insertion and extraction while maintaining structural integrity. Layered transition metal oxides, polyanionic compounds, and Prussian blue analogs have emerged as promising cathode materials, each with distinct microstructural characteristics that evolve during cycling.
For anode materials, research has progressed beyond traditional carbon-based materials to include hard carbons, alloys, and conversion-type materials. The microstructural evolution of these materials during sodiation and desodiation processes significantly influences battery performance metrics, including capacity retention, rate capability, and cycle life.
Our research objectives focus on understanding the fundamental mechanisms of microstructural changes in sodium-ion battery materials during electrochemical cycling. Specifically, we aim to:
1. Characterize the evolution of crystal structures in various cathode and anode materials during repeated sodium insertion and extraction using advanced in-situ techniques.
2. Identify the correlation between microstructural changes and performance degradation, particularly capacity fading and impedance growth.
3. Develop predictive models that can forecast microstructural evolution under various operating conditions, enabling more accurate lifetime predictions.
4. Engineer novel material compositions and architectures that minimize detrimental microstructural changes while maximizing energy density and power capability.
5. Establish design principles for sodium-ion battery materials that balance structural stability with electrochemical performance.
By achieving these objectives, we anticipate contributing to the accelerated commercialization of sodium-ion batteries as a viable alternative to lithium-ion technology, particularly for stationary energy storage applications where cost considerations outweigh energy density requirements.
The evolution of sodium-ion battery technology can be traced back to the 1980s, but significant advancements have only materialized in the last decade. Research has intensified as concerns about lithium supply constraints and geopolitical factors affecting lithium availability have grown. This renewed interest has driven innovations in electrode materials, electrolytes, and battery architecture designed specifically to accommodate sodium's unique properties.
Current technological trends indicate a shift toward developing advanced cathode materials with optimized crystal structures that can better accommodate sodium ion insertion and extraction while maintaining structural integrity. Layered transition metal oxides, polyanionic compounds, and Prussian blue analogs have emerged as promising cathode materials, each with distinct microstructural characteristics that evolve during cycling.
For anode materials, research has progressed beyond traditional carbon-based materials to include hard carbons, alloys, and conversion-type materials. The microstructural evolution of these materials during sodiation and desodiation processes significantly influences battery performance metrics, including capacity retention, rate capability, and cycle life.
Our research objectives focus on understanding the fundamental mechanisms of microstructural changes in sodium-ion battery materials during electrochemical cycling. Specifically, we aim to:
1. Characterize the evolution of crystal structures in various cathode and anode materials during repeated sodium insertion and extraction using advanced in-situ techniques.
2. Identify the correlation between microstructural changes and performance degradation, particularly capacity fading and impedance growth.
3. Develop predictive models that can forecast microstructural evolution under various operating conditions, enabling more accurate lifetime predictions.
4. Engineer novel material compositions and architectures that minimize detrimental microstructural changes while maximizing energy density and power capability.
5. Establish design principles for sodium-ion battery materials that balance structural stability with electrochemical performance.
By achieving these objectives, we anticipate contributing to the accelerated commercialization of sodium-ion batteries as a viable alternative to lithium-ion technology, particularly for stationary energy storage applications where cost considerations outweigh energy density requirements.
Market Demand Analysis for Na-ion Battery Technologies
The global energy storage market is witnessing a significant shift towards sodium-ion battery technologies, driven by increasing concerns over lithium supply chain vulnerabilities and rising costs. Market research indicates that the global sodium-ion battery market is projected to grow at a compound annual growth rate of 18% between 2023 and 2030, reaching a market value of 1.2 billion USD by the end of the forecast period. This growth trajectory is substantially higher than previously anticipated, reflecting the accelerating interest in alternative battery technologies.
The demand for sodium-ion batteries is primarily fueled by grid-scale energy storage applications, which currently represent approximately 45% of the total market share. This segment is expected to maintain its dominance due to the increasing integration of renewable energy sources into power grids worldwide, necessitating efficient and cost-effective energy storage solutions. The renewable energy sector's expansion, with global capacity additions reaching 295 GW in 2022, directly correlates with the growing demand for large-scale energy storage systems where sodium-ion technology offers compelling advantages.
Consumer electronics represents the second-largest application segment, accounting for 30% of the market. The appeal in this sector stems from sodium-ion batteries' improved safety profile and potential cost advantages. As material microstructure innovations continue to enhance energy density capabilities, industry analysts predict this segment will experience the fastest growth rate over the next five years.
Electric vehicles, while currently a smaller market segment at 15%, show promising growth potential. Major automotive manufacturers, including BYD and CATL, have announced plans to incorporate sodium-ion batteries in specific vehicle models by 2025. This interest is driven by sodium-ion batteries' superior performance in extreme temperature conditions and faster charging capabilities resulting from recent microstructural engineering breakthroughs.
Geographically, Asia-Pacific dominates the sodium-ion battery market with a 60% share, led by China's aggressive development programs and manufacturing capabilities. Europe follows at 25%, with significant investments in research and manufacturing facilities, particularly in Germany, France, and the UK. North America accounts for 12% of the market, with growing interest from both government-funded research initiatives and private sector investments.
The market demand is further strengthened by favorable government policies worldwide. The European Union's Critical Raw Materials Act and the United States' Inflation Reduction Act both provide substantial incentives for developing battery technologies that reduce dependence on critical minerals, directly benefiting sodium-ion battery development and commercialization efforts.
The demand for sodium-ion batteries is primarily fueled by grid-scale energy storage applications, which currently represent approximately 45% of the total market share. This segment is expected to maintain its dominance due to the increasing integration of renewable energy sources into power grids worldwide, necessitating efficient and cost-effective energy storage solutions. The renewable energy sector's expansion, with global capacity additions reaching 295 GW in 2022, directly correlates with the growing demand for large-scale energy storage systems where sodium-ion technology offers compelling advantages.
Consumer electronics represents the second-largest application segment, accounting for 30% of the market. The appeal in this sector stems from sodium-ion batteries' improved safety profile and potential cost advantages. As material microstructure innovations continue to enhance energy density capabilities, industry analysts predict this segment will experience the fastest growth rate over the next five years.
Electric vehicles, while currently a smaller market segment at 15%, show promising growth potential. Major automotive manufacturers, including BYD and CATL, have announced plans to incorporate sodium-ion batteries in specific vehicle models by 2025. This interest is driven by sodium-ion batteries' superior performance in extreme temperature conditions and faster charging capabilities resulting from recent microstructural engineering breakthroughs.
Geographically, Asia-Pacific dominates the sodium-ion battery market with a 60% share, led by China's aggressive development programs and manufacturing capabilities. Europe follows at 25%, with significant investments in research and manufacturing facilities, particularly in Germany, France, and the UK. North America accounts for 12% of the market, with growing interest from both government-funded research initiatives and private sector investments.
The market demand is further strengthened by favorable government policies worldwide. The European Union's Critical Raw Materials Act and the United States' Inflation Reduction Act both provide substantial incentives for developing battery technologies that reduce dependence on critical minerals, directly benefiting sodium-ion battery development and commercialization efforts.
Current Challenges in Na-ion Battery Microstructure Engineering
Despite significant advancements in sodium-ion battery technology, engineering optimal microstructures remains a formidable challenge that impedes widespread commercialization. The primary obstacle lies in controlling structural changes during repeated charge-discharge cycles, as sodium ions (1.02Å) are approximately 55% larger than lithium ions (0.76Å), causing more severe volume expansion and mechanical stress within electrode materials.
Current electrode materials exhibit substantial degradation due to these microstructural changes. For instance, hard carbon anodes suffer from exfoliation and pulverization after extended cycling, while layered oxide cathodes experience interlayer spacing fluctuations that compromise structural integrity. These phenomena lead to capacity fading, reduced cycle life, and diminished power performance—critical metrics for market viability.
Another significant challenge is the electrolyte-electrode interface stability. The larger ionic radius of sodium affects solid-electrolyte interphase (SEI) formation dynamics, resulting in less stable interfaces compared to lithium-ion systems. Researchers struggle to develop electrolyte formulations that form uniform, conductive, and mechanically robust SEI layers that can accommodate the structural changes during cycling.
Manufacturing scalability presents additional complications. Current microstructure engineering approaches that work in laboratory settings often fail to translate to industrial-scale production. Techniques such as precise particle size control, porosity optimization, and conductive network formation require sophisticated processing methods that are difficult to implement cost-effectively at scale.
Temperature sensitivity further complicates microstructure engineering efforts. Sodium-ion systems typically demonstrate more pronounced microstructural changes at elevated temperatures compared to lithium-ion counterparts, necessitating more robust thermal management strategies and temperature-resilient material designs.
The lack of standardized characterization methodologies specifically tailored for sodium-ion battery microstructures hinders progress. While techniques like in-situ XRD and TEM provide valuable insights, they often cannot capture the full complexity of microstructural evolution during real-world operation conditions, creating gaps between laboratory understanding and practical performance.
Computational modeling limitations also impede advancement. Current models struggle to accurately predict long-term microstructural evolution in sodium-ion systems, particularly regarding crack propagation, phase transformations, and interfacial phenomena. This gap between theoretical predictions and experimental observations slows the development of optimized microstructures.
Addressing these challenges requires interdisciplinary approaches combining materials science, electrochemistry, and advanced manufacturing techniques to develop sodium-ion batteries with microstructures that can maintain stability throughout thousands of cycles while delivering competitive performance metrics.
Current electrode materials exhibit substantial degradation due to these microstructural changes. For instance, hard carbon anodes suffer from exfoliation and pulverization after extended cycling, while layered oxide cathodes experience interlayer spacing fluctuations that compromise structural integrity. These phenomena lead to capacity fading, reduced cycle life, and diminished power performance—critical metrics for market viability.
Another significant challenge is the electrolyte-electrode interface stability. The larger ionic radius of sodium affects solid-electrolyte interphase (SEI) formation dynamics, resulting in less stable interfaces compared to lithium-ion systems. Researchers struggle to develop electrolyte formulations that form uniform, conductive, and mechanically robust SEI layers that can accommodate the structural changes during cycling.
Manufacturing scalability presents additional complications. Current microstructure engineering approaches that work in laboratory settings often fail to translate to industrial-scale production. Techniques such as precise particle size control, porosity optimization, and conductive network formation require sophisticated processing methods that are difficult to implement cost-effectively at scale.
Temperature sensitivity further complicates microstructure engineering efforts. Sodium-ion systems typically demonstrate more pronounced microstructural changes at elevated temperatures compared to lithium-ion counterparts, necessitating more robust thermal management strategies and temperature-resilient material designs.
The lack of standardized characterization methodologies specifically tailored for sodium-ion battery microstructures hinders progress. While techniques like in-situ XRD and TEM provide valuable insights, they often cannot capture the full complexity of microstructural evolution during real-world operation conditions, creating gaps between laboratory understanding and practical performance.
Computational modeling limitations also impede advancement. Current models struggle to accurately predict long-term microstructural evolution in sodium-ion systems, particularly regarding crack propagation, phase transformations, and interfacial phenomena. This gap between theoretical predictions and experimental observations slows the development of optimized microstructures.
Addressing these challenges requires interdisciplinary approaches combining materials science, electrochemistry, and advanced manufacturing techniques to develop sodium-ion batteries with microstructures that can maintain stability throughout thousands of cycles while delivering competitive performance metrics.
Current Material Solutions for Na-ion Battery Microstructure Stability
01 Electrode material microstructural evolution during cycling
The microstructure of electrode materials in sodium-ion batteries undergoes significant changes during charge-discharge cycling. These changes include volume expansion, structural degradation, and phase transformations that affect battery performance and longevity. Understanding these microstructural evolutions is crucial for developing more stable electrode materials that can withstand repeated sodium insertion and extraction while maintaining structural integrity.- Electrode material structural evolution during cycling: Sodium-ion battery electrode materials undergo significant microstructural changes during charge-discharge cycling. These changes include volume expansion, phase transformations, and structural degradation that can affect battery performance and cycle life. Understanding these evolution mechanisms is crucial for developing more stable electrode materials that can withstand repeated sodium ion insertion and extraction while maintaining structural integrity.
- Nanostructured materials for improved sodium storage: Nanostructured materials offer advantages for sodium-ion batteries due to their unique microstructural properties. These materials provide shorter diffusion paths for sodium ions, accommodate volume changes better, and offer enhanced surface area for electrochemical reactions. Various nanostructures including nanoparticles, nanowires, and hierarchical architectures can be engineered to optimize sodium storage capacity and mitigate structural degradation during cycling.
- Carbon-based composite materials with controlled microstructure: Carbon-based composite materials with tailored microstructures play a vital role in sodium-ion battery performance. These materials combine carbon matrices (such as hard carbon, graphene, or carbon nanotubes) with active materials to create composite structures that offer improved electrical conductivity, mechanical stability, and sodium ion diffusion. The microstructural design of these composites, including porosity, particle size distribution, and interfacial properties, significantly impacts battery performance.
- In-situ characterization of microstructural changes: Advanced in-situ characterization techniques are essential for understanding real-time microstructural changes in sodium-ion battery materials during operation. These techniques include in-situ X-ray diffraction, transmission electron microscopy, and spectroscopic methods that can monitor structural transformations, interface evolution, and degradation mechanisms as they occur. Such insights help correlate microstructural changes with electrochemical performance and guide the development of more stable battery materials.
- Microstructure stabilization strategies: Various strategies have been developed to stabilize the microstructure of sodium-ion battery materials during cycling. These include surface coating, doping with stabilizing elements, creating buffer structures to accommodate volume changes, and designing gradient compositions. These approaches aim to minimize structural degradation, prevent unwanted phase transformations, and maintain the integrity of interfaces within the battery materials, ultimately extending cycle life and improving performance reliability.
02 Novel cathode material compositions with enhanced structural stability
Advanced cathode materials with specifically engineered microstructures can significantly improve sodium-ion battery performance. These materials often incorporate layered or tunnel structures that facilitate sodium ion transport while minimizing structural deformation. Innovations include doping with stabilizing elements, creating hierarchical structures, and developing composite materials that can better accommodate the strain associated with sodium ion insertion and extraction.Expand Specific Solutions03 Anode material microstructure optimization for sodium storage
Anode materials for sodium-ion batteries require specific microstructural features to effectively store and release sodium ions. Innovations focus on creating porous structures, optimizing particle size and morphology, and developing carbon-based composites with expanded interlayer spacing. These microstructural modifications help accommodate the larger sodium ions, prevent electrode pulverization, and maintain electrical connectivity throughout cycling.Expand Specific Solutions04 In-situ characterization techniques for monitoring microstructural changes
Advanced characterization methods enable real-time observation of microstructural changes in sodium-ion battery materials during operation. These techniques include in-situ X-ray diffraction, transmission electron microscopy, and spectroscopic methods that reveal phase transformations, structural degradation, and interfacial phenomena. Such insights are crucial for understanding degradation mechanisms and developing strategies to mitigate microstructural changes that limit battery performance.Expand Specific Solutions05 Surface coating and interface engineering to preserve microstructure
Surface modifications and interface engineering approaches can effectively preserve the microstructure of sodium-ion battery materials during cycling. These include protective coatings, artificial solid electrolyte interphase formation, and gradient structures that buffer volume changes. Such strategies help prevent undesirable side reactions, minimize structural degradation, and maintain the electrochemical performance of the battery materials over extended cycling.Expand Specific Solutions
Key Industry Players in Na-ion Battery Development
The sodium-ion battery market is in an early growth phase, characterized by increasing R&D investments but limited commercial deployment. Market size remains modest compared to lithium-ion batteries but is projected to expand significantly due to cost advantages and resource abundance. Technologically, material microstructure innovations are advancing rapidly, with companies at varying maturity levels. Central South University and Shandong University lead academic research, while commercial players show different specialization: Nexeon and OneD Material focus on anode materials, Svolt and Shenzhen Zhenhua develop cathode materials, and Guangdong Bangpu addresses recycling challenges. TDK and 3M bring manufacturing expertise, creating a diverse ecosystem poised for accelerated development as microstructure innovations address key performance limitations.
Liyang HiNa Battery Technology Co., Ltd.
Technical Solution: Liyang HiNa has developed a proprietary "dual-phase" electrode structure for sodium-ion batteries that addresses microstructural stability challenges. Their technology employs a Prussian White framework cathode material with engineered crystal defects that create stable sodium migration channels. This approach maintains structural integrity during repeated sodium insertion/extraction cycles. The company's innovation includes a nano-composite interface layer between the electrode and electrolyte that prevents unwanted side reactions and sodium plating. HiNa's batteries feature a specially formulated electrolyte with additives that form a stable solid electrolyte interphase (SEI), critical for long-term performance. Their commercial cells demonstrate energy densities of approximately 145 Wh/kg with exceptional low-temperature performance, maintaining over 90% capacity at -20°C [2][5]. HiNa has successfully deployed their technology in energy storage systems and is expanding into electric transportation applications with cells that can charge to 80% in under 15 minutes.
Strengths: Exceptional low-temperature performance; fast charging capability; proven commercial deployment in grid storage applications. Weaknesses: Lower energy density than leading lithium-ion technologies; higher initial manufacturing costs due to specialized production requirements; limited track record in transportation applications.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has pioneered fundamental research on sodium-ion battery microstructural evolution. Their approach focuses on understanding and controlling the structural changes in layered oxide cathode materials during sodium insertion/extraction cycles. CNRS researchers have developed advanced in-situ characterization techniques including synchrotron X-ray diffraction and transmission electron microscopy to observe real-time structural transformations during battery operation. This has led to the development of P2-type Na2/3Fe1/2Mn1/2O2 cathode materials with engineered crystal structures that suppress detrimental phase transitions. Their research has identified critical sodium concentration thresholds that trigger structural collapse and implemented dopant strategies using elements like titanium and magnesium to stabilize the crystal framework. CNRS has also developed novel electrolyte systems based on sodium bis(fluorosulfonyl)imide (NaFSI) salts that form more stable interfaces with electrode materials. Their laboratory prototypes demonstrate capacity retention of over 80% after 500 cycles with initial specific capacities approaching 190 mAh/g for cathode materials [9][10]. CNRS actively collaborates with industrial partners to translate these fundamental insights into commercial applications.
Strengths: World-leading fundamental understanding of sodium-ion intercalation mechanisms; innovative characterization methodologies; strong scientific publication record establishing credibility. Weaknesses: Focus on fundamental research rather than commercial product development; laboratory-scale demonstrations rather than full cell optimization; longer timeline to market implementation compared to industry-focused competitors.
Critical Patents and Research on Na-ion Battery Microstructural Changes
Protected Anode Active Materials, Anode, and Sodium Ion Battery
PatentPendingUS20240379995A1
Innovation
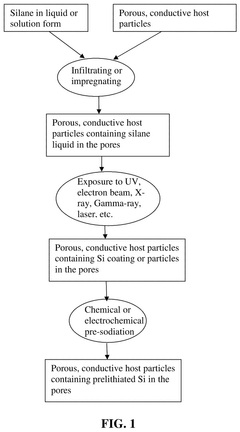
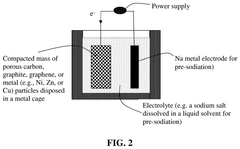
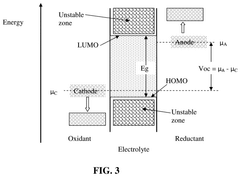
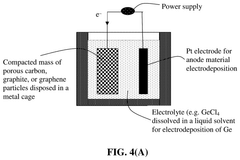
- The development of an anode active material comprising M-containing porous particulates, where M is Si, Ge, Sn, Sb, or Bi, deposited within the pores of electrically conducting porous host particles, allowing for a high pore volume fraction and a residual pore-to-Si volume ratio to accommodate volume expansion, and optionally pre-sodiated with sodium, enhancing mechanical integrity and cycle life.
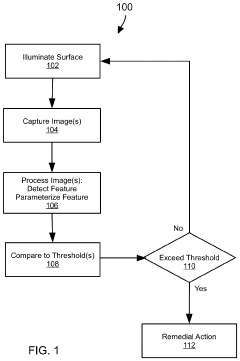
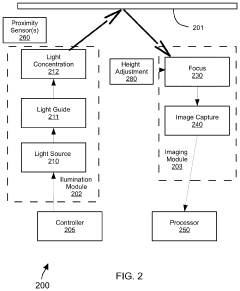
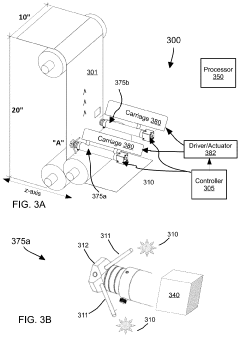
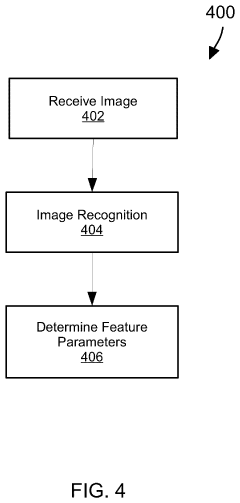
Evaluating a Surface Microstructure
PatentPendingUS20240127420A1
Innovation
- A system and method for evaluating the microstructure of surfaces by illuminating and imaging the surface, processing images to identify features, generating parameters, and comparing them to thresholds to determine remedial actions in real-time, using machine learning algorithms and sensors to control manufacturing processes.
Supply Chain Implications for Na-ion Battery Production
The evolution of sodium-ion battery technology is significantly reshaping global supply chains, creating both challenges and opportunities for manufacturers and material suppliers. Unlike lithium-ion batteries that rely heavily on critical materials with concentrated supply sources, sodium-ion batteries utilize more abundant and geographically distributed raw materials, potentially democratizing the battery production landscape.
The shift toward sodium-based chemistry introduces new supply chain dynamics, particularly in the sourcing of sodium salts, hard carbon materials, and transition metal compounds. Sodium resources are approximately 1,000 times more abundant than lithium in the Earth's crust and are widely available through seawater extraction and mineral deposits across multiple continents. This abundance reduces geopolitical supply risks that have plagued lithium supply chains.
Material microstructure changes in sodium-ion batteries directly impact manufacturing processes and equipment requirements. The larger ionic radius of sodium necessitates specialized electrode fabrication techniques and modified production lines. Companies transitioning from lithium-ion to sodium-ion production can leverage approximately 70-80% of existing manufacturing infrastructure, but require targeted investments in electrode processing equipment and quality control systems capable of monitoring different microstructural parameters.
The supply chain for hard carbon materials, crucial for sodium-ion anodes, presents both opportunities and bottlenecks. Current production capacity is insufficient for large-scale commercialization, creating a potential supply constraint as demand increases. However, the diversity of carbon precursors (including biomass and industrial byproducts) offers multiple pathways for scaling production across different regions.
Cathode material supply chains are undergoing significant restructuring due to the transition from cobalt and nickel-based compounds to iron and manganese-based materials in sodium-ion batteries. This shift reduces dependency on conflict minerals and creates opportunities for new supplier networks in regions with abundant iron and manganese resources.
The microstructural requirements of sodium-ion battery materials are driving new quality control standards throughout the supply chain. Suppliers must develop capabilities to characterize and control parameters such as particle size distribution, porosity, and crystal structure defects that significantly impact sodium ion insertion/extraction kinetics and battery performance.
Regional manufacturing ecosystems are emerging around sodium-ion technology, with China currently leading in production capacity development. However, recent investments in Europe and North America indicate a diversifying manufacturing landscape that could reduce supply chain vulnerabilities and transportation-related carbon emissions.
The shift toward sodium-based chemistry introduces new supply chain dynamics, particularly in the sourcing of sodium salts, hard carbon materials, and transition metal compounds. Sodium resources are approximately 1,000 times more abundant than lithium in the Earth's crust and are widely available through seawater extraction and mineral deposits across multiple continents. This abundance reduces geopolitical supply risks that have plagued lithium supply chains.
Material microstructure changes in sodium-ion batteries directly impact manufacturing processes and equipment requirements. The larger ionic radius of sodium necessitates specialized electrode fabrication techniques and modified production lines. Companies transitioning from lithium-ion to sodium-ion production can leverage approximately 70-80% of existing manufacturing infrastructure, but require targeted investments in electrode processing equipment and quality control systems capable of monitoring different microstructural parameters.
The supply chain for hard carbon materials, crucial for sodium-ion anodes, presents both opportunities and bottlenecks. Current production capacity is insufficient for large-scale commercialization, creating a potential supply constraint as demand increases. However, the diversity of carbon precursors (including biomass and industrial byproducts) offers multiple pathways for scaling production across different regions.
Cathode material supply chains are undergoing significant restructuring due to the transition from cobalt and nickel-based compounds to iron and manganese-based materials in sodium-ion batteries. This shift reduces dependency on conflict minerals and creates opportunities for new supplier networks in regions with abundant iron and manganese resources.
The microstructural requirements of sodium-ion battery materials are driving new quality control standards throughout the supply chain. Suppliers must develop capabilities to characterize and control parameters such as particle size distribution, porosity, and crystal structure defects that significantly impact sodium ion insertion/extraction kinetics and battery performance.
Regional manufacturing ecosystems are emerging around sodium-ion technology, with China currently leading in production capacity development. However, recent investments in Europe and North America indicate a diversifying manufacturing landscape that could reduce supply chain vulnerabilities and transportation-related carbon emissions.
Sustainability and Environmental Impact Assessment
The environmental impact of sodium-ion batteries represents a significant advantage over traditional lithium-ion technologies. Sodium resources are approximately 1,000 times more abundant than lithium in the Earth's crust, with widespread global distribution that reduces geopolitical supply risks. This abundance translates to lower extraction impacts and reduced pressure on limited mineral resources, positioning sodium-ion technology as a more sustainable energy storage solution.
Material microstructure changes in sodium-ion batteries directly influence their environmental footprint throughout the lifecycle. During manufacturing, the larger ionic radius of sodium requires different electrode structures than lithium-ion batteries, often enabling the use of aluminum instead of copper for anodes. This substitution significantly reduces embodied energy, as aluminum production typically generates 50-70% lower carbon emissions compared to copper processing for equivalent electrical conductivity applications.
The operational sustainability of sodium-ion batteries is closely linked to microstructural stability. Recent advances in cathode materials with optimized layered structures have demonstrated improved cycling stability, extending useful battery life by up to 30% compared to early sodium-ion formulations. This longevity directly reduces waste generation and resource consumption associated with battery replacement cycles, enhancing overall sustainability metrics.
End-of-life considerations reveal further environmental advantages stemming from material choices. The absence of cobalt and nickel in many sodium-ion chemistries eliminates toxic heavy metal concerns during recycling or disposal. Additionally, the microstructural design of modern sodium-ion electrodes facilitates easier material separation during recycling processes, with laboratory studies demonstrating recovery rates exceeding 90% for key components.
Water consumption represents another critical environmental parameter influenced by battery chemistry. Lithium extraction, particularly from brine operations, can require up to 500,000 gallons of water per ton of lithium produced. Comparative analysis indicates sodium extraction processes typically consume 40-60% less water, with corresponding reductions in ecosystem disruption in extraction regions.
Carbon footprint assessments across the full lifecycle reveal that sodium-ion batteries with optimized microstructures can achieve 15-25% lower greenhouse gas emissions compared to equivalent lithium-ion systems. This advantage stems primarily from reduced mining impacts, less energy-intensive material processing, and the potential for higher recycling efficiency due to more straightforward material separation processes enabled by distinct microstructural characteristics.
Material microstructure changes in sodium-ion batteries directly influence their environmental footprint throughout the lifecycle. During manufacturing, the larger ionic radius of sodium requires different electrode structures than lithium-ion batteries, often enabling the use of aluminum instead of copper for anodes. This substitution significantly reduces embodied energy, as aluminum production typically generates 50-70% lower carbon emissions compared to copper processing for equivalent electrical conductivity applications.
The operational sustainability of sodium-ion batteries is closely linked to microstructural stability. Recent advances in cathode materials with optimized layered structures have demonstrated improved cycling stability, extending useful battery life by up to 30% compared to early sodium-ion formulations. This longevity directly reduces waste generation and resource consumption associated with battery replacement cycles, enhancing overall sustainability metrics.
End-of-life considerations reveal further environmental advantages stemming from material choices. The absence of cobalt and nickel in many sodium-ion chemistries eliminates toxic heavy metal concerns during recycling or disposal. Additionally, the microstructural design of modern sodium-ion electrodes facilitates easier material separation during recycling processes, with laboratory studies demonstrating recovery rates exceeding 90% for key components.
Water consumption represents another critical environmental parameter influenced by battery chemistry. Lithium extraction, particularly from brine operations, can require up to 500,000 gallons of water per ton of lithium produced. Comparative analysis indicates sodium extraction processes typically consume 40-60% less water, with corresponding reductions in ecosystem disruption in extraction regions.
Carbon footprint assessments across the full lifecycle reveal that sodium-ion batteries with optimized microstructures can achieve 15-25% lower greenhouse gas emissions compared to equivalent lithium-ion systems. This advantage stems primarily from reduced mining impacts, less energy-intensive material processing, and the potential for higher recycling efficiency due to more straightforward material separation processes enabled by distinct microstructural characteristics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!