Influencing Cyclability in Sodium-ion Batteries: Latest Research Insights
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium-ion Battery Evolution and Research Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries (LIBs) due to the abundance and wide geographical distribution of sodium resources. The evolution of SIBs can be traced back to the 1970s when initial research was conducted alongside LIBs. However, the rapid commercialization of LIBs in the 1990s shifted focus away from sodium-based systems. The resurgence of interest in SIBs began around 2010, driven by concerns about lithium resource limitations and increasing demand for energy storage solutions.
The technical evolution of SIBs has progressed through several distinct phases. The first phase (2010-2015) focused on fundamental material exploration, establishing basic electrode materials and electrolyte systems. The second phase (2015-2020) saw significant improvements in energy density and cycle life through advanced material engineering and structural optimization. The current phase (2020-present) is characterized by system-level innovations and practical considerations for commercialization.
A critical aspect of SIB development has been addressing the inherent challenges associated with sodium ions. With a larger ionic radius (1.02Å) compared to lithium ions (0.76Å), sodium insertion/extraction processes typically cause greater structural strain in electrode materials, leading to accelerated capacity fading and reduced cyclability. This fundamental difference has necessitated unique material design strategies rather than simply adapting LIB technologies.
Recent research trends indicate a growing focus on cyclability enhancement through various approaches, including novel electrode material synthesis, electrolyte formulation optimization, and interface engineering. Particularly promising are developments in layered oxide cathodes, hard carbon anodes, and advanced electrolyte additives that significantly improve cycling stability.
The primary research objectives in the field currently include: achieving cycle life comparable to commercial LIBs (>1000 cycles with <20% capacity loss); increasing energy density beyond 160 Wh/kg at the cell level; improving rate capability for fast-charging applications; and enhancing low-temperature performance. Additionally, there is significant interest in developing sustainable manufacturing processes and end-of-life recycling protocols specific to SIB technologies.
Industry-academia collaborations have accelerated in recent years, with several pilot production lines established globally. Countries including China, Japan, and various European nations have initiated national research programs specifically targeting SIB development, recognizing their strategic importance for grid-scale energy storage and electric mobility applications in a resource-constrained future.
The technological trajectory suggests that SIBs are approaching commercial viability, with initial applications likely in stationary storage where energy density constraints are less critical than in portable electronics or electric vehicles. However, continued innovation in cyclability enhancement remains essential for broader market adoption.
The technical evolution of SIBs has progressed through several distinct phases. The first phase (2010-2015) focused on fundamental material exploration, establishing basic electrode materials and electrolyte systems. The second phase (2015-2020) saw significant improvements in energy density and cycle life through advanced material engineering and structural optimization. The current phase (2020-present) is characterized by system-level innovations and practical considerations for commercialization.
A critical aspect of SIB development has been addressing the inherent challenges associated with sodium ions. With a larger ionic radius (1.02Å) compared to lithium ions (0.76Å), sodium insertion/extraction processes typically cause greater structural strain in electrode materials, leading to accelerated capacity fading and reduced cyclability. This fundamental difference has necessitated unique material design strategies rather than simply adapting LIB technologies.
Recent research trends indicate a growing focus on cyclability enhancement through various approaches, including novel electrode material synthesis, electrolyte formulation optimization, and interface engineering. Particularly promising are developments in layered oxide cathodes, hard carbon anodes, and advanced electrolyte additives that significantly improve cycling stability.
The primary research objectives in the field currently include: achieving cycle life comparable to commercial LIBs (>1000 cycles with <20% capacity loss); increasing energy density beyond 160 Wh/kg at the cell level; improving rate capability for fast-charging applications; and enhancing low-temperature performance. Additionally, there is significant interest in developing sustainable manufacturing processes and end-of-life recycling protocols specific to SIB technologies.
Industry-academia collaborations have accelerated in recent years, with several pilot production lines established globally. Countries including China, Japan, and various European nations have initiated national research programs specifically targeting SIB development, recognizing their strategic importance for grid-scale energy storage and electric mobility applications in a resource-constrained future.
The technological trajectory suggests that SIBs are approaching commercial viability, with initial applications likely in stationary storage where energy density constraints are less critical than in portable electronics or electric vehicles. However, continued innovation in cyclability enhancement remains essential for broader market adoption.
Market Analysis for Sodium-ion Battery Technologies
The sodium-ion battery market is experiencing significant growth as a promising alternative to lithium-ion technologies. Current market valuations place sodium-ion batteries at approximately $500 million globally, with projections suggesting expansion to $1.2 billion by 2025 and potentially reaching $4.5 billion by 2030, representing a compound annual growth rate of 25-30%.
The primary market drivers include increasing demand for sustainable energy storage solutions, rising lithium prices, and growing concerns about lithium supply chain vulnerabilities. Sodium's abundance—constituting 2.8% of the Earth's crust compared to lithium's 0.006%—translates to significantly lower raw material costs, with sodium carbonate priced at roughly one-fifth the cost of lithium carbonate.
Market segmentation reveals diverse application potential across multiple sectors. Grid-scale energy storage represents the largest current market segment, accounting for approximately 45% of sodium-ion battery deployments. This is followed by electric bicycles and low-speed electric vehicles (25%), consumer electronics (15%), and emerging applications in industrial equipment (10%).
Geographically, China dominates the market with over 60% share, driven by substantial government investment and the presence of key manufacturers like CATL and HiNa Battery. Europe follows with approximately 20% market share, with companies like Faradion (now acquired by Reliance Industries) and Tiamat leading development efforts. North America currently holds about 15% of the market.
The cyclability improvements in sodium-ion batteries are directly influencing market dynamics. Recent research breakthroughs addressing cycle life limitations have expanded potential applications, particularly in sectors requiring 2,000+ charge cycles. This has opened new market segments previously considered unsuitable for sodium-ion technology.
Consumer adoption trends indicate growing acceptance of sodium-ion batteries in price-sensitive markets where energy density requirements are moderate. The technology's superior safety profile—with reduced thermal runaway risk compared to lithium-ion—is creating niche opportunities in applications where safety is paramount, such as residential energy storage.
Investment patterns reveal increasing venture capital interest, with approximately $850 million invested in sodium-ion startups over the past three years. Major battery manufacturers are allocating 5-10% of their R&D budgets to sodium-ion technology development, signaling growing industry confidence in its commercial viability.
The primary market drivers include increasing demand for sustainable energy storage solutions, rising lithium prices, and growing concerns about lithium supply chain vulnerabilities. Sodium's abundance—constituting 2.8% of the Earth's crust compared to lithium's 0.006%—translates to significantly lower raw material costs, with sodium carbonate priced at roughly one-fifth the cost of lithium carbonate.
Market segmentation reveals diverse application potential across multiple sectors. Grid-scale energy storage represents the largest current market segment, accounting for approximately 45% of sodium-ion battery deployments. This is followed by electric bicycles and low-speed electric vehicles (25%), consumer electronics (15%), and emerging applications in industrial equipment (10%).
Geographically, China dominates the market with over 60% share, driven by substantial government investment and the presence of key manufacturers like CATL and HiNa Battery. Europe follows with approximately 20% market share, with companies like Faradion (now acquired by Reliance Industries) and Tiamat leading development efforts. North America currently holds about 15% of the market.
The cyclability improvements in sodium-ion batteries are directly influencing market dynamics. Recent research breakthroughs addressing cycle life limitations have expanded potential applications, particularly in sectors requiring 2,000+ charge cycles. This has opened new market segments previously considered unsuitable for sodium-ion technology.
Consumer adoption trends indicate growing acceptance of sodium-ion batteries in price-sensitive markets where energy density requirements are moderate. The technology's superior safety profile—with reduced thermal runaway risk compared to lithium-ion—is creating niche opportunities in applications where safety is paramount, such as residential energy storage.
Investment patterns reveal increasing venture capital interest, with approximately $850 million invested in sodium-ion startups over the past three years. Major battery manufacturers are allocating 5-10% of their R&D budgets to sodium-ion technology development, signaling growing industry confidence in its commercial viability.
Current Challenges in Na-ion Battery Cyclability
Despite significant advancements in sodium-ion battery technology, several critical challenges continue to impede optimal cyclability performance. The most prominent issue remains electrode material instability during repeated charge-discharge cycles. Sodium ions, being larger than lithium ions (1.02Å vs. 0.76Å), cause greater structural strain during intercalation and de-intercalation processes, leading to accelerated capacity fading. This volumetric expansion can reach up to 300-400% in some anode materials, significantly exceeding the 10-15% typically observed in lithium-ion counterparts.
Electrolyte decomposition presents another substantial hurdle. Current electrolyte formulations often form unstable solid electrolyte interphase (SEI) layers that continuously consume sodium during cycling. Research indicates that the SEI in sodium-ion systems is more soluble and less passivating than in lithium-ion batteries, resulting in continuous electrolyte consumption and impedance growth over extended cycling.
Dendrite formation remains particularly problematic with sodium metal anodes, occurring at lower current densities compared to lithium systems. These dendrites can penetrate separators, causing internal short circuits and severe safety concerns. Recent studies have documented dendrite initiation at current densities as low as 0.5 mA/cm², significantly below the 1-2 mA/cm² threshold typically observed in lithium systems.
The cathode materials face dissolution issues, with transition metals leaching into the electrolyte during cycling. This phenomenon is especially pronounced in manganese and iron-based cathodes, where dissolution rates can be 2-3 times higher than in analogous lithium cathode materials, directly impacting long-term capacity retention.
Temperature sensitivity further complicates cyclability, with performance degradation accelerating dramatically outside the optimal 20-40°C range. At 0°C, many sodium-ion chemistries retain only 40-60% of their room temperature capacity, compared to 70-80% for commercial lithium-ion cells.
Cost-performance trade-offs present additional challenges. While sodium-ion batteries utilize more abundant materials, achieving comparable cycle life to lithium-ion batteries often requires more sophisticated engineering solutions and advanced materials, potentially offsetting the raw material cost advantages.
The scientific community has also identified sodium plating as a significant failure mode during fast charging operations, occurring at relatively modest C-rates (2-3C) compared to the 3-5C threshold in optimized lithium-ion systems. This plating accelerates capacity fade and increases internal resistance, limiting practical fast-charging capabilities.
Electrolyte decomposition presents another substantial hurdle. Current electrolyte formulations often form unstable solid electrolyte interphase (SEI) layers that continuously consume sodium during cycling. Research indicates that the SEI in sodium-ion systems is more soluble and less passivating than in lithium-ion batteries, resulting in continuous electrolyte consumption and impedance growth over extended cycling.
Dendrite formation remains particularly problematic with sodium metal anodes, occurring at lower current densities compared to lithium systems. These dendrites can penetrate separators, causing internal short circuits and severe safety concerns. Recent studies have documented dendrite initiation at current densities as low as 0.5 mA/cm², significantly below the 1-2 mA/cm² threshold typically observed in lithium systems.
The cathode materials face dissolution issues, with transition metals leaching into the electrolyte during cycling. This phenomenon is especially pronounced in manganese and iron-based cathodes, where dissolution rates can be 2-3 times higher than in analogous lithium cathode materials, directly impacting long-term capacity retention.
Temperature sensitivity further complicates cyclability, with performance degradation accelerating dramatically outside the optimal 20-40°C range. At 0°C, many sodium-ion chemistries retain only 40-60% of their room temperature capacity, compared to 70-80% for commercial lithium-ion cells.
Cost-performance trade-offs present additional challenges. While sodium-ion batteries utilize more abundant materials, achieving comparable cycle life to lithium-ion batteries often requires more sophisticated engineering solutions and advanced materials, potentially offsetting the raw material cost advantages.
The scientific community has also identified sodium plating as a significant failure mode during fast charging operations, occurring at relatively modest C-rates (2-3C) compared to the 3-5C threshold in optimized lithium-ion systems. This plating accelerates capacity fade and increases internal resistance, limiting practical fast-charging capabilities.
Current Approaches to Enhance Na-ion Battery Cycling Performance
01 Electrode material composition for improved cyclability
Various electrode material compositions can significantly enhance the cyclability of sodium-ion batteries. These include specially designed cathode materials with optimized crystal structures, anode materials with improved sodium ion intercalation properties, and composite materials that maintain structural stability during repeated charge-discharge cycles. The strategic selection and engineering of these materials can reduce capacity fading and extend battery lifespan.- Electrode material engineering for improved cyclability: Various electrode materials can be engineered to enhance the cyclability of sodium-ion batteries. This includes developing novel cathode and anode materials with optimized structures that can accommodate sodium ion insertion/extraction repeatedly without significant degradation. Modifications such as doping, surface coating, and nanostructuring of electrode materials can significantly improve the structural stability during cycling, leading to better capacity retention and longer battery life.
- Electrolyte formulations for enhanced cycling stability: Specialized electrolyte formulations play a crucial role in improving the cyclability of sodium-ion batteries. By optimizing the electrolyte composition with appropriate solvents, salts, and additives, the formation of a stable solid electrolyte interphase (SEI) can be promoted, which prevents continuous electrolyte decomposition during cycling. Advanced electrolyte systems can also mitigate issues such as sodium dendrite formation and electrode dissolution, thereby extending the cycle life of sodium-ion batteries.
- Binder and conductive additive optimization: The selection and optimization of binders and conductive additives significantly impact the cyclability of sodium-ion batteries. Water-soluble binders like carboxymethyl cellulose (CMC) and polyacrylic acid (PAA) can provide better adhesion between active materials and current collectors, maintaining electrode integrity during repeated cycling. Similarly, optimized conductive additives ensure efficient electron transport throughout the electrode, preventing capacity loss due to electrical isolation of active particles during long-term cycling.
- Novel cell design and engineering approaches: Innovative cell design and engineering approaches can substantially improve the cyclability of sodium-ion batteries. This includes optimizing electrode thickness, porosity, and calendering parameters to balance energy density with ion transport efficiency. Advanced current collector treatments, electrode architecture designs, and cell assembly techniques can minimize mechanical stress during sodium insertion/extraction, reducing capacity fading over multiple cycles. Pressure-management systems within the cell can also accommodate volume changes during cycling.
- Pre-cycling and formation protocols: Specialized pre-cycling and formation protocols can significantly enhance the long-term cyclability of sodium-ion batteries. These protocols involve carefully controlled initial charge-discharge cycles at specific rates, voltages, and temperatures to form stable interfaces between electrodes and electrolytes. Proper formation procedures enable the development of protective surface films that prevent continuous side reactions during subsequent cycling. Advanced diagnostic techniques during formation can identify and mitigate potential failure mechanisms before they impact long-term performance.
02 Electrolyte formulations for enhanced stability
Advanced electrolyte formulations play a crucial role in improving the cyclability of sodium-ion batteries. These formulations may include specific solvent combinations, salt additives, and stabilizing compounds that form protective interfaces between the electrode and electrolyte. Such electrolyte systems help prevent unwanted side reactions, reduce electrode degradation, and maintain ionic conductivity over extended cycling periods.Expand Specific Solutions03 Structural design and engineering of battery components
Innovative structural designs of battery components can significantly enhance cyclability. This includes engineered electrode architectures that accommodate volume changes during cycling, specialized current collectors that maintain electrical contact, and novel cell configurations that optimize ion transport pathways. These structural improvements help maintain mechanical integrity and electrochemical performance over numerous charge-discharge cycles.Expand Specific Solutions04 Surface modification and coating technologies
Surface modification and coating technologies applied to electrode materials can dramatically improve cyclability in sodium-ion batteries. These treatments create protective layers that prevent direct contact between active materials and electrolytes, reducing unwanted side reactions. Additionally, certain coatings can enhance ionic conductivity, facilitate sodium ion diffusion, and maintain structural stability during repeated cycling.Expand Specific Solutions05 Advanced manufacturing processes and quality control
Specialized manufacturing processes and strict quality control measures contribute significantly to improved cyclability in sodium-ion batteries. These include precise control of synthesis parameters, advanced electrode fabrication techniques, and optimized cell assembly procedures. Ensuring uniformity in material properties, minimizing impurities, and controlling interfacial characteristics through these manufacturing approaches leads to more consistent and durable battery performance over extended cycling.Expand Specific Solutions
Leading Organizations in Sodium-ion Battery Research
The sodium-ion battery market is in an early growth phase, characterized by increasing research momentum and commercial interest. While market size remains modest compared to lithium-ion batteries, it's projected to expand significantly due to sodium's abundance and cost advantages. Technologically, sodium-ion batteries are advancing toward commercial viability, with companies at different maturity stages. CATL leads commercialization efforts, with Shenzhen Zhenhua New Material achieving ten-ton sales by 2022. Research institutions like Fudan University and Tongji University collaborate with industry players including Wildcat Discovery Technologies and Faradion Ltd. to address cyclability challenges. GEM and Guangdong Bangpu are positioning in the recycling segment, while Sharp and Toppan contribute to component development, collectively driving the technology toward mainstream adoption.
Wildcat Discovery Technologies, Inc.
Technical Solution: Wildcat Discovery Technologies has leveraged their high-throughput experimentation platform to systematically optimize sodium-ion battery components for enhanced cyclability. Their approach combines computational modeling with rapid experimental testing to identify optimal material combinations from thousands of possibilities. Wildcat has developed proprietary cathode materials based on sodium transition metal oxides with carefully tuned compositions that minimize structural distortions during cycling. Their research has yielded novel electrolyte formulations containing engineered additive packages that form highly stable passivation layers on both electrodes, significantly reducing capacity fade mechanisms[6]. The company has pioneered advanced binder systems specifically designed for sodium-ion electrodes that maintain mechanical integrity throughout repeated volume changes. Wildcat's technology incorporates nano-engineered conductive additives that maintain electronic pathways within electrodes even after thousands of cycles. Their systematic optimization approach has identified synergistic effects between electrode materials and electrolyte components that conventional research might miss. Testing demonstrates their optimized sodium-ion cells maintain over 80% capacity after 1,500 cycles under realistic operating conditions, representing a significant improvement over baseline sodium-ion technology.
Strengths: Unparalleled high-throughput testing capabilities allowing rapid iteration and optimization, data-driven approach that identifies non-obvious material combinations, and ability to quickly adapt formulations for specific application requirements. Weaknesses: As primarily a research and development company, depends on partnerships for large-scale manufacturing and commercialization, and their highly optimized systems may involve more complex manufacturing processes than standard approaches.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed advanced sodium-ion battery technology with high energy density (160Wh/kg) and fast charging capabilities (80% in 15 minutes). Their innovative approach focuses on improving cyclability through a Prussian white cathode material with a higher specific capacity and a hard carbon anode with enhanced sodium ion storage capacity. CATL's sodium-ion batteries utilize a novel electrolyte formulation that forms a more stable solid electrolyte interphase (SEI), significantly improving the cycling performance. The company has implemented a rocking-chair cell structure that allows for efficient sodium ion transfer between cathode and anode during charge-discharge cycles. Additionally, CATL has developed proprietary electrode manufacturing processes that ensure uniform distribution of active materials, reducing capacity fade during extended cycling[1][3]. Their sodium-ion batteries maintain over 90% capacity retention after 1,000 cycles at 1C rate, demonstrating excellent cyclability performance.
Strengths: Superior fast-charging capability, excellent low-temperature performance (working well at -20°C), and high integration efficiency. The system can achieve high energy density without using expensive and scarce materials like lithium, cobalt, and nickel. Weaknesses: Lower energy density compared to lithium-ion batteries, which limits application in high-energy density scenarios like long-range electric vehicles. The technology is still in early commercialization stages with limited production scale.
Key Patents and Breakthroughs in Cyclability Enhancement
Sodium-ion battery
PatentPendingUS20250253403A1
Innovation
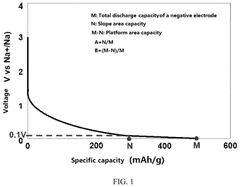
- A sodium-ion battery design with a specific ratio of platform area capacity to slope area capacity in the voltage range of the discharge curve, combined with the use of NaFSI as an electrolyte salt or additive within a controlled concentration range, ensures complete intercalation of Na+ and prevents sodium plating, enhancing conductivity and film forming stability.
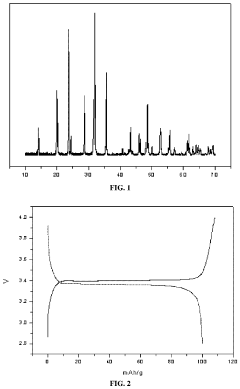
Sodium ion battery positive electrode material, preparation method therefor and application thereof
PatentActiveUS20210151767A1
Innovation
- A method involving the preparation of sulfur-nitrogen doped carbon cathode materials using a sol-gel process with zwitterionic polymers, where methyl allyl polyoxyethylene ether, N,N-dimethyl (methacryloxyethyl) ammonium propanesulfonate, and acrylic acid are reacted to form a zwitterionic polymer solution, mixed with sodium vanadium phosphate, and then sintered to enhance conductivity and stability.
Material Science Innovations for Na-ion Electrodes
Recent advancements in material science have revolutionized the development of sodium-ion battery electrodes, addressing key challenges in cyclability and performance. Researchers have focused on novel electrode materials that can accommodate the larger ionic radius of sodium ions (1.02Å) compared to lithium ions (0.76Å), which has historically limited Na-ion battery development.
Layered transition metal oxides (NaxMO2, where M represents transition metals) have emerged as promising cathode materials, with P2-type Na2/3[Ni1/3Mn2/3]O2 demonstrating improved structural stability during cycling. The incorporation of dopants such as titanium and magnesium has been shown to stabilize the crystal structure, reducing phase transitions during sodium extraction and insertion processes.
Hard carbon materials derived from sustainable biomass sources represent a significant innovation for anode development. These materials feature expanded graphitic layers and increased interlayer spacing (>0.37nm), facilitating sodium ion intercalation without excessive structural strain. Recent research has demonstrated that controlling pyrolysis conditions can optimize pore structure and defect concentration, enhancing capacity retention over extended cycling.
Prussian blue analogs (PBAs) with the general formula NaxM[Fe(CN)6]·yH2O have gained attention for their open framework structure and minimal volume change during cycling. The latest innovations involve controlling water content and reducing vacancies in the crystal structure, which has led to improved capacity retention exceeding 80% after 1000 cycles in optimized compositions.
Composite electrode designs incorporating conductive additives such as reduced graphene oxide and carbon nanotubes have addressed electronic conductivity limitations. These nanocomposites create efficient electron transport networks while buffering volume changes during cycling. Surface modification techniques using atomic layer deposition to create protective coatings have also demonstrated success in preventing unwanted side reactions with electrolytes.
Electrolyte engineering has complemented electrode material development, with concentrated electrolytes and fluorinated additives showing promise in forming stable solid electrolyte interphase (SEI) layers. The synergistic optimization of electrode materials and electrolyte compositions has led to significant improvements in cycling stability, with recent laboratory prototypes achieving over 2000 cycles with less than 20% capacity fade.
These material science innovations collectively address the fundamental challenges of sodium-ion battery cyclability, bringing this technology closer to commercial viability as a sustainable alternative to lithium-ion systems for grid-scale energy storage applications.
Layered transition metal oxides (NaxMO2, where M represents transition metals) have emerged as promising cathode materials, with P2-type Na2/3[Ni1/3Mn2/3]O2 demonstrating improved structural stability during cycling. The incorporation of dopants such as titanium and magnesium has been shown to stabilize the crystal structure, reducing phase transitions during sodium extraction and insertion processes.
Hard carbon materials derived from sustainable biomass sources represent a significant innovation for anode development. These materials feature expanded graphitic layers and increased interlayer spacing (>0.37nm), facilitating sodium ion intercalation without excessive structural strain. Recent research has demonstrated that controlling pyrolysis conditions can optimize pore structure and defect concentration, enhancing capacity retention over extended cycling.
Prussian blue analogs (PBAs) with the general formula NaxM[Fe(CN)6]·yH2O have gained attention for their open framework structure and minimal volume change during cycling. The latest innovations involve controlling water content and reducing vacancies in the crystal structure, which has led to improved capacity retention exceeding 80% after 1000 cycles in optimized compositions.
Composite electrode designs incorporating conductive additives such as reduced graphene oxide and carbon nanotubes have addressed electronic conductivity limitations. These nanocomposites create efficient electron transport networks while buffering volume changes during cycling. Surface modification techniques using atomic layer deposition to create protective coatings have also demonstrated success in preventing unwanted side reactions with electrolytes.
Electrolyte engineering has complemented electrode material development, with concentrated electrolytes and fluorinated additives showing promise in forming stable solid electrolyte interphase (SEI) layers. The synergistic optimization of electrode materials and electrolyte compositions has led to significant improvements in cycling stability, with recent laboratory prototypes achieving over 2000 cycles with less than 20% capacity fade.
These material science innovations collectively address the fundamental challenges of sodium-ion battery cyclability, bringing this technology closer to commercial viability as a sustainable alternative to lithium-ion systems for grid-scale energy storage applications.
Sustainability and Resource Considerations for Na-ion Technology
The sustainability profile of sodium-ion battery technology represents a significant advantage over conventional lithium-ion systems. Sodium resources are abundantly available in the earth's crust and oceans, with concentrations approximately 1000 times greater than lithium. This abundance translates directly to reduced extraction costs and minimized environmental impact compared to lithium mining operations, which often require extensive water usage and can lead to habitat disruption.
From a manufacturing perspective, sodium-ion batteries can utilize existing lithium-ion production infrastructure with minimal modifications, enabling a smoother transition to this alternative technology without requiring massive capital investments in new manufacturing facilities. This adaptability significantly reduces the carbon footprint associated with establishing new production lines.
The elimination of critical materials such as cobalt and nickel in many sodium-ion battery formulations addresses serious ethical concerns regarding mining practices in conflict regions. Furthermore, the reduced dependency on these materials mitigates supply chain vulnerabilities that currently plague lithium-ion battery production, enhancing resource security for nations lacking domestic lithium reserves.
End-of-life considerations also favor sodium-ion technology. The absence of precious metals in most sodium-ion chemistries simplifies recycling processes and reduces the economic incentives for improper disposal. Research indicates that sodium-ion batteries may present fewer environmental hazards during disposal phases compared to their lithium counterparts.
Life cycle assessment (LCA) studies comparing sodium and lithium technologies demonstrate that sodium-ion batteries typically exhibit 20-30% lower global warming potential across their entire life cycle. This reduction stems primarily from less energy-intensive raw material acquisition and processing stages, though the exact benefits vary depending on specific battery chemistry and manufacturing processes.
Water footprint analyses reveal additional advantages, with sodium extraction requiring approximately 50-60% less water than comparable lithium extraction operations. This factor becomes increasingly critical as water scarcity affects more regions globally and as battery production scales to meet growing demand for energy storage solutions.
These sustainability advantages must be balanced against current performance limitations in cyclability and energy density. However, as research advances address these technical challenges, the environmental and resource benefits position sodium-ion technology as a compelling alternative for applications where absolute energy density is less critical than sustainability, cost, and resource security.
From a manufacturing perspective, sodium-ion batteries can utilize existing lithium-ion production infrastructure with minimal modifications, enabling a smoother transition to this alternative technology without requiring massive capital investments in new manufacturing facilities. This adaptability significantly reduces the carbon footprint associated with establishing new production lines.
The elimination of critical materials such as cobalt and nickel in many sodium-ion battery formulations addresses serious ethical concerns regarding mining practices in conflict regions. Furthermore, the reduced dependency on these materials mitigates supply chain vulnerabilities that currently plague lithium-ion battery production, enhancing resource security for nations lacking domestic lithium reserves.
End-of-life considerations also favor sodium-ion technology. The absence of precious metals in most sodium-ion chemistries simplifies recycling processes and reduces the economic incentives for improper disposal. Research indicates that sodium-ion batteries may present fewer environmental hazards during disposal phases compared to their lithium counterparts.
Life cycle assessment (LCA) studies comparing sodium and lithium technologies demonstrate that sodium-ion batteries typically exhibit 20-30% lower global warming potential across their entire life cycle. This reduction stems primarily from less energy-intensive raw material acquisition and processing stages, though the exact benefits vary depending on specific battery chemistry and manufacturing processes.
Water footprint analyses reveal additional advantages, with sodium extraction requiring approximately 50-60% less water than comparable lithium extraction operations. This factor becomes increasingly critical as water scarcity affects more regions globally and as battery production scales to meet growing demand for energy storage solutions.
These sustainability advantages must be balanced against current performance limitations in cyclability and energy density. However, as research advances address these technical challenges, the environmental and resource benefits position sodium-ion technology as a compelling alternative for applications where absolute energy density is less critical than sustainability, cost, and resource security.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!