Electrolyte technologies in advancing solid oxide electrolysis cells
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolyte Development History and Objectives
Solid oxide electrolysis cells (SOECs) have evolved significantly since their conceptual inception in the mid-20th century. The development of electrolyte materials has been central to this evolution, with early research focusing primarily on stabilized zirconia systems. The 1960s marked a pivotal moment when NASA's interest in oxygen generation for space applications accelerated research in this field, leading to the first practical demonstrations of solid oxide-based electrolysis.
The 1980s witnessed a paradigm shift with the introduction of yttria-stabilized zirconia (YSZ) as the standard electrolyte material, offering improved ionic conductivity and thermal stability. This period established the foundation for modern SOEC technology, though operational temperatures remained prohibitively high at 800-1000°C, limiting practical applications and component durability.
The 1990s through early 2000s saw intensive efforts to reduce operating temperatures while maintaining performance, leading to the exploration of alternative electrolyte materials such as gadolinium-doped ceria (GDC) and scandium-stabilized zirconia (ScSZ). These materials demonstrated superior ionic conductivity at intermediate temperatures (600-800°C), expanding the potential application scope of SOECs.
Recent developments have focused on thin-film electrolyte technologies, enabling significant reductions in ohmic resistance and allowing operation at lower temperatures without compromising efficiency. Advanced manufacturing techniques, including atomic layer deposition and pulsed laser deposition, have facilitated the creation of electrolyte layers with thicknesses below 1 μm, representing a remarkable engineering achievement in the field.
The primary objectives of current electrolyte research center on several critical parameters: achieving higher ionic conductivity at temperatures below 600°C; enhancing long-term stability under high-current operation; improving mechanical robustness to withstand thermal cycling; and developing cost-effective manufacturing processes suitable for industrial scale production.
Additionally, researchers aim to develop electrolyte materials with enhanced resistance to chromium poisoning and carbon deposition, two significant degradation mechanisms in SOECs. The ultimate goal is to create electrolyte systems that enable efficient operation at temperatures as low as 500°C while maintaining current densities above 1 A/cm² and demonstrating operational lifetimes exceeding 40,000 hours.
Future objectives include the development of proton-conducting electrolytes for SOECs, which theoretically offer higher efficiency for hydrogen production compared to oxygen-ion conducting systems. Research is also increasingly focused on composite electrolyte structures that combine the advantages of different materials to achieve previously unattainable performance metrics.
The 1980s witnessed a paradigm shift with the introduction of yttria-stabilized zirconia (YSZ) as the standard electrolyte material, offering improved ionic conductivity and thermal stability. This period established the foundation for modern SOEC technology, though operational temperatures remained prohibitively high at 800-1000°C, limiting practical applications and component durability.
The 1990s through early 2000s saw intensive efforts to reduce operating temperatures while maintaining performance, leading to the exploration of alternative electrolyte materials such as gadolinium-doped ceria (GDC) and scandium-stabilized zirconia (ScSZ). These materials demonstrated superior ionic conductivity at intermediate temperatures (600-800°C), expanding the potential application scope of SOECs.
Recent developments have focused on thin-film electrolyte technologies, enabling significant reductions in ohmic resistance and allowing operation at lower temperatures without compromising efficiency. Advanced manufacturing techniques, including atomic layer deposition and pulsed laser deposition, have facilitated the creation of electrolyte layers with thicknesses below 1 μm, representing a remarkable engineering achievement in the field.
The primary objectives of current electrolyte research center on several critical parameters: achieving higher ionic conductivity at temperatures below 600°C; enhancing long-term stability under high-current operation; improving mechanical robustness to withstand thermal cycling; and developing cost-effective manufacturing processes suitable for industrial scale production.
Additionally, researchers aim to develop electrolyte materials with enhanced resistance to chromium poisoning and carbon deposition, two significant degradation mechanisms in SOECs. The ultimate goal is to create electrolyte systems that enable efficient operation at temperatures as low as 500°C while maintaining current densities above 1 A/cm² and demonstrating operational lifetimes exceeding 40,000 hours.
Future objectives include the development of proton-conducting electrolytes for SOECs, which theoretically offer higher efficiency for hydrogen production compared to oxygen-ion conducting systems. Research is also increasingly focused on composite electrolyte structures that combine the advantages of different materials to achieve previously unattainable performance metrics.
Market Analysis for Solid Oxide Electrolysis Applications
The global market for solid oxide electrolysis cell (SOEC) applications is experiencing significant growth, driven by increasing demand for clean hydrogen production and carbon-neutral energy solutions. Current market valuations indicate that the SOEC technology sector is projected to grow at a compound annual growth rate of 15-20% through 2030, with the hydrogen production segment representing the largest market share.
The primary market drivers for SOEC technology include the global push for decarbonization, renewable energy integration, and industrial sector transformation. Government policies supporting hydrogen economies, particularly in Europe, Japan, South Korea, and increasingly in the United States and China, are creating favorable market conditions. The European Union's Hydrogen Strategy, which aims to install at least 40 GW of electrolyzer capacity by 2030, represents a substantial market opportunity for SOEC technology.
Market segmentation reveals diverse application areas beyond pure hydrogen production. Power-to-X applications, where excess renewable electricity is converted to storable chemical energy carriers, represent a rapidly growing segment with projected market expansion of 25-30% annually. Industrial applications for syngas production and carbon utilization are emerging as significant market opportunities, particularly in chemical manufacturing and steel production sectors.
Regional analysis shows Europe leading the SOEC market development, accounting for approximately 45% of current installations, followed by Asia-Pacific at 30% and North America at 20%. China's recent policy shifts toward hydrogen energy have created a rapidly expanding market expected to grow substantially over the next decade.
End-user industries demonstrate varying adoption rates and requirements. The energy storage sector values SOEC's high efficiency and integration capabilities with renewable power sources. Industrial users prioritize scalability and durability for continuous operation. Transportation and mobility applications focus on hydrogen purity and production costs.
Market challenges include high capital costs compared to alternative electrolysis technologies, with SOEC systems currently commanding a premium of 30-40% over alkaline electrolyzers. However, this gap is narrowing as manufacturing scales increase and materials science advances improve electrolyte performance and durability.
Customer demand analysis indicates growing interest in integrated energy systems where SOEC technology serves multiple functions, including electricity storage, heat recovery, and carbon utilization. This trend toward multi-functional systems is expected to expand the addressable market for SOEC technology by 35-40% over the next five years as energy system integration becomes a priority for industrial and utility customers.
The primary market drivers for SOEC technology include the global push for decarbonization, renewable energy integration, and industrial sector transformation. Government policies supporting hydrogen economies, particularly in Europe, Japan, South Korea, and increasingly in the United States and China, are creating favorable market conditions. The European Union's Hydrogen Strategy, which aims to install at least 40 GW of electrolyzer capacity by 2030, represents a substantial market opportunity for SOEC technology.
Market segmentation reveals diverse application areas beyond pure hydrogen production. Power-to-X applications, where excess renewable electricity is converted to storable chemical energy carriers, represent a rapidly growing segment with projected market expansion of 25-30% annually. Industrial applications for syngas production and carbon utilization are emerging as significant market opportunities, particularly in chemical manufacturing and steel production sectors.
Regional analysis shows Europe leading the SOEC market development, accounting for approximately 45% of current installations, followed by Asia-Pacific at 30% and North America at 20%. China's recent policy shifts toward hydrogen energy have created a rapidly expanding market expected to grow substantially over the next decade.
End-user industries demonstrate varying adoption rates and requirements. The energy storage sector values SOEC's high efficiency and integration capabilities with renewable power sources. Industrial users prioritize scalability and durability for continuous operation. Transportation and mobility applications focus on hydrogen purity and production costs.
Market challenges include high capital costs compared to alternative electrolysis technologies, with SOEC systems currently commanding a premium of 30-40% over alkaline electrolyzers. However, this gap is narrowing as manufacturing scales increase and materials science advances improve electrolyte performance and durability.
Customer demand analysis indicates growing interest in integrated energy systems where SOEC technology serves multiple functions, including electricity storage, heat recovery, and carbon utilization. This trend toward multi-functional systems is expected to expand the addressable market for SOEC technology by 35-40% over the next five years as energy system integration becomes a priority for industrial and utility customers.
Current Electrolyte Technologies and Barriers
Solid oxide electrolysis cells (SOECs) rely heavily on electrolyte materials that can efficiently conduct ions at high operating temperatures. Currently, the most widely used electrolyte material is yttria-stabilized zirconia (YSZ), which offers good ionic conductivity at temperatures above 800°C. YSZ has been extensively studied and optimized, with various compositions containing 8-10 mol% Y2O3 showing the best performance balance between conductivity and stability.
Scandium-stabilized zirconia (ScSZ) represents another significant advancement, demonstrating superior ionic conductivity compared to YSZ at equivalent temperatures. However, its widespread adoption remains limited due to the scarcity and high cost of scandium, making it economically viable only for specialized applications where performance requirements justify the increased expense.
Gadolinium-doped ceria (GDC) and samarium-doped ceria (SDC) have emerged as promising intermediate-temperature electrolytes, operating effectively between 500-700°C. These ceria-based materials offer higher ionic conductivity at lower temperatures compared to zirconia-based alternatives. However, they suffer from electronic conductivity under reducing conditions, which can lead to internal short-circuiting and decreased efficiency.
Lanthanum gallate-based electrolytes, particularly lanthanum strontium gallium magnesium oxide (LSGM), demonstrate excellent ionic conductivity at intermediate temperatures. Despite these advantages, LSGM faces challenges including difficult synthesis procedures, mechanical fragility, and potential reactivity with electrode materials during long-term operation.
Several critical barriers impede further advancement of electrolyte technologies in SOECs. The mechanical integrity of thin electrolytes remains problematic, as reducing thickness to minimize ohmic resistance often compromises structural stability. This trade-off between conductivity and mechanical strength presents a significant engineering challenge.
Thermal cycling durability represents another major obstacle, as the repeated heating and cooling cycles in practical applications induce thermal stresses that can lead to microcracking and eventual failure of the electrolyte layer. This is particularly problematic for large-scale industrial implementations where system reliability is paramount.
Chemical compatibility between electrolytes and electrode materials continues to challenge researchers, with interdiffusion and unwanted phase formation at interfaces degrading performance over time. Additionally, manufacturing scalability remains limited, with techniques capable of producing high-quality, defect-free thin electrolytes at industrial scales still under development.
Addressing these barriers requires interdisciplinary approaches combining materials science, electrochemistry, and manufacturing engineering to develop next-generation electrolytes that can enable more efficient, durable, and economically viable solid oxide electrolysis cells.
Scandium-stabilized zirconia (ScSZ) represents another significant advancement, demonstrating superior ionic conductivity compared to YSZ at equivalent temperatures. However, its widespread adoption remains limited due to the scarcity and high cost of scandium, making it economically viable only for specialized applications where performance requirements justify the increased expense.
Gadolinium-doped ceria (GDC) and samarium-doped ceria (SDC) have emerged as promising intermediate-temperature electrolytes, operating effectively between 500-700°C. These ceria-based materials offer higher ionic conductivity at lower temperatures compared to zirconia-based alternatives. However, they suffer from electronic conductivity under reducing conditions, which can lead to internal short-circuiting and decreased efficiency.
Lanthanum gallate-based electrolytes, particularly lanthanum strontium gallium magnesium oxide (LSGM), demonstrate excellent ionic conductivity at intermediate temperatures. Despite these advantages, LSGM faces challenges including difficult synthesis procedures, mechanical fragility, and potential reactivity with electrode materials during long-term operation.
Several critical barriers impede further advancement of electrolyte technologies in SOECs. The mechanical integrity of thin electrolytes remains problematic, as reducing thickness to minimize ohmic resistance often compromises structural stability. This trade-off between conductivity and mechanical strength presents a significant engineering challenge.
Thermal cycling durability represents another major obstacle, as the repeated heating and cooling cycles in practical applications induce thermal stresses that can lead to microcracking and eventual failure of the electrolyte layer. This is particularly problematic for large-scale industrial implementations where system reliability is paramount.
Chemical compatibility between electrolytes and electrode materials continues to challenge researchers, with interdiffusion and unwanted phase formation at interfaces degrading performance over time. Additionally, manufacturing scalability remains limited, with techniques capable of producing high-quality, defect-free thin electrolytes at industrial scales still under development.
Addressing these barriers requires interdisciplinary approaches combining materials science, electrochemistry, and manufacturing engineering to develop next-generation electrolytes that can enable more efficient, durable, and economically viable solid oxide electrolysis cells.
State-of-the-Art Electrolyte Solutions for SOECs
01 Ceramic-based electrolyte materials
Ceramic-based materials are widely used as electrolytes in solid oxide electrolysis cells due to their high ionic conductivity at elevated temperatures. These materials include yttria-stabilized zirconia (YSZ), scandia-stabilized zirconia (ScSZ), and gadolinium-doped ceria (GDC). The doping of these ceramic materials enhances their oxygen ion conductivity and stability under operating conditions, making them suitable for high-temperature electrolysis applications.- Ceramic-based electrolyte materials: Ceramic-based materials are widely used as electrolytes in solid oxide electrolysis cells due to their high ionic conductivity at elevated temperatures. These materials include yttria-stabilized zirconia (YSZ), scandia-stabilized zirconia (ScSZ), and gadolinium-doped ceria (GDC). The doping of these ceramic materials enhances their oxygen ion conductivity and stability under operating conditions, making them suitable for efficient electrolysis operations.
- Thin-film electrolyte fabrication techniques: Advanced thin-film fabrication techniques are employed to create electrolytes with reduced thickness, which decreases the ohmic resistance and improves overall cell efficiency. These techniques include physical vapor deposition, chemical vapor deposition, and various printing methods. Thin-film electrolytes allow for lower operating temperatures while maintaining adequate ionic conductivity, which extends the cell lifetime and reduces material degradation.
- Composite and multi-layer electrolyte structures: Composite and multi-layer electrolyte structures combine different materials to leverage their complementary properties. These structures typically consist of a dense electrolyte layer sandwiched between functional layers that enhance performance at the electrode interfaces. By using multiple materials, these electrolytes can achieve both high ionic conductivity and improved mechanical stability, while also mitigating issues related to thermal expansion mismatches between components.
- Proton-conducting electrolytes: Proton-conducting electrolytes offer an alternative to traditional oxygen-ion conducting materials for solid oxide electrolysis cells. These materials, including doped barium cerates and zirconates, allow for proton transport at intermediate temperatures (400-700°C). The lower operating temperature reduces thermal stress and degradation while potentially improving energy efficiency. Proton-conducting electrolytes are particularly advantageous for hydrogen production applications.
- Electrolyte interface engineering: Engineering the interfaces between the electrolyte and electrodes is crucial for optimizing cell performance. This involves modifying the surface chemistry, microstructure, and composition of the electrolyte at the interface regions. Techniques such as infiltration of catalytic materials, gradient compositions, and nanoscale structuring are employed to reduce interfacial resistance and enhance electrochemical activity. Proper interface engineering mitigates degradation mechanisms such as delamination and chemical reactions between cell components.
02 Thin-film electrolyte fabrication techniques
Advanced fabrication techniques for thin-film electrolytes can significantly improve the performance of solid oxide electrolysis cells. Methods such as physical vapor deposition, chemical vapor deposition, and tape casting allow for the creation of thin, dense electrolyte layers that reduce ohmic resistance while maintaining gas-tightness. These techniques enable the production of electrolytes with thicknesses in the micrometer range, enhancing overall cell efficiency.Expand Specific Solutions03 Composite and multi-layer electrolyte structures
Composite and multi-layer electrolyte structures combine different materials to optimize performance characteristics. These structures typically consist of layers with complementary properties, such as a high ionic conductivity layer sandwiched between more chemically stable layers. This approach helps overcome limitations of single-material electrolytes, providing improved durability, reduced operating temperatures, and enhanced ionic conductivity across a wider temperature range.Expand Specific Solutions04 Proton-conducting electrolytes
Proton-conducting electrolytes offer an alternative to traditional oxygen-ion conducting materials for solid oxide electrolysis cells. These materials, including doped barium cerates and zirconates, allow for proton transport at intermediate temperatures (400-700°C), potentially reducing operating temperatures compared to conventional systems. Proton-conducting electrolytes can improve efficiency in hydrogen production applications and may offer advantages in terms of selectivity and energy consumption.Expand Specific Solutions05 Electrolyte interface engineering
Engineering the interfaces between the electrolyte and electrodes is crucial for optimizing solid oxide electrolysis cell performance. Techniques include surface modification, introduction of buffer layers, and controlled elemental diffusion to reduce interfacial resistance. These approaches minimize degradation mechanisms such as delamination and chemical reactions at interfaces, leading to improved long-term stability and reduced polarization losses during operation.Expand Specific Solutions
Leading Companies and Research Institutions in SOEC Electrolytes
The solid oxide electrolysis cell (SOEC) market is in its growth phase, characterized by increasing research intensity and commercial development. The global market is projected to expand significantly as hydrogen economy initiatives gain momentum worldwide. Technologically, electrolyte development remains a critical focus area with varying maturity levels across different approaches. Leading academic institutions (Tsinghua University, KAIST, Technical University of Denmark) are driving fundamental research, while industrial players demonstrate different specialization levels. Energy corporations (Sinopec, Saudi Aramco, Hyundai) are investing in large-scale applications, while materials specialists (CoorsTek, BASF, Kyocera) focus on component optimization. Research institutes (Dalian Institute of Chemical Physics, KIST) bridge fundamental science and commercial applications, creating a competitive landscape where collaboration between academia and industry accelerates technology commercialization.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced composite electrolytes for solid oxide electrolysis cells (SOECs) that combine yttria-stabilized zirconia (YSZ) with gadolinium-doped ceria (GDC) to enhance ionic conductivity while maintaining mechanical stability. Their innovative approach involves creating a functionally graded electrolyte structure where the composition gradually transitions from YSZ to GDC, effectively minimizing thermal expansion coefficient mismatches and reducing interfacial resistance. DICP researchers have also pioneered the incorporation of proton-conducting materials like BaZr0.8Y0.2O3-δ (BZY) into traditional oxygen-ion conducting electrolytes, creating dual-ion conducting systems that operate efficiently at intermediate temperatures (600-750°C). Their electrolyte fabrication techniques include advanced methods such as tape casting and plasma spraying to achieve ultra-thin electrolyte layers (5-15 μm), significantly reducing ohmic resistance while maintaining gas-tightness.
Strengths: Their composite electrolyte designs achieve superior ionic conductivity (0.1-0.2 S/cm at 750°C) while maintaining excellent mechanical durability. The functionally graded approach effectively addresses thermal expansion mismatches that typically lead to delamination in conventional designs. Weaknesses: The complex fabrication processes for their advanced electrolytes may present challenges for large-scale manufacturing and cost-effective production.
Technical University of Denmark
Technical Solution: The Technical University of Denmark (DTU) has pioneered innovative electrolyte technologies for solid oxide electrolysis cells through their Department of Energy Conversion and Storage. Their research focuses on metal-supported SOECs with advanced thin-film electrolytes that enable operation at intermediate temperatures (600-750°C). DTU has developed a proprietary co-sintering process for creating scandia and ceria co-doped zirconia electrolytes (ScCeSZ) with thickness below 10 μm while maintaining excellent gas-tightness. Their electrolyte formulations incorporate carefully controlled grain boundary engineering to enhance ionic conductivity while suppressing electronic conductivity, crucial for high-efficiency electrolysis operation. DTU researchers have also developed infiltration techniques to introduce nanostructured catalytic materials at the electrolyte-electrode interfaces, significantly reducing polarization resistance without compromising electrolyte integrity. Their recent breakthrough involves the development of proton-conducting ceramic electrolytes based on Y-doped BaZrO3 that enable efficient operation at temperatures as low as 500°C, representing a significant advancement for SOEC technology. DTU's electrolyte technologies have demonstrated exceptional durability in long-term testing, maintaining stable performance for over 5,000 hours of continuous operation.
Strengths: DTU's electrolyte technologies enable operation at lower temperatures than conventional SOECs, reducing thermal stress and extending operational lifetime while maintaining high efficiency. Their metal-supported cell architecture with thin-film electrolytes offers excellent mechanical robustness and thermal cycling capability. Weaknesses: The complex manufacturing processes required for their advanced electrolyte structures may present challenges for cost-effective scaling to industrial production volumes.
Critical Patents and Research on Advanced Electrolyte Materials
Enhanced proton conduction and steam tolerance of a donor doped electrolyte for solid oxide electrolysis cells
PatentWO2022245710A3
Innovation
- Development of donor doped electrolytes with enhanced proton conduction for solid oxide electrolysis cells (SOECs), improving overall hydrogen production efficiency.
- Creation of electrolyte materials with improved steam tolerance, addressing a critical challenge in SOEC operation where high steam concentrations are present.
- Design of SOEC electrolytes with dual benefits of enhanced conductivity and stability, enabling higher performance in electrolysis cells, fuel cells, and reversible cell applications.
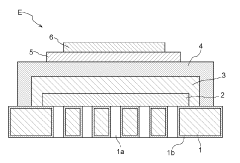
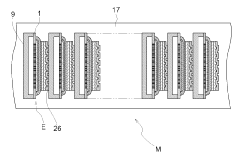
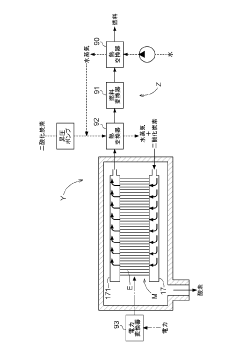
Solid oxide type electrolysis cell, manufacturing method for solid oxide type electrolysis cell, solid oxide type electrolytic module, electrochemical device and energy system
PatentPendingJP2023144948A
Innovation
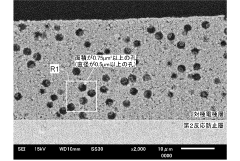
- A solid oxide electrolytic cell design with specific pore configurations in the electrode layers, including micron- and nano-order pores, and a reaction prevention layer to manage oxygen release and prevent peeling, supported by a metal substrate to maintain structural integrity.
Sustainability Impact of Advanced Electrolyte Technologies
Advanced electrolyte technologies in solid oxide electrolysis cells (SOECs) are making significant contributions to global sustainability goals through multiple pathways. The environmental impact of these technologies extends far beyond their immediate application in hydrogen production, creating cascading benefits across energy systems and industrial processes.
The primary sustainability advantage comes from enabling efficient renewable energy storage through power-to-gas conversion. By utilizing excess renewable electricity during peak production periods, SOECs with advanced electrolytes can convert this otherwise curtailed energy into storable hydrogen or syngas, effectively increasing the utilization rate of intermittent renewable sources like solar and wind. This capability is critical for grid stabilization and reducing the need for fossil fuel backup generation.
Carbon footprint reduction represents another crucial sustainability dimension. When powered by renewable electricity, SOECs with high-performance electrolytes can produce hydrogen with near-zero emissions, compared to conventional steam methane reforming which generates 9-12 kg CO₂ per kg H₂. Furthermore, these systems can directly utilize CO₂ as a feedstock in co-electrolysis mode, converting industrial emissions into valuable chemical precursors and effectively creating carbon-neutral or even carbon-negative production pathways.
Resource efficiency improvements are equally significant. Advanced electrolyte materials that operate at intermediate temperatures (600-800°C) rather than traditional high temperatures (>850°C) reduce thermal stress on system components, extending operational lifetimes and decreasing material replacement frequency. This translates to lower lifecycle resource consumption and waste generation. Additionally, newer electrolyte compositions often reduce or eliminate dependency on critical raw materials like rare earth elements, improving supply chain sustainability.
The economic sustainability aspects cannot be overlooked. As electrolyte technologies advance, manufacturing costs decrease while performance improves, driving down the levelized cost of hydrogen production. Current projections indicate that SOEC-produced green hydrogen could reach cost parity with fossil-derived hydrogen by 2030 in regions with low-cost renewable electricity, creating economically sustainable pathways for industrial decarbonization.
From a circular economy perspective, advanced electrolyte technologies are increasingly designed with end-of-life considerations. Research into recyclable ceramic compositions and recovery processes for valuable elements from spent electrolytes is progressing, though significant challenges remain in scaling these approaches commercially. The development of standardized recycling protocols for these specialized materials represents an important frontier for maximizing their full sustainability potential.
The primary sustainability advantage comes from enabling efficient renewable energy storage through power-to-gas conversion. By utilizing excess renewable electricity during peak production periods, SOECs with advanced electrolytes can convert this otherwise curtailed energy into storable hydrogen or syngas, effectively increasing the utilization rate of intermittent renewable sources like solar and wind. This capability is critical for grid stabilization and reducing the need for fossil fuel backup generation.
Carbon footprint reduction represents another crucial sustainability dimension. When powered by renewable electricity, SOECs with high-performance electrolytes can produce hydrogen with near-zero emissions, compared to conventional steam methane reforming which generates 9-12 kg CO₂ per kg H₂. Furthermore, these systems can directly utilize CO₂ as a feedstock in co-electrolysis mode, converting industrial emissions into valuable chemical precursors and effectively creating carbon-neutral or even carbon-negative production pathways.
Resource efficiency improvements are equally significant. Advanced electrolyte materials that operate at intermediate temperatures (600-800°C) rather than traditional high temperatures (>850°C) reduce thermal stress on system components, extending operational lifetimes and decreasing material replacement frequency. This translates to lower lifecycle resource consumption and waste generation. Additionally, newer electrolyte compositions often reduce or eliminate dependency on critical raw materials like rare earth elements, improving supply chain sustainability.
The economic sustainability aspects cannot be overlooked. As electrolyte technologies advance, manufacturing costs decrease while performance improves, driving down the levelized cost of hydrogen production. Current projections indicate that SOEC-produced green hydrogen could reach cost parity with fossil-derived hydrogen by 2030 in regions with low-cost renewable electricity, creating economically sustainable pathways for industrial decarbonization.
From a circular economy perspective, advanced electrolyte technologies are increasingly designed with end-of-life considerations. Research into recyclable ceramic compositions and recovery processes for valuable elements from spent electrolytes is progressing, though significant challenges remain in scaling these approaches commercially. The development of standardized recycling protocols for these specialized materials represents an important frontier for maximizing their full sustainability potential.
Manufacturing Scalability of Novel Electrolyte Materials
The manufacturing scalability of novel electrolyte materials represents a critical challenge in the commercial deployment of solid oxide electrolysis cells (SOECs). Current production methods for advanced electrolytes such as yttria-stabilized zirconia (YSZ), gadolinium-doped ceria (GDC), and emerging proton-conducting ceramics face significant barriers when transitioning from laboratory to industrial scale.
Traditional ceramic processing techniques including tape casting, screen printing, and spray coating have demonstrated reliability for conventional electrolyte materials but encounter limitations when applied to novel compositions with complex stoichiometry or nanostructured features. These limitations manifest as inconsistent material properties, thickness variations, and defect formation during scale-up processes.
Recent advancements in manufacturing technologies show promising pathways toward industrial-scale production. Atomic layer deposition (ALD) enables precise control over electrolyte thickness and composition at the nanoscale, though throughput remains a challenge for large-area applications. Pulsed laser deposition (PLD) offers excellent control over stoichiometry but faces similar throughput limitations and high equipment costs.
Solution-based processing routes, including sol-gel methods and colloidal processing, present more economically viable approaches for large-scale manufacturing. These methods allow for better control of precursor chemistry and can be adapted to continuous production processes. However, they require careful optimization of rheological properties and drying conditions to prevent cracking and delamination during scale-up.
The integration of additive manufacturing techniques represents a paradigm shift in electrolyte fabrication. Direct ink writing and robocasting enable complex geometries and compositional gradients previously unattainable through conventional methods. These approaches reduce material waste and processing steps but require specialized formulations with precise viscoelastic properties.
Quality control and characterization present additional challenges at industrial scales. In-line monitoring techniques such as optical coherence tomography and impedance spectroscopy are being developed to provide real-time feedback during manufacturing, enabling adaptive process control to maintain consistent electrolyte properties.
Cost considerations remain paramount for commercial viability. Current estimates suggest that novel electrolyte materials can represent 15-30% of total SOEC stack costs. Economies of scale and process optimization could potentially reduce these costs by 40-60% over the next decade, particularly through the development of continuous manufacturing processes and precursor recycling systems.
Environmental and safety considerations must also be addressed as production scales increase. Many advanced electrolyte materials contain rare earth elements with supply chain vulnerabilities, driving research toward more abundant alternatives or improved material utilization efficiency in manufacturing processes.
Traditional ceramic processing techniques including tape casting, screen printing, and spray coating have demonstrated reliability for conventional electrolyte materials but encounter limitations when applied to novel compositions with complex stoichiometry or nanostructured features. These limitations manifest as inconsistent material properties, thickness variations, and defect formation during scale-up processes.
Recent advancements in manufacturing technologies show promising pathways toward industrial-scale production. Atomic layer deposition (ALD) enables precise control over electrolyte thickness and composition at the nanoscale, though throughput remains a challenge for large-area applications. Pulsed laser deposition (PLD) offers excellent control over stoichiometry but faces similar throughput limitations and high equipment costs.
Solution-based processing routes, including sol-gel methods and colloidal processing, present more economically viable approaches for large-scale manufacturing. These methods allow for better control of precursor chemistry and can be adapted to continuous production processes. However, they require careful optimization of rheological properties and drying conditions to prevent cracking and delamination during scale-up.
The integration of additive manufacturing techniques represents a paradigm shift in electrolyte fabrication. Direct ink writing and robocasting enable complex geometries and compositional gradients previously unattainable through conventional methods. These approaches reduce material waste and processing steps but require specialized formulations with precise viscoelastic properties.
Quality control and characterization present additional challenges at industrial scales. In-line monitoring techniques such as optical coherence tomography and impedance spectroscopy are being developed to provide real-time feedback during manufacturing, enabling adaptive process control to maintain consistent electrolyte properties.
Cost considerations remain paramount for commercial viability. Current estimates suggest that novel electrolyte materials can represent 15-30% of total SOEC stack costs. Economies of scale and process optimization could potentially reduce these costs by 40-60% over the next decade, particularly through the development of continuous manufacturing processes and precursor recycling systems.
Environmental and safety considerations must also be addressed as production scales increase. Many advanced electrolyte materials contain rare earth elements with supply chain vulnerabilities, driving research toward more abundant alternatives or improved material utilization efficiency in manufacturing processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!