Solid oxide electrolysis cells and emerging energy landscape analysis
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SOEC Technology Background and Objectives
Solid oxide electrolysis cells (SOECs) represent a transformative technology in the evolving energy landscape, with roots dating back to the 1980s when researchers first explored high-temperature electrolysis for hydrogen production. The technology has evolved significantly over the past four decades, transitioning from laboratory curiosity to commercial viability, particularly as global energy systems pivot toward decarbonization and renewable integration.
The fundamental principle of SOECs involves the electrochemical conversion of steam and/or carbon dioxide into hydrogen and/or carbon monoxide at elevated temperatures (700-900°C), effectively storing electrical energy in chemical bonds. This reverse operation of solid oxide fuel cells offers remarkable theoretical efficiency advantages over low-temperature electrolysis technologies, with potential electrical-to-chemical energy conversion efficiencies exceeding 85%.
Recent technological advancements have accelerated SOEC development, particularly in materials science, where more durable electrodes and electrolytes have extended operational lifetimes from hundreds to thousands of hours. Concurrently, manufacturing innovations have reduced production costs by approximately 60% over the past decade, bringing commercial viability within reach for specific applications.
The primary technical objective for SOEC development centers on achieving economic competitiveness with conventional hydrogen production methods while offering superior environmental performance. This requires simultaneous progress in durability, efficiency, and cost reduction. Current targets include achieving degradation rates below 0.5% per 1000 hours, capital cost reduction to under $500/kW, and system lifetimes exceeding 40,000 hours for industrial deployment.
Within the broader energy landscape, SOECs aim to serve as a critical link between renewable electricity generation and sectors difficult to electrify directly. The technology's ability to produce hydrogen and synthesis gas positions it as an enabler for sector coupling—connecting power generation with industrial processes, transportation fuels, and chemical manufacturing through power-to-X pathways.
The technology evolution trajectory suggests SOECs will progress from current demonstration projects (1-5 MW scale) to commercial deployment (10-100 MW) by 2025-2030, with gigawatt-scale implementation possible by 2035-2040. This progression aligns with projected renewable energy expansion and hydrogen economy development, particularly in regions with ambitious decarbonization targets like the European Union, Japan, and increasingly, China and the United States.
The fundamental principle of SOECs involves the electrochemical conversion of steam and/or carbon dioxide into hydrogen and/or carbon monoxide at elevated temperatures (700-900°C), effectively storing electrical energy in chemical bonds. This reverse operation of solid oxide fuel cells offers remarkable theoretical efficiency advantages over low-temperature electrolysis technologies, with potential electrical-to-chemical energy conversion efficiencies exceeding 85%.
Recent technological advancements have accelerated SOEC development, particularly in materials science, where more durable electrodes and electrolytes have extended operational lifetimes from hundreds to thousands of hours. Concurrently, manufacturing innovations have reduced production costs by approximately 60% over the past decade, bringing commercial viability within reach for specific applications.
The primary technical objective for SOEC development centers on achieving economic competitiveness with conventional hydrogen production methods while offering superior environmental performance. This requires simultaneous progress in durability, efficiency, and cost reduction. Current targets include achieving degradation rates below 0.5% per 1000 hours, capital cost reduction to under $500/kW, and system lifetimes exceeding 40,000 hours for industrial deployment.
Within the broader energy landscape, SOECs aim to serve as a critical link between renewable electricity generation and sectors difficult to electrify directly. The technology's ability to produce hydrogen and synthesis gas positions it as an enabler for sector coupling—connecting power generation with industrial processes, transportation fuels, and chemical manufacturing through power-to-X pathways.
The technology evolution trajectory suggests SOECs will progress from current demonstration projects (1-5 MW scale) to commercial deployment (10-100 MW) by 2025-2030, with gigawatt-scale implementation possible by 2035-2040. This progression aligns with projected renewable energy expansion and hydrogen economy development, particularly in regions with ambitious decarbonization targets like the European Union, Japan, and increasingly, China and the United States.
Market Demand for Green Hydrogen Production
The global market for green hydrogen production is experiencing unprecedented growth, driven by the urgent need to decarbonize energy-intensive industries and meet ambitious climate targets. Current estimates value the green hydrogen market at approximately $2.5 billion in 2022, with projections indicating a compound annual growth rate of 39.5% through 2030. This remarkable expansion is primarily fueled by increasing governmental commitments to net-zero emissions and substantial investments in renewable energy infrastructure.
Solid oxide electrolysis cells (SOECs) are emerging as a critical technology in this landscape, offering higher efficiency compared to conventional alkaline and PEM electrolyzers. The market specifically for SOEC technology is expected to grow from $320 million in 2023 to potentially reach $1.8 billion by 2030, representing a significant segment of the broader hydrogen production equipment market.
Industrial sectors constitute the largest demand drivers for green hydrogen, with chemical manufacturing, particularly ammonia and methanol production, leading consumption patterns. These industries alone account for approximately 45% of current hydrogen usage. Steel manufacturing follows closely, with major producers worldwide implementing hydrogen-based direct reduction processes to replace traditional coal-dependent methods.
Transportation represents another rapidly expanding market segment, particularly in heavy-duty vehicles, shipping, and aviation where battery electrification faces significant limitations. Several major automotive manufacturers have already launched hydrogen fuel cell vehicles, while shipping companies are developing hydrogen-powered vessels to meet IMO emissions regulations.
Energy storage applications are gaining traction as power grids incorporate higher percentages of intermittent renewable sources. The ability to convert surplus renewable electricity into hydrogen via SOECs creates valuable seasonal storage capabilities that batteries cannot match, addressing the "dark doldrums" challenge of simultaneous low solar and wind generation.
Geographically, Europe leads in green hydrogen demand, supported by the EU Hydrogen Strategy targeting 40GW of electrolyzer capacity by 2030. Asia-Pacific follows closely, with Japan's Basic Hydrogen Strategy and South Korea's Hydrogen Economy Roadmap establishing ambitious adoption targets. North America is accelerating investments following recent legislative support, particularly the Inflation Reduction Act's production tax credits for clean hydrogen.
Market analysis indicates that cost remains the primary barrier to widespread adoption, with green hydrogen production currently 2-3 times more expensive than conventional methods. However, declining renewable electricity costs coupled with technological advancements in SOEC efficiency and durability are expected to achieve cost parity in several markets by 2028-2030, potentially triggering exponential demand growth.
Solid oxide electrolysis cells (SOECs) are emerging as a critical technology in this landscape, offering higher efficiency compared to conventional alkaline and PEM electrolyzers. The market specifically for SOEC technology is expected to grow from $320 million in 2023 to potentially reach $1.8 billion by 2030, representing a significant segment of the broader hydrogen production equipment market.
Industrial sectors constitute the largest demand drivers for green hydrogen, with chemical manufacturing, particularly ammonia and methanol production, leading consumption patterns. These industries alone account for approximately 45% of current hydrogen usage. Steel manufacturing follows closely, with major producers worldwide implementing hydrogen-based direct reduction processes to replace traditional coal-dependent methods.
Transportation represents another rapidly expanding market segment, particularly in heavy-duty vehicles, shipping, and aviation where battery electrification faces significant limitations. Several major automotive manufacturers have already launched hydrogen fuel cell vehicles, while shipping companies are developing hydrogen-powered vessels to meet IMO emissions regulations.
Energy storage applications are gaining traction as power grids incorporate higher percentages of intermittent renewable sources. The ability to convert surplus renewable electricity into hydrogen via SOECs creates valuable seasonal storage capabilities that batteries cannot match, addressing the "dark doldrums" challenge of simultaneous low solar and wind generation.
Geographically, Europe leads in green hydrogen demand, supported by the EU Hydrogen Strategy targeting 40GW of electrolyzer capacity by 2030. Asia-Pacific follows closely, with Japan's Basic Hydrogen Strategy and South Korea's Hydrogen Economy Roadmap establishing ambitious adoption targets. North America is accelerating investments following recent legislative support, particularly the Inflation Reduction Act's production tax credits for clean hydrogen.
Market analysis indicates that cost remains the primary barrier to widespread adoption, with green hydrogen production currently 2-3 times more expensive than conventional methods. However, declining renewable electricity costs coupled with technological advancements in SOEC efficiency and durability are expected to achieve cost parity in several markets by 2028-2030, potentially triggering exponential demand growth.
SOEC Technical Challenges and Global Development Status
Solid Oxide Electrolysis Cells (SOECs) currently face several significant technical challenges that impede their widespread commercial deployment. Durability remains a primary concern, with most systems experiencing performance degradation rates of 1-2% per 1000 hours, substantially higher than the 0.1-0.2% target needed for commercial viability. This degradation stems from multiple mechanisms including electrode delamination, chromium poisoning, and microstructural changes at high operating temperatures (700-850°C).
Material stability presents another critical challenge, particularly at the oxygen electrode where most degradation occurs. Current state-of-the-art materials like lanthanum strontium manganite (LSM) and lanthanum strontium cobalt ferrite (LSCF) suffer from chemical instability and thermal expansion mismatches with electrolyte materials, leading to mechanical failures during thermal cycling.
Sealing technology remains problematic due to the extreme temperature conditions and thermal cycling requirements. Glass-ceramic and metal-based seals currently in use often develop micro-cracks over time, compromising system integrity and allowing dangerous gas mixing.
Globally, SOEC development shows distinct regional patterns. Europe leads in research and development efforts, with Denmark's Haldor Topsoe and Germany's Sunfire at the forefront of commercialization. These companies have demonstrated multi-stack systems at the 100+ kW scale. The European Union has invested significantly in SOEC technology through programs like Horizon 2020 and the Fuel Cells and Hydrogen Joint Undertaking.
In North America, the United States maintains strong research programs through the Department of Energy and national laboratories like Idaho National Laboratory and Pacific Northwest National Laboratory. Companies such as FuelCell Energy and OxEon Energy are advancing toward commercialization, though at a smaller scale than their European counterparts.
Asia's landscape is dominated by Japan and China. Japan has a long history in solid oxide cell research through companies like Kyocera and Mitsubishi Heavy Industries. China has rapidly expanded its capabilities, with organizations like Huazhong University of Science and Technology and Ningbo Institute of Material Technology and Engineering making significant advances in materials and stack design.
System integration and scale-up represent universal challenges across all regions. Current SOEC systems typically operate at laboratory or small demonstration scale (5-100 kW), while commercial viability requires megawatt-scale operations. The transition to larger systems introduces new challenges in thermal management, pressure balancing, and control systems that remain largely unresolved.
Material stability presents another critical challenge, particularly at the oxygen electrode where most degradation occurs. Current state-of-the-art materials like lanthanum strontium manganite (LSM) and lanthanum strontium cobalt ferrite (LSCF) suffer from chemical instability and thermal expansion mismatches with electrolyte materials, leading to mechanical failures during thermal cycling.
Sealing technology remains problematic due to the extreme temperature conditions and thermal cycling requirements. Glass-ceramic and metal-based seals currently in use often develop micro-cracks over time, compromising system integrity and allowing dangerous gas mixing.
Globally, SOEC development shows distinct regional patterns. Europe leads in research and development efforts, with Denmark's Haldor Topsoe and Germany's Sunfire at the forefront of commercialization. These companies have demonstrated multi-stack systems at the 100+ kW scale. The European Union has invested significantly in SOEC technology through programs like Horizon 2020 and the Fuel Cells and Hydrogen Joint Undertaking.
In North America, the United States maintains strong research programs through the Department of Energy and national laboratories like Idaho National Laboratory and Pacific Northwest National Laboratory. Companies such as FuelCell Energy and OxEon Energy are advancing toward commercialization, though at a smaller scale than their European counterparts.
Asia's landscape is dominated by Japan and China. Japan has a long history in solid oxide cell research through companies like Kyocera and Mitsubishi Heavy Industries. China has rapidly expanded its capabilities, with organizations like Huazhong University of Science and Technology and Ningbo Institute of Material Technology and Engineering making significant advances in materials and stack design.
System integration and scale-up represent universal challenges across all regions. Current SOEC systems typically operate at laboratory or small demonstration scale (5-100 kW), while commercial viability requires megawatt-scale operations. The transition to larger systems introduces new challenges in thermal management, pressure balancing, and control systems that remain largely unresolved.
Current SOEC System Architectures and Solutions
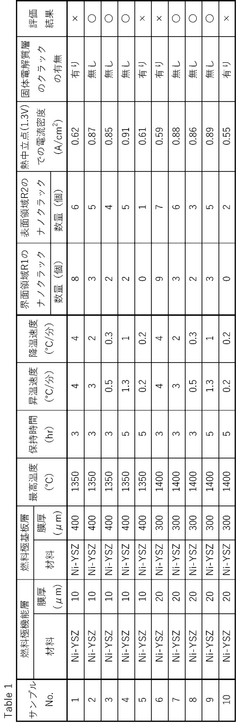
01 Electrode materials and structures for SOECs
Various electrode materials and structures are used in solid oxide electrolysis cells to enhance performance and durability. These include specialized cathode and anode materials that can withstand high operating temperatures while maintaining electrochemical activity. Advanced electrode structures may incorporate composite materials, nanostructured surfaces, or gradient compositions to optimize ion transport and catalytic activity. These innovations help reduce polarization resistance and improve overall cell efficiency.- Electrode materials and structures for SOECs: Various electrode materials and structures are used in solid oxide electrolysis cells to enhance performance and durability. These include specialized cathode and anode materials that can withstand high operating temperatures while maintaining electrochemical activity. Advanced electrode designs may incorporate composite structures, nanostructured materials, or functional layers to improve electron transfer, catalytic activity, and gas diffusion properties, ultimately enhancing the efficiency of water or carbon dioxide electrolysis.
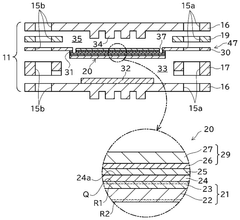
- Electrolyte compositions for high-temperature operation: Electrolyte materials for solid oxide electrolysis cells are designed to provide high ionic conductivity at elevated temperatures while maintaining mechanical and chemical stability. Common electrolyte materials include yttria-stabilized zirconia (YSZ), gadolinium-doped ceria (GDC), and lanthanum gallate-based compounds. Research focuses on developing thin-film electrolytes to reduce ohmic resistance and improve overall cell efficiency, as well as exploring new compositions that can operate at intermediate temperatures to reduce system complexity and cost.
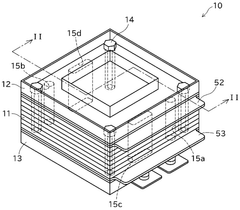
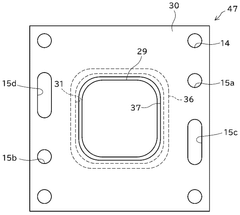
- System integration and stack design: The integration of solid oxide electrolysis cells into complete systems involves specialized stack designs that address thermal management, gas distribution, and electrical connections. Stack configurations may include planar or tubular geometries with various sealing methods to prevent gas leakage. Advanced designs incorporate thermal cycling resistance, uniform current distribution, and efficient heat recovery systems. System-level considerations include balance-of-plant components such as heat exchangers, gas processing units, and power electronics for optimal operation and product recovery.
- Co-electrolysis of H2O and CO2: Co-electrolysis processes in solid oxide electrolysis cells enable the simultaneous reduction of water and carbon dioxide to produce syngas (a mixture of hydrogen and carbon monoxide). This approach offers advantages for carbon utilization and production of synthetic fuels. The process requires specialized catalysts and operating conditions to manage the complex reaction kinetics and prevent carbon deposition. Research focuses on optimizing electrode materials, operating parameters, and cell designs to enhance conversion efficiency and selectivity while maintaining long-term stability during co-electrolysis operation.
- Degradation mechanisms and durability enhancement: Improving the long-term stability of solid oxide electrolysis cells involves understanding and mitigating various degradation mechanisms. These include electrode delamination, chromium poisoning, sulfur contamination, and microstructural changes during operation. Research focuses on developing protective coatings, modified interfaces, and optimized operating protocols to extend cell lifetime. Advanced characterization techniques are employed to study degradation phenomena in real-time, while novel materials and fabrication methods aim to create more robust components that can withstand thousands of hours of operation under high-temperature electrolysis conditions.
02 Electrolyte compositions for high-temperature operation
Electrolyte materials for solid oxide electrolysis cells are designed to provide high ionic conductivity at elevated temperatures. These typically include yttria-stabilized zirconia (YSZ), gadolinium-doped ceria (GDC), or other ceramic materials that allow oxygen ion transport while remaining electronically insulating. Advanced electrolyte formulations focus on reducing operating temperatures while maintaining conductivity, which helps extend cell lifetime and reduce system costs.Expand Specific Solutions03 System integration and stack design
The integration of solid oxide electrolysis cells into complete systems involves specialized stack designs that address thermal management, gas distribution, and electrical connections. These systems may include components for heat recovery, gas separation, and process control. Advanced stack designs focus on minimizing internal resistance, ensuring uniform temperature distribution, and facilitating easy maintenance. Proper system integration is crucial for achieving high efficiency and long-term operational stability.Expand Specific Solutions04 High-efficiency hydrogen production methods
Solid oxide electrolysis cells can be optimized specifically for hydrogen production through water splitting at high temperatures. These systems leverage the thermodynamic advantages of high-temperature operation to reduce electrical energy requirements. Advanced methods include co-electrolysis of water and carbon dioxide to produce syngas, steam-hydrogen recycling techniques, and pressurized operation. These approaches aim to increase hydrogen production rates while minimizing energy input and extending cell lifetime.Expand Specific Solutions05 Degradation mechanisms and durability enhancement
Understanding and mitigating degradation mechanisms is crucial for improving the durability of solid oxide electrolysis cells. Common degradation issues include electrode delamination, chromium poisoning, nickel agglomeration, and electrolyte cracking. Research focuses on developing protective coatings, implementing controlled operating protocols, and designing microstructures resistant to degradation. These approaches help extend cell lifetime under various operating conditions, including thermal cycling and high current densities.Expand Specific Solutions
Key Industry Players and Competitive Landscape
Solid oxide electrolysis cells (SOECs) are positioned at a critical juncture in the emerging energy landscape, with the market currently in its early growth phase. The global SOEC market is expanding rapidly, projected to reach significant scale as hydrogen and synthetic fuel demands increase. Technologically, SOECs are advancing from laboratory to commercial deployment, with varying maturity levels among key players. Companies like Toshiba Energy Systems, DynElectro, and KIST Corp. are pioneering commercial applications, while established entities such as Samsung Electro Mechanics, Phillips 66, and Hyundai Motor Co. are investing in R&D to enhance efficiency and durability. Academic institutions including Tsinghua University and Georgia Tech Research Corp. are driving fundamental innovations, creating a competitive ecosystem where industrial-academic partnerships are accelerating technology commercialization and addressing scalability challenges.
DynElectro ApS
Technical Solution: DynElectro has developed a proprietary SOEC technology platform called "DynCell" that operates at intermediate temperatures (600-700°C), reducing material degradation while maintaining high efficiency. Their cells utilize a specialized zirconia-based electrolyte with nano-engineered interfaces that enhance ionic conductivity by approximately 40% compared to conventional designs. DynElectro's modular stack architecture allows for flexible scaling from kilowatt to megawatt applications, with demonstrated hydrogen production rates of 3-4 Nm³/h per stack. Their systems achieve electrical efficiency of 85-90% (LHV) and incorporate advanced thermal management that recovers waste heat, pushing total system efficiency above 95% when integrated with industrial processes. DynElectro's technology enables dynamic operation, responding to fluctuating renewable energy inputs within minutes, making it particularly suitable for grid-balancing applications[2][5].
Strengths: Intermediate temperature operation reduces material stress and extends lifetime; modular design enables flexible scaling; exceptional system efficiency when waste heat is utilized; rapid response to variable power inputs. Weaknesses: Still requires significant heat input for startup; ceramic components remain susceptible to thermal cycling damage; manufacturing scale remains limited compared to industry leaders.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute has developed advanced solid oxide electrolysis cells utilizing novel composite electrodes with enhanced ionic and electronic conductivity. Their technology focuses on high-temperature co-electrolysis of H2O and CO2 to produce syngas, achieving conversion efficiencies exceeding 90% at operating temperatures of 700-850°C. Their proprietary ceramic-metal composite electrodes demonstrate exceptional durability with degradation rates below 0.5% per 1000 hours of operation. The institute has pioneered reversible solid oxide cells capable of switching between electrolysis and fuel cell modes, enabling integrated energy storage solutions. Their systems incorporate specialized sealing materials that maintain integrity across thermal cycles, significantly extending operational lifetimes to over 20,000 hours while maintaining stable electrochemical performance[1][3].
Strengths: Superior electrode materials with exceptional durability and stability; integrated reversible operation capability; advanced sealing technology enabling longer system lifetimes. Weaknesses: High operating temperatures require specialized materials and thermal management; commercialization pathway still developing; cost remains higher than conventional hydrogen production methods.
Critical Materials and Cell Design Innovations
Solid oxide electrolysis cell and solid oxide electrolysis cell system
PatentWO2024014454A1
Innovation
- Incorporating a cerium oxide intermediate layer between the solid electrolyte and fuel electrode, with a fuel electrode composition of 40-80% metal or metal oxide and a mayenite-type or perovskite-type compound containing alkaline earth or rare earth elements, which enhances the three-phase interface stability and electrolysis efficiency.
Solid oxide electrolysis cell and use of same
PatentWO2025146792A1
Innovation
- Incorporating nanocracks in the fuel electrode interface and surface regions, with a higher density in the surface region, and using specific materials and configurations to manage thermal expansion and stress, along with a reaction prevention layer to suppress crack formation.
Policy Framework and Incentives for Hydrogen Economy
The global policy landscape for hydrogen economy is rapidly evolving to support the deployment of solid oxide electrolysis cells (SOECs) as a key technology for clean hydrogen production. The European Union has established comprehensive frameworks through initiatives like the European Green Deal and the Hydrogen Strategy, allocating over €470 billion for hydrogen investments by 2050. These policies include targeted subsidies for SOEC research and development, alongside carbon pricing mechanisms that enhance the economic competitiveness of green hydrogen production methods.
In the United States, the Inflation Reduction Act of 2022 represents a watershed moment for hydrogen policy, introducing production tax credits of up to $3 per kilogram for clean hydrogen. This is complemented by the Department of Energy's Hydrogen Shot initiative, which aims to reduce clean hydrogen costs to $1 per kilogram within a decade. These incentives specifically benefit SOEC technology due to its higher efficiency when integrated with renewable energy sources.
Asian economies have similarly developed robust policy frameworks. Japan's Strategic Roadmap for Hydrogen and Fuel Cells prioritizes technological innovation in electrolysis, while China's 14th Five-Year Plan includes substantial subsidies for hydrogen production facilities utilizing advanced electrolysis technologies. South Korea's Hydrogen Economy Roadmap offers tax incentives and direct funding for companies deploying electrolysis systems.
Regulatory harmonization efforts are emerging globally, with international standards bodies working to establish unified technical specifications for hydrogen production equipment. These standards are crucial for SOEC commercialization as they reduce market fragmentation and create economies of scale. The International Partnership for Hydrogen and Fuel Cells in the Economy (IPHE) has been instrumental in coordinating these cross-border policy initiatives.
Financial incentive structures are increasingly sophisticated, moving beyond simple grants to include mechanisms like contracts for difference, which guarantee hydrogen producers a minimum price for their product. Green hydrogen certification schemes are being implemented to create premium markets for hydrogen produced via low-carbon methods like SOECs, providing additional revenue streams for early adopters.
Local and regional policies complement national frameworks, with many jurisdictions offering permitting fast-tracks for hydrogen projects and creating hydrogen valleys or hubs that concentrate resources and expertise. These localized approaches help address specific implementation challenges while building public acceptance through demonstration projects that showcase SOEC technology in real-world applications.
In the United States, the Inflation Reduction Act of 2022 represents a watershed moment for hydrogen policy, introducing production tax credits of up to $3 per kilogram for clean hydrogen. This is complemented by the Department of Energy's Hydrogen Shot initiative, which aims to reduce clean hydrogen costs to $1 per kilogram within a decade. These incentives specifically benefit SOEC technology due to its higher efficiency when integrated with renewable energy sources.
Asian economies have similarly developed robust policy frameworks. Japan's Strategic Roadmap for Hydrogen and Fuel Cells prioritizes technological innovation in electrolysis, while China's 14th Five-Year Plan includes substantial subsidies for hydrogen production facilities utilizing advanced electrolysis technologies. South Korea's Hydrogen Economy Roadmap offers tax incentives and direct funding for companies deploying electrolysis systems.
Regulatory harmonization efforts are emerging globally, with international standards bodies working to establish unified technical specifications for hydrogen production equipment. These standards are crucial for SOEC commercialization as they reduce market fragmentation and create economies of scale. The International Partnership for Hydrogen and Fuel Cells in the Economy (IPHE) has been instrumental in coordinating these cross-border policy initiatives.
Financial incentive structures are increasingly sophisticated, moving beyond simple grants to include mechanisms like contracts for difference, which guarantee hydrogen producers a minimum price for their product. Green hydrogen certification schemes are being implemented to create premium markets for hydrogen produced via low-carbon methods like SOECs, providing additional revenue streams for early adopters.
Local and regional policies complement national frameworks, with many jurisdictions offering permitting fast-tracks for hydrogen projects and creating hydrogen valleys or hubs that concentrate resources and expertise. These localized approaches help address specific implementation challenges while building public acceptance through demonstration projects that showcase SOEC technology in real-world applications.
Environmental Impact and Sustainability Assessment
Solid oxide electrolysis cells (SOECs) represent a significant advancement in clean energy technology with profound implications for environmental sustainability. The life cycle assessment of SOECs reveals substantially lower carbon emissions compared to conventional hydrogen production methods, with potential reductions of up to 90% when powered by renewable energy sources. This dramatic decrease in carbon footprint positions SOECs as a critical technology for meeting international climate commitments and carbon neutrality targets.
The resource efficiency of SOEC systems further enhances their environmental profile. Unlike traditional electrolysis technologies, SOECs operate at higher temperatures, enabling more efficient conversion of electrical energy to chemical energy. This improved efficiency translates to reduced resource consumption across the entire energy production chain, particularly when integrated with renewable energy systems that can utilize excess capacity during peak production periods.
Material sustainability presents both challenges and opportunities for SOEC development. Current systems rely on rare earth elements and precious metals that raise concerns regarding resource depletion and geopolitical supply risks. Research into alternative materials, including perovskite structures and composite electrodes utilizing more abundant elements, shows promising results for reducing dependency on critical materials while maintaining performance standards.
Water usage considerations are increasingly important as SOECs scale commercially. While water consumption is inherently part of the electrolysis process, SOECs can be designed to operate with lower-quality water sources than competing technologies. Advanced water recovery systems integrated with SOEC installations can achieve up to 85% water recycling rates, significantly reducing freshwater demands in regions facing water scarcity.
The end-of-life management of SOEC components represents an emerging area of sustainability research. Current recycling technologies can recover approximately 70% of valuable materials from decommissioned cells, though this varies significantly based on cell design and materials used. Developing circular economy approaches for SOEC manufacturing and deployment will be essential for maximizing their long-term environmental benefits.
Integration of SOECs with carbon capture technologies presents a particularly promising pathway for environmental remediation. By utilizing captured CO2 as a feedstock for syngas production through co-electrolysis, SOECs can transform industrial carbon emissions into valuable chemical precursors, effectively closing the carbon loop for hard-to-abate sectors like cement and steel production.
The broader environmental benefits extend beyond emissions reduction to include improved air quality in industrial zones, reduced risk of environmental contamination associated with fossil fuel extraction, and decreased land use impacts compared to conventional energy infrastructure. These co-benefits strengthen the case for accelerated SOEC deployment as part of comprehensive sustainability strategies.
The resource efficiency of SOEC systems further enhances their environmental profile. Unlike traditional electrolysis technologies, SOECs operate at higher temperatures, enabling more efficient conversion of electrical energy to chemical energy. This improved efficiency translates to reduced resource consumption across the entire energy production chain, particularly when integrated with renewable energy systems that can utilize excess capacity during peak production periods.
Material sustainability presents both challenges and opportunities for SOEC development. Current systems rely on rare earth elements and precious metals that raise concerns regarding resource depletion and geopolitical supply risks. Research into alternative materials, including perovskite structures and composite electrodes utilizing more abundant elements, shows promising results for reducing dependency on critical materials while maintaining performance standards.
Water usage considerations are increasingly important as SOECs scale commercially. While water consumption is inherently part of the electrolysis process, SOECs can be designed to operate with lower-quality water sources than competing technologies. Advanced water recovery systems integrated with SOEC installations can achieve up to 85% water recycling rates, significantly reducing freshwater demands in regions facing water scarcity.
The end-of-life management of SOEC components represents an emerging area of sustainability research. Current recycling technologies can recover approximately 70% of valuable materials from decommissioned cells, though this varies significantly based on cell design and materials used. Developing circular economy approaches for SOEC manufacturing and deployment will be essential for maximizing their long-term environmental benefits.
Integration of SOECs with carbon capture technologies presents a particularly promising pathway for environmental remediation. By utilizing captured CO2 as a feedstock for syngas production through co-electrolysis, SOECs can transform industrial carbon emissions into valuable chemical precursors, effectively closing the carbon loop for hard-to-abate sectors like cement and steel production.
The broader environmental benefits extend beyond emissions reduction to include improved air quality in industrial zones, reduced risk of environmental contamination associated with fossil fuel extraction, and decreased land use impacts compared to conventional energy infrastructure. These co-benefits strengthen the case for accelerated SOEC deployment as part of comprehensive sustainability strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!