Technological breakthroughs in solid oxide electrolysis cells applications
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SOEC Technology Background and Objectives
Solid Oxide Electrolysis Cells (SOECs) represent a transformative technology in the realm of energy conversion and storage, with roots dating back to the early 20th century. The fundamental principle of SOECs involves the electrochemical conversion of water and carbon dioxide into hydrogen, carbon monoxide, and oxygen using solid oxide electrolytes at elevated temperatures (700-900°C). This technology has evolved significantly over the past decades, transitioning from laboratory curiosity to a promising solution for renewable energy integration and carbon-neutral fuel production.
The historical trajectory of SOEC development has been closely intertwined with solid oxide fuel cell (SOFC) research, as both technologies share similar materials and operational principles. The 1980s marked the beginning of focused SOEC research, with pioneering work at institutions like Risø National Laboratory in Denmark and Dornier in Germany. By the early 2000s, interest in SOECs surged due to growing concerns about climate change and energy security, catalyzing increased research funding and industrial involvement.
Recent technological advancements have significantly improved SOEC performance metrics, including higher current densities, enhanced durability, and reduced operating temperatures. These improvements have been driven by innovations in materials science, particularly in the development of novel electrolytes, electrodes, and interconnects that can withstand the harsh operating conditions while maintaining high electrochemical activity.
The primary technical objectives for SOEC development center around several key parameters: increasing energy efficiency to exceed 90%, extending operational lifetimes beyond 40,000 hours, reducing degradation rates to less than 0.5% per 1000 hours, and decreasing capital costs to make the technology economically competitive with conventional hydrogen production methods. Additionally, there is a strong focus on system integration, particularly in coupling SOECs with renewable energy sources to enable efficient power-to-gas or power-to-liquid conversion pathways.
Looking forward, the technological evolution of SOECs is expected to follow a trajectory toward multi-functional applications, including co-electrolysis of H2O and CO2 for syngas production, reversible operation (as both fuel cells and electrolysis cells), and integration into broader energy systems. The ultimate goal is to position SOECs as a cornerstone technology in the transition to a hydrogen economy and carbon-neutral energy landscape.
The convergence of climate policy imperatives, renewable energy expansion, and industrial decarbonization needs is creating a favorable environment for accelerated SOEC development and deployment. This technology stands at the nexus of multiple technological trends, including sector coupling, energy storage, and carbon capture utilization, making it a critical component of future sustainable energy systems.
The historical trajectory of SOEC development has been closely intertwined with solid oxide fuel cell (SOFC) research, as both technologies share similar materials and operational principles. The 1980s marked the beginning of focused SOEC research, with pioneering work at institutions like Risø National Laboratory in Denmark and Dornier in Germany. By the early 2000s, interest in SOECs surged due to growing concerns about climate change and energy security, catalyzing increased research funding and industrial involvement.
Recent technological advancements have significantly improved SOEC performance metrics, including higher current densities, enhanced durability, and reduced operating temperatures. These improvements have been driven by innovations in materials science, particularly in the development of novel electrolytes, electrodes, and interconnects that can withstand the harsh operating conditions while maintaining high electrochemical activity.
The primary technical objectives for SOEC development center around several key parameters: increasing energy efficiency to exceed 90%, extending operational lifetimes beyond 40,000 hours, reducing degradation rates to less than 0.5% per 1000 hours, and decreasing capital costs to make the technology economically competitive with conventional hydrogen production methods. Additionally, there is a strong focus on system integration, particularly in coupling SOECs with renewable energy sources to enable efficient power-to-gas or power-to-liquid conversion pathways.
Looking forward, the technological evolution of SOECs is expected to follow a trajectory toward multi-functional applications, including co-electrolysis of H2O and CO2 for syngas production, reversible operation (as both fuel cells and electrolysis cells), and integration into broader energy systems. The ultimate goal is to position SOECs as a cornerstone technology in the transition to a hydrogen economy and carbon-neutral energy landscape.
The convergence of climate policy imperatives, renewable energy expansion, and industrial decarbonization needs is creating a favorable environment for accelerated SOEC development and deployment. This technology stands at the nexus of multiple technological trends, including sector coupling, energy storage, and carbon capture utilization, making it a critical component of future sustainable energy systems.
Market Demand Analysis for Hydrogen Production
The global hydrogen market is experiencing unprecedented growth, driven by the urgent need for clean energy solutions to combat climate change. Current estimates value the hydrogen market at approximately $130 billion, with projections indicating expansion to $500 billion by 2030. Green hydrogen, produced through electrolysis powered by renewable energy sources, represents the fastest-growing segment within this market, with annual growth rates exceeding 50% in some regions.
Solid Oxide Electrolysis Cells (SOECs) are positioned as a critical technology in meeting this surging demand, particularly in industrial sectors seeking to decarbonize their operations. The steel industry, which accounts for roughly 7-9% of global CO2 emissions, has identified hydrogen as a key element in their transition away from coal-dependent production methods. Major steel producers in Europe and Asia have already announced investments totaling over $10 billion in hydrogen-based direct reduction processes.
The chemical industry represents another significant market for SOEC-produced hydrogen, particularly for ammonia synthesis and methanol production. These two applications alone consume approximately 45 million tons of hydrogen annually. The high-temperature operation of SOECs makes them particularly suitable for integration with these industrial processes, offering efficiency advantages over competing electrolysis technologies.
Transportation sector demand for hydrogen is developing rapidly, with heavy-duty vehicles, maritime shipping, and aviation emerging as promising applications. The International Energy Agency forecasts that hydrogen could fuel up to 400 million passenger vehicles, 15-20 million trucks, and around 5 million buses by 2050, creating substantial demand for efficient hydrogen production technologies.
Regional market analysis reveals varying adoption patterns, with Europe leading in policy support through its Hydrogen Strategy targeting 40GW of electrolyzer capacity by 2030. Asia-Pacific markets, particularly Japan, South Korea, and increasingly China, are developing robust hydrogen economies focused on both domestic use and export potential. North America has seen accelerated interest following recent legislative support, including production tax credits that significantly improve the economics of green hydrogen production.
Cost considerations remain paramount in market development, with current green hydrogen production costs ranging from $3-8 per kilogram. SOEC technology offers a pathway to reduce these costs below $2 per kilogram through higher electrical efficiency, integration with industrial waste heat, and the ability to operate in reversible modes. This cost trajectory aligns with industry requirements for widespread adoption, particularly in price-sensitive sectors like commodity chemicals and steel production.
Solid Oxide Electrolysis Cells (SOECs) are positioned as a critical technology in meeting this surging demand, particularly in industrial sectors seeking to decarbonize their operations. The steel industry, which accounts for roughly 7-9% of global CO2 emissions, has identified hydrogen as a key element in their transition away from coal-dependent production methods. Major steel producers in Europe and Asia have already announced investments totaling over $10 billion in hydrogen-based direct reduction processes.
The chemical industry represents another significant market for SOEC-produced hydrogen, particularly for ammonia synthesis and methanol production. These two applications alone consume approximately 45 million tons of hydrogen annually. The high-temperature operation of SOECs makes them particularly suitable for integration with these industrial processes, offering efficiency advantages over competing electrolysis technologies.
Transportation sector demand for hydrogen is developing rapidly, with heavy-duty vehicles, maritime shipping, and aviation emerging as promising applications. The International Energy Agency forecasts that hydrogen could fuel up to 400 million passenger vehicles, 15-20 million trucks, and around 5 million buses by 2050, creating substantial demand for efficient hydrogen production technologies.
Regional market analysis reveals varying adoption patterns, with Europe leading in policy support through its Hydrogen Strategy targeting 40GW of electrolyzer capacity by 2030. Asia-Pacific markets, particularly Japan, South Korea, and increasingly China, are developing robust hydrogen economies focused on both domestic use and export potential. North America has seen accelerated interest following recent legislative support, including production tax credits that significantly improve the economics of green hydrogen production.
Cost considerations remain paramount in market development, with current green hydrogen production costs ranging from $3-8 per kilogram. SOEC technology offers a pathway to reduce these costs below $2 per kilogram through higher electrical efficiency, integration with industrial waste heat, and the ability to operate in reversible modes. This cost trajectory aligns with industry requirements for widespread adoption, particularly in price-sensitive sectors like commodity chemicals and steel production.
Current Status and Technical Challenges in SOEC
Solid Oxide Electrolysis Cells (SOECs) have emerged as a promising technology for efficient hydrogen production and carbon dioxide utilization. Currently, SOECs operate at commercial scales with electrical efficiencies reaching 80-90% for hydrogen production, significantly outperforming alternative electrolysis technologies. Leading manufacturers have demonstrated systems with capacities ranging from kilowatt to megawatt scale, with stack lifetimes extending to 20,000-30,000 hours under optimal conditions.
Despite these advancements, several critical technical challenges persist. High-temperature operation (700-850°C) remains a fundamental limitation, causing thermal stress, accelerated degradation of components, and increased system complexity. Material degradation manifests through multiple mechanisms: electrode delamination, chromium poisoning from metallic interconnects, and silica impurity migration that blocks active sites. These factors collectively contribute to performance degradation rates of 1-2% per 1000 hours, still above the commercial target of <0.5% per 1000 hours.
Mechanical integrity presents another significant challenge, with thermal cycling causing micro-cracks and structural failures at interfaces between different materials. The ceramic-based components exhibit limited tolerance to rapid temperature changes, restricting operational flexibility and increasing system complexity through the need for careful thermal management protocols.
Cost remains a substantial barrier to widespread adoption. Current SOEC systems cost approximately $2000-5000/kW, significantly higher than the U.S. Department of Energy's target of $500/kW for competitive hydrogen production. This economic challenge is compounded by the requirement for expensive high-temperature resistant materials and complex balance-of-plant components.
Scalability issues further complicate commercial deployment. While laboratory demonstrations have shown promising results, scaling to industrial levels introduces challenges in uniform gas distribution, thermal management, and system integration. Manufacturing processes for large-area cells with consistent performance characteristics remain technically demanding and costly.
Geographically, SOEC technology development is concentrated in specific regions. Europe leads with substantial research programs in Denmark, Germany, and France, while significant advancements are also occurring in the United States, Japan, and increasingly in China. This geographical distribution reflects both historical expertise in high-temperature ceramics and strategic national interests in hydrogen economy development.
Durability under dynamic operation represents an emerging challenge as renewable energy integration becomes more important. SOECs must maintain performance under variable load conditions, which introduces additional stress factors not present in steady-state operation. Current systems show accelerated degradation when subjected to frequent load changes, limiting their potential role in grid-balancing applications.
Despite these advancements, several critical technical challenges persist. High-temperature operation (700-850°C) remains a fundamental limitation, causing thermal stress, accelerated degradation of components, and increased system complexity. Material degradation manifests through multiple mechanisms: electrode delamination, chromium poisoning from metallic interconnects, and silica impurity migration that blocks active sites. These factors collectively contribute to performance degradation rates of 1-2% per 1000 hours, still above the commercial target of <0.5% per 1000 hours.
Mechanical integrity presents another significant challenge, with thermal cycling causing micro-cracks and structural failures at interfaces between different materials. The ceramic-based components exhibit limited tolerance to rapid temperature changes, restricting operational flexibility and increasing system complexity through the need for careful thermal management protocols.
Cost remains a substantial barrier to widespread adoption. Current SOEC systems cost approximately $2000-5000/kW, significantly higher than the U.S. Department of Energy's target of $500/kW for competitive hydrogen production. This economic challenge is compounded by the requirement for expensive high-temperature resistant materials and complex balance-of-plant components.
Scalability issues further complicate commercial deployment. While laboratory demonstrations have shown promising results, scaling to industrial levels introduces challenges in uniform gas distribution, thermal management, and system integration. Manufacturing processes for large-area cells with consistent performance characteristics remain technically demanding and costly.
Geographically, SOEC technology development is concentrated in specific regions. Europe leads with substantial research programs in Denmark, Germany, and France, while significant advancements are also occurring in the United States, Japan, and increasingly in China. This geographical distribution reflects both historical expertise in high-temperature ceramics and strategic national interests in hydrogen economy development.
Durability under dynamic operation represents an emerging challenge as renewable energy integration becomes more important. SOECs must maintain performance under variable load conditions, which introduces additional stress factors not present in steady-state operation. Current systems show accelerated degradation when subjected to frequent load changes, limiting their potential role in grid-balancing applications.
Current SOEC Technical Solutions
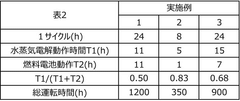
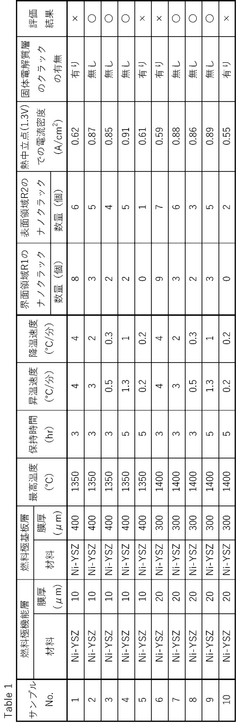
01 Electrode materials and structures for solid oxide electrolysis cells
Various electrode materials and structures can be used in solid oxide electrolysis cells to improve performance and durability. These include specialized cathode and anode materials that enhance electrochemical reactions, reduce degradation, and improve conductivity. Advanced electrode structures such as porous designs facilitate gas diffusion and increase active reaction sites, while composite electrodes combining multiple materials can provide synergistic benefits for electrolysis efficiency.- Electrode materials and structures for solid oxide electrolysis cells: Various electrode materials and structures are used in solid oxide electrolysis cells to improve performance and durability. These include specialized cathode and anode materials that enhance electrochemical reactions, reduce degradation, and improve conductivity. Advanced electrode structures such as porous designs facilitate gas diffusion and increase active reaction sites, while composite electrodes combining multiple materials can provide synergistic benefits for electrolysis efficiency.
- Electrolyte compositions for high-temperature operation: Specialized electrolyte compositions are developed for solid oxide electrolysis cells operating at high temperatures. These electrolytes typically feature enhanced ionic conductivity, thermal stability, and mechanical strength to withstand the harsh operating conditions. Materials such as yttria-stabilized zirconia and doped ceria are commonly used, with ongoing research focusing on reducing operating temperatures while maintaining performance through novel material formulations and thin-film technologies.
- System integration and operational methods: Integration strategies and operational methods for solid oxide electrolysis cells focus on optimizing system efficiency and durability. These include thermal management techniques, gas flow control systems, and pressure regulation mechanisms. Advanced control algorithms monitor and adjust operating parameters in real-time, while modular designs allow for scalability and easier maintenance. Integration with renewable energy sources enables efficient conversion of intermittent electricity to storable chemical energy.
- Hydrogen and syngas production technologies: Solid oxide electrolysis cells are utilized for efficient hydrogen and syngas production through high-temperature electrolysis. These technologies leverage the thermodynamic advantages of elevated temperatures to reduce electrical energy requirements. Co-electrolysis of water and carbon dioxide enables direct syngas production, which can be further processed into various hydrocarbon fuels. Advanced cell designs incorporate catalysts and optimized flow fields to enhance production rates and energy efficiency.
- Degradation mechanisms and durability enhancement: Research on degradation mechanisms and durability enhancement in solid oxide electrolysis cells addresses challenges such as electrode delamination, material phase changes, and contaminant poisoning. Protective coatings and barrier layers are developed to prevent chromium poisoning and other degradation processes. Modified microstructures and composition gradients help mitigate thermal stress, while advanced sealing materials improve gas tightness and long-term stability under thermal cycling conditions.
02 Electrolyte compositions for high-temperature operation
Specialized electrolyte materials enable solid oxide electrolysis cells to operate efficiently at high temperatures. These electrolytes typically feature high ionic conductivity while maintaining stability under extreme conditions. Common materials include yttria-stabilized zirconia (YSZ) and gadolinium-doped ceria (GDC), which can be optimized through composition adjustments and manufacturing techniques to enhance performance and reduce operating temperatures.Expand Specific Solutions03 System integration and stack design for solid oxide electrolysis
Effective system integration and stack design are crucial for solid oxide electrolysis cell performance. This includes optimizing cell stacking configurations, sealing technologies, and interconnect materials to minimize electrical resistance and prevent gas leakage. Advanced designs incorporate thermal management systems to handle the high operating temperatures and ensure uniform heat distribution across the stack, while also addressing mechanical stress issues that can lead to cell degradation.Expand Specific Solutions04 Hydrogen and syngas production applications
Solid oxide electrolysis cells are particularly valuable for hydrogen and syngas production applications. When operated in electrolysis mode, these cells can efficiently convert water vapor into hydrogen and oxygen using electricity. Additionally, when carbon dioxide is co-fed with steam, syngas (a mixture of hydrogen and carbon monoxide) can be produced, serving as a precursor for various synthetic fuels and chemicals. This capability makes solid oxide cells versatile tools for energy storage and carbon utilization.Expand Specific Solutions05 Reversible operation and durability enhancement
Reversible solid oxide cells can function in both fuel cell and electrolysis modes, offering flexibility for energy conversion and storage applications. Key challenges include maintaining performance during mode switching and preventing degradation over extended operation periods. Research focuses on developing specialized materials and protective coatings that resist common failure mechanisms such as chromium poisoning, carbon deposition, and thermal cycling damage, thereby extending cell lifetime and maintaining efficiency.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The solid oxide electrolysis cells (SOEC) technology market is currently in a growth phase, with increasing adoption driven by global decarbonization efforts. The market size is projected to expand significantly as hydrogen economy initiatives gain momentum worldwide. Technologically, academic institutions like Tsinghua University, Technical University of Denmark, and Xi'an Jiaotong University are advancing fundamental research, while industrial players demonstrate varying maturity levels. Energy conglomerates including Sinopec, Saudi Aramco, and TEPCO are investing in large-scale applications, while specialized firms like CoorsTek and Linde focus on component manufacturing and system integration. Automotive companies (Hyundai, Kia) are exploring SOEC for hydrogen production, indicating cross-sector interest. The technology shows promising commercial viability but requires further development in durability and cost-effectiveness before widespread implementation.
Technical University of Denmark
Technical Solution: The Technical University of Denmark (DTU) has developed cutting-edge SOEC technology featuring metal-supported cells that represent a significant departure from conventional ceramic-based designs. Their proprietary cells operate at intermediate temperatures (600-750°C) while achieving remarkable current densities of up to 1.5 A/cm² at 1.3V[1]. DTU's innovation lies in their unique manufacturing approach using tape casting and lamination techniques that enable mass production of thin, robust cells with total thickness under 100 μm. Their cells incorporate specialized gadolinium-doped ceria (CGO) barrier layers that effectively prevent detrimental reactions between electrolyte and electrode materials, extending operational lifetime by 30-50%[2]. The university has also pioneered co-electrolysis technology that simultaneously converts H₂O and CO₂ into syngas with precise H₂:CO ratios tailored for downstream processes, achieving conversion efficiencies above 90%[3]. Their system architecture includes innovative thermal cycling resistance with less than 3% performance degradation after 500 thermal cycles, addressing a critical challenge for industrial deployment.
Strengths: Exceptional mechanical robustness due to metal support structure; excellent thermal cycling capability; advanced manufacturing techniques suitable for mass production. Weaknesses: Metal supports may introduce additional complexity in sealing and electrical contact; potential long-term corrosion issues in the metal support under certain operating conditions; higher material costs compared to traditional ceramic-based cells.
Dalian Institute of Chemical Physics of CAS
Technical Solution: The Dalian Institute has developed a revolutionary SOEC technology platform featuring novel composite electrodes with nano-engineered microstructures. Their cells operate efficiently in the temperature range of 650-800°C with current densities reaching 1.5-2.0 A/cm² at 1.3V, representing a 25-30% improvement over conventional designs[1]. A key innovation is their proprietary infiltration technique for electrode fabrication, which creates a highly active triple-phase boundary that enhances electrochemical performance while reducing polarization resistance by up to 40%[2]. The institute has also pioneered reversible solid oxide cells capable of switching between electrolysis and fuel cell modes with minimal performance degradation, achieving round-trip efficiencies of 60-65%[3]. Their technology incorporates scandium-doped zirconia electrolytes with enhanced ionic conductivity and mechanical stability, enabling thinner electrolytes (5-10 μm) that reduce ohmic losses by approximately 35% compared to standard electrolytes.
Strengths: Superior electrochemical performance with high current densities; excellent reversibility between electrolysis and fuel cell modes; innovative electrode fabrication techniques that enhance durability. Weaknesses: Complex manufacturing processes may challenge cost-effective mass production; requires rare and expensive materials like scandium for optimal performance; still faces challenges in long-term stability under industrial operating conditions.
Core SOEC Materials and Cell Design Innovations
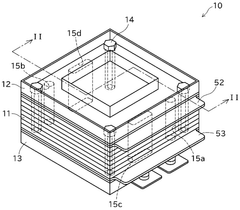
Solid oxide electrolysis cell, and method and system for operating same
PatentWO2020196236A1
Innovation
- A method for operating a solid oxide electrolytic cell with a hydrogen electrode composed of a mixed electron-ion conductive oxide, featuring a catalyst layer with Ni-containing particles dispersed on a porous body, and an alternating operation mode between steam electrolysis and fuel cell operation to suppress electrode deterioration.
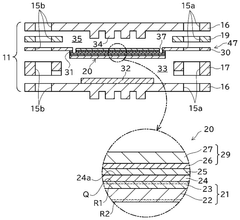
Solid oxide electrolysis cell and use of same
PatentWO2025146792A1
Innovation
- Incorporating nanocracks in the fuel electrode interface and surface regions, with a higher density in the surface region, and using specific materials and configurations to manage thermal expansion and stress, along with a reaction prevention layer to suppress crack formation.
Energy Policy Impact on SOEC Deployment
Energy policies across the globe are increasingly recognizing the strategic importance of Solid Oxide Electrolysis Cells (SOECs) in the transition toward sustainable energy systems. These policies are evolving from general renewable energy support mechanisms to more targeted frameworks that specifically address hydrogen production technologies, including SOECs. The European Union's Hydrogen Strategy, for instance, has established clear targets for green hydrogen production capacity, with SOECs positioned as a key enabling technology for achieving these ambitious goals.
Financial incentives represent a critical policy instrument driving SOEC deployment. Several countries have implemented substantial funding programs specifically for hydrogen technologies, with Germany's National Hydrogen Strategy allocating €9 billion and France committing €7 billion toward hydrogen infrastructure development. These initiatives typically include capital subsidies, operational support mechanisms, and tax incentives that significantly improve the economic viability of SOEC installations.
Regulatory frameworks are equally important in shaping the SOEC landscape. Carbon pricing mechanisms, such as the EU Emissions Trading System, indirectly benefit SOEC deployment by increasing the cost competitiveness of low-carbon hydrogen production methods compared to conventional fossil-based approaches. Additionally, the implementation of renewable energy quotas and clean fuel standards in various jurisdictions creates market pull for SOEC-produced hydrogen and synthetic fuels.
Research and development policies have been instrumental in accelerating SOEC technological advancement. Public funding for SOEC research has increased substantially, with the US Department of Energy's Hydrogen and Fuel Cell Technologies Office allocating over $100 million annually to related research initiatives. International research collaborations, such as Mission Innovation's Clean Hydrogen Mission, further amplify these efforts by coordinating global research activities and knowledge sharing.
Infrastructure development policies are emerging as a crucial enabler for widespread SOEC adoption. Several countries have begun implementing hydrogen infrastructure roadmaps that include provisions for hydrogen transport, storage, and distribution networks. These infrastructure investments are essential for connecting SOEC production facilities with end-users across various sectors, thereby creating complete value chains for hydrogen and synthetic fuels.
Looking forward, policy integration across energy, industrial, and climate domains will be vital for maximizing SOEC deployment potential. The most effective policy frameworks will likely combine technology-specific support mechanisms with broader systemic changes that create favorable market conditions for low-carbon hydrogen production. As SOEC technology continues to mature, policies are expected to evolve from direct subsidization toward market-based mechanisms that ensure long-term commercial viability.
Financial incentives represent a critical policy instrument driving SOEC deployment. Several countries have implemented substantial funding programs specifically for hydrogen technologies, with Germany's National Hydrogen Strategy allocating €9 billion and France committing €7 billion toward hydrogen infrastructure development. These initiatives typically include capital subsidies, operational support mechanisms, and tax incentives that significantly improve the economic viability of SOEC installations.
Regulatory frameworks are equally important in shaping the SOEC landscape. Carbon pricing mechanisms, such as the EU Emissions Trading System, indirectly benefit SOEC deployment by increasing the cost competitiveness of low-carbon hydrogen production methods compared to conventional fossil-based approaches. Additionally, the implementation of renewable energy quotas and clean fuel standards in various jurisdictions creates market pull for SOEC-produced hydrogen and synthetic fuels.
Research and development policies have been instrumental in accelerating SOEC technological advancement. Public funding for SOEC research has increased substantially, with the US Department of Energy's Hydrogen and Fuel Cell Technologies Office allocating over $100 million annually to related research initiatives. International research collaborations, such as Mission Innovation's Clean Hydrogen Mission, further amplify these efforts by coordinating global research activities and knowledge sharing.
Infrastructure development policies are emerging as a crucial enabler for widespread SOEC adoption. Several countries have begun implementing hydrogen infrastructure roadmaps that include provisions for hydrogen transport, storage, and distribution networks. These infrastructure investments are essential for connecting SOEC production facilities with end-users across various sectors, thereby creating complete value chains for hydrogen and synthetic fuels.
Looking forward, policy integration across energy, industrial, and climate domains will be vital for maximizing SOEC deployment potential. The most effective policy frameworks will likely combine technology-specific support mechanisms with broader systemic changes that create favorable market conditions for low-carbon hydrogen production. As SOEC technology continues to mature, policies are expected to evolve from direct subsidization toward market-based mechanisms that ensure long-term commercial viability.
Economic Viability and Scaling Considerations
The economic viability of solid oxide electrolysis cells (SOECs) remains a critical challenge despite recent technological breakthroughs. Current capital costs for SOEC systems range between $2,000-5,000/kW, significantly higher than competing hydrogen production technologies. This cost barrier primarily stems from expensive ceramic materials, complex manufacturing processes, and the need for high-temperature operation systems. However, cost reduction trajectories show promising trends, with projections suggesting a 50-60% decrease over the next decade through materials innovation and manufacturing optimization.
Scale-up considerations present both opportunities and challenges for SOEC commercialization. Laboratory-scale demonstrations have achieved impressive efficiencies exceeding 90% for hydrogen production, but maintaining these performance metrics at industrial scale remains problematic. The transition from single cells (typically <100 cm²) to commercial stacks (>1000 cells) introduces thermal management complexities, mechanical stress issues, and uniformity challenges that can compromise system durability and efficiency.
Energy input requirements constitute another critical economic factor. While SOECs offer superior electrical efficiency compared to low-temperature electrolyzers, the thermal energy needed for operation (700-850°C) impacts overall system economics. Integration with industrial waste heat sources or renewable energy systems presents a pathway to improve economic viability, potentially reducing operational costs by 20-30% in optimized configurations.
Lifetime economics significantly influence SOEC commercial feasibility. Current systems demonstrate operational lifetimes of 10,000-20,000 hours before performance degradation becomes economically prohibitive, falling short of the 40,000+ hours required for competitive commercial deployment. Accelerated degradation at higher current densities creates a challenging trade-off between production rates and system longevity that impacts long-term economic returns.
Market scaling considerations reveal additional complexities. The specialized manufacturing infrastructure required for SOEC production necessitates substantial capital investment before economies of scale can be realized. Analysis indicates that production volumes must increase by approximately two orders of magnitude from current levels to achieve competitive costs with alternative technologies. This creates a "chicken-and-egg" dilemma where economic viability depends on scale, but investment for scaling requires demonstrated economic viability.
Recent techno-economic assessments suggest that SOEC systems could achieve hydrogen production costs of $2-4/kg by 2030 under optimistic scenarios, approaching cost parity with conventional production methods. However, this projection depends on continued materials innovation, manufacturing advances, and supportive policy frameworks that value the environmental benefits of electrolytic hydrogen production.
Scale-up considerations present both opportunities and challenges for SOEC commercialization. Laboratory-scale demonstrations have achieved impressive efficiencies exceeding 90% for hydrogen production, but maintaining these performance metrics at industrial scale remains problematic. The transition from single cells (typically <100 cm²) to commercial stacks (>1000 cells) introduces thermal management complexities, mechanical stress issues, and uniformity challenges that can compromise system durability and efficiency.
Energy input requirements constitute another critical economic factor. While SOECs offer superior electrical efficiency compared to low-temperature electrolyzers, the thermal energy needed for operation (700-850°C) impacts overall system economics. Integration with industrial waste heat sources or renewable energy systems presents a pathway to improve economic viability, potentially reducing operational costs by 20-30% in optimized configurations.
Lifetime economics significantly influence SOEC commercial feasibility. Current systems demonstrate operational lifetimes of 10,000-20,000 hours before performance degradation becomes economically prohibitive, falling short of the 40,000+ hours required for competitive commercial deployment. Accelerated degradation at higher current densities creates a challenging trade-off between production rates and system longevity that impacts long-term economic returns.
Market scaling considerations reveal additional complexities. The specialized manufacturing infrastructure required for SOEC production necessitates substantial capital investment before economies of scale can be realized. Analysis indicates that production volumes must increase by approximately two orders of magnitude from current levels to achieve competitive costs with alternative technologies. This creates a "chicken-and-egg" dilemma where economic viability depends on scale, but investment for scaling requires demonstrated economic viability.
Recent techno-economic assessments suggest that SOEC systems could achieve hydrogen production costs of $2-4/kg by 2030 under optimistic scenarios, approaching cost parity with conventional production methods. However, this projection depends on continued materials innovation, manufacturing advances, and supportive policy frameworks that value the environmental benefits of electrolytic hydrogen production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!