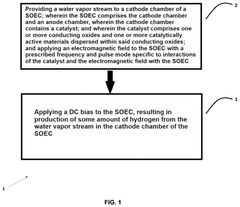
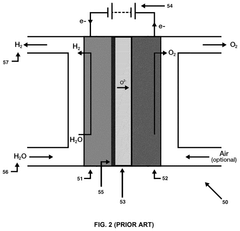
The interaction of catalysts and solid oxide electrolysis cells
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Catalyst-SOEC Integration Background and Objectives
Solid oxide electrolysis cells (SOECs) have emerged as a promising technology for efficient conversion of electrical energy into chemical energy, particularly for hydrogen production and carbon dioxide reduction. The integration of catalysts with SOECs represents a critical intersection of materials science, electrochemistry, and energy technology that has evolved significantly over the past decades. Initially developed as a reverse operation of solid oxide fuel cells (SOFCs), SOEC technology has gradually established its own research trajectory with unique challenges and opportunities.
The evolution of catalyst-SOEC integration can be traced back to the 1980s when researchers began exploring high-temperature electrolysis using ceramic materials. However, significant advancements occurred in the early 2000s with the growing interest in hydrogen economy and renewable energy storage solutions. The field has since witnessed accelerated development, driven by the global push for decarbonization and the need for efficient energy conversion technologies.
Current technological trends in this domain focus on enhancing electrochemical performance, improving durability, and reducing operating temperatures. Catalyst development has shifted from traditional metal-based systems to advanced nanostructured materials, composite catalysts, and novel synthesis methods that enable precise control over catalyst morphology and distribution within the SOEC architecture.
The primary technical objectives in catalyst-SOEC integration include reducing activation energy barriers at electrode-electrolyte interfaces, enhancing selectivity for target reactions, mitigating degradation mechanisms, and enabling stable operation under various conditions. Researchers aim to develop catalysts that can effectively promote desired electrochemical reactions while maintaining compatibility with the high-temperature ceramic components of SOECs.
Long-term goals encompass the development of robust, scalable, and economically viable SOEC systems that can operate efficiently at intermediate temperatures (600-800°C) with minimal degradation over extended periods. This includes designing catalyst systems that remain active and stable under thermal cycling, redox conditions, and in the presence of contaminants that may be present in real-world applications.
The interdisciplinary nature of catalyst-SOEC integration necessitates collaborative approaches across materials science, surface chemistry, electrochemistry, and process engineering. Recent advances in computational modeling and in-situ characterization techniques have accelerated understanding of reaction mechanisms and degradation processes, enabling more rational design of catalyst-electrode assemblies.
As global energy systems transition toward greater electrification and renewable integration, catalyst-SOEC technology stands at a critical juncture with potential applications extending beyond hydrogen production to include synthetic fuel production, carbon utilization, and industrial process decarbonization. The technical trajectory suggests continued innovation in materials design, manufacturing processes, and system integration to realize the full potential of this transformative technology.
The evolution of catalyst-SOEC integration can be traced back to the 1980s when researchers began exploring high-temperature electrolysis using ceramic materials. However, significant advancements occurred in the early 2000s with the growing interest in hydrogen economy and renewable energy storage solutions. The field has since witnessed accelerated development, driven by the global push for decarbonization and the need for efficient energy conversion technologies.
Current technological trends in this domain focus on enhancing electrochemical performance, improving durability, and reducing operating temperatures. Catalyst development has shifted from traditional metal-based systems to advanced nanostructured materials, composite catalysts, and novel synthesis methods that enable precise control over catalyst morphology and distribution within the SOEC architecture.
The primary technical objectives in catalyst-SOEC integration include reducing activation energy barriers at electrode-electrolyte interfaces, enhancing selectivity for target reactions, mitigating degradation mechanisms, and enabling stable operation under various conditions. Researchers aim to develop catalysts that can effectively promote desired electrochemical reactions while maintaining compatibility with the high-temperature ceramic components of SOECs.
Long-term goals encompass the development of robust, scalable, and economically viable SOEC systems that can operate efficiently at intermediate temperatures (600-800°C) with minimal degradation over extended periods. This includes designing catalyst systems that remain active and stable under thermal cycling, redox conditions, and in the presence of contaminants that may be present in real-world applications.
The interdisciplinary nature of catalyst-SOEC integration necessitates collaborative approaches across materials science, surface chemistry, electrochemistry, and process engineering. Recent advances in computational modeling and in-situ characterization techniques have accelerated understanding of reaction mechanisms and degradation processes, enabling more rational design of catalyst-electrode assemblies.
As global energy systems transition toward greater electrification and renewable integration, catalyst-SOEC technology stands at a critical juncture with potential applications extending beyond hydrogen production to include synthetic fuel production, carbon utilization, and industrial process decarbonization. The technical trajectory suggests continued innovation in materials design, manufacturing processes, and system integration to realize the full potential of this transformative technology.
Market Analysis for Catalyst-Enhanced SOEC Technologies
The global market for catalyst-enhanced Solid Oxide Electrolysis Cell (SOEC) technologies is experiencing significant growth, driven by increasing demand for clean hydrogen production and carbon utilization solutions. Current market valuations place the SOEC sector at approximately $320 million in 2023, with catalyst-enhanced systems representing about 40% of this market. Industry forecasts project a compound annual growth rate of 22.7% through 2030, potentially reaching a market size of $1.3 billion.
Regional analysis reveals distinct market characteristics across different territories. Europe leads adoption with approximately 45% market share, supported by aggressive decarbonization policies and substantial government funding through initiatives like the European Hydrogen Strategy. North America follows at 30% market share, with particular strength in industrial applications and growing interest from the natural gas sector for syngas production.
Asia-Pacific represents the fastest-growing region with 18% current market share but projected growth rates exceeding 25% annually. China's recent five-year plan specifically targets SOEC technology advancement, while Japan and South Korea focus on integration with existing industrial infrastructure.
Customer segmentation shows three primary market segments driving demand. Industrial hydrogen producers represent 52% of current demand, particularly in ammonia production, refining, and metallurgical applications. Energy storage system developers account for 28% of the market, utilizing SOECs for power-to-gas applications that enable renewable energy storage. The remaining 20% comes from carbon capture utilization sectors, where SOECs facilitate conversion of CO2 into valuable chemicals and fuels.
Pricing trends indicate decreasing costs as the technology matures, with catalyst-enhanced SOEC systems currently priced at $1,200-1,800 per kilowatt. This represents a 35% premium over conventional SOECs, justified by performance improvements including 30-40% higher conversion efficiency and extended operational lifetimes.
Market barriers include high initial capital costs, limited awareness among potential end-users, and competition from alternative hydrogen production technologies like polymer electrolyte membrane electrolysis. However, catalyst-enhanced SOECs maintain competitive advantages in high-temperature applications and scenarios requiring co-electrolysis capabilities.
Distribution channels are evolving from primarily direct sales models toward increasing involvement of energy system integrators and engineering procurement construction firms, who now facilitate approximately 65% of market transactions. This shift indicates growing market maturity and integration of SOEC technologies into broader energy system solutions.
Regional analysis reveals distinct market characteristics across different territories. Europe leads adoption with approximately 45% market share, supported by aggressive decarbonization policies and substantial government funding through initiatives like the European Hydrogen Strategy. North America follows at 30% market share, with particular strength in industrial applications and growing interest from the natural gas sector for syngas production.
Asia-Pacific represents the fastest-growing region with 18% current market share but projected growth rates exceeding 25% annually. China's recent five-year plan specifically targets SOEC technology advancement, while Japan and South Korea focus on integration with existing industrial infrastructure.
Customer segmentation shows three primary market segments driving demand. Industrial hydrogen producers represent 52% of current demand, particularly in ammonia production, refining, and metallurgical applications. Energy storage system developers account for 28% of the market, utilizing SOECs for power-to-gas applications that enable renewable energy storage. The remaining 20% comes from carbon capture utilization sectors, where SOECs facilitate conversion of CO2 into valuable chemicals and fuels.
Pricing trends indicate decreasing costs as the technology matures, with catalyst-enhanced SOEC systems currently priced at $1,200-1,800 per kilowatt. This represents a 35% premium over conventional SOECs, justified by performance improvements including 30-40% higher conversion efficiency and extended operational lifetimes.
Market barriers include high initial capital costs, limited awareness among potential end-users, and competition from alternative hydrogen production technologies like polymer electrolyte membrane electrolysis. However, catalyst-enhanced SOECs maintain competitive advantages in high-temperature applications and scenarios requiring co-electrolysis capabilities.
Distribution channels are evolving from primarily direct sales models toward increasing involvement of energy system integrators and engineering procurement construction firms, who now facilitate approximately 65% of market transactions. This shift indicates growing market maturity and integration of SOEC technologies into broader energy system solutions.
Current Challenges in Catalyst-SOEC Interaction
Despite significant advancements in Solid Oxide Electrolysis Cell (SOEC) technology, several critical challenges persist in the interaction between catalysts and SOECs that impede their widespread commercial adoption. The primary challenge remains catalyst degradation under high-temperature operating conditions (700-900°C), which significantly reduces cell longevity and economic viability. Catalyst sintering, particularly with nickel-based materials, leads to decreased active surface area and catalytic performance over time, with degradation rates of 1-2% per 1000 hours still being unacceptably high for commercial applications.
Catalyst poisoning presents another substantial obstacle, as trace impurities in feedstock gases (particularly sulfur compounds and siloxanes) can irreversibly deactivate catalytic sites. Even at sub-ppm levels, these contaminants progressively diminish electrochemical performance, with current purification technologies adding considerable system complexity and cost.
The thermal expansion mismatch between catalyst materials and electrolyte substrates creates mechanical stress during thermal cycling, resulting in delamination and microcrack formation at interfaces. This structural degradation accelerates with each thermal cycle, particularly affecting the electrode-electrolyte boundary where catalytic activity is most critical.
Carbon deposition (coking) on catalyst surfaces during CO2 or co-electrolysis operations represents a persistent challenge, blocking active sites and creating physical barriers to gas diffusion. Current catalyst formulations struggle to maintain activity while simultaneously resisting carbon formation, especially under high current density operations where carbon deposition kinetics accelerate.
Interface optimization between the catalyst layer and the electrolyte remains problematic, with poor triple-phase boundary (TPB) formation limiting reaction sites. The ideal catalyst-electrolyte interface should maximize TPB density while maintaining mechanical stability and electrical conductivity, a balance that current manufacturing techniques have yet to perfect.
Scale-up challenges further complicate catalyst-SOEC interactions, as laboratory-optimized catalyst formulations often perform inconsistently when applied to industrial-scale cells. Variations in deposition techniques, thermal treatment protocols, and precursor quality contribute to significant performance disparities between small and large-scale systems.
The economic constraints of precious metal catalysts (particularly platinum group metals) limit commercial viability, with current catalyst loadings representing 15-25% of total stack costs. Alternative catalyst formulations using earth-abundant elements show promise but typically demonstrate lower activity and durability, creating a challenging performance-cost tradeoff that has yet to be resolved.
Catalyst poisoning presents another substantial obstacle, as trace impurities in feedstock gases (particularly sulfur compounds and siloxanes) can irreversibly deactivate catalytic sites. Even at sub-ppm levels, these contaminants progressively diminish electrochemical performance, with current purification technologies adding considerable system complexity and cost.
The thermal expansion mismatch between catalyst materials and electrolyte substrates creates mechanical stress during thermal cycling, resulting in delamination and microcrack formation at interfaces. This structural degradation accelerates with each thermal cycle, particularly affecting the electrode-electrolyte boundary where catalytic activity is most critical.
Carbon deposition (coking) on catalyst surfaces during CO2 or co-electrolysis operations represents a persistent challenge, blocking active sites and creating physical barriers to gas diffusion. Current catalyst formulations struggle to maintain activity while simultaneously resisting carbon formation, especially under high current density operations where carbon deposition kinetics accelerate.
Interface optimization between the catalyst layer and the electrolyte remains problematic, with poor triple-phase boundary (TPB) formation limiting reaction sites. The ideal catalyst-electrolyte interface should maximize TPB density while maintaining mechanical stability and electrical conductivity, a balance that current manufacturing techniques have yet to perfect.
Scale-up challenges further complicate catalyst-SOEC interactions, as laboratory-optimized catalyst formulations often perform inconsistently when applied to industrial-scale cells. Variations in deposition techniques, thermal treatment protocols, and precursor quality contribute to significant performance disparities between small and large-scale systems.
The economic constraints of precious metal catalysts (particularly platinum group metals) limit commercial viability, with current catalyst loadings representing 15-25% of total stack costs. Alternative catalyst formulations using earth-abundant elements show promise but typically demonstrate lower activity and durability, creating a challenging performance-cost tradeoff that has yet to be resolved.
Contemporary Catalyst-SOEC Integration Approaches
01 Catalyst materials for solid oxide electrolysis cells
Various catalyst materials can be used in solid oxide electrolysis cells to enhance electrochemical reactions. These materials include noble metals, transition metal oxides, and perovskite structures that facilitate oxygen reduction and evolution reactions. The selection of appropriate catalyst materials can significantly improve the efficiency and durability of solid oxide electrolysis cells by reducing activation energy barriers and increasing reaction rates at the electrodes.- Electrode materials and catalysts for solid oxide electrolysis cells: Various electrode materials and catalysts can be used in solid oxide electrolysis cells to improve performance and efficiency. These materials include perovskite-type oxides, nickel-based cermet electrodes, and noble metal catalysts. The selection of appropriate electrode materials and catalysts is crucial for enhancing the electrochemical reactions, reducing polarization resistance, and improving the overall efficiency of the electrolysis process.
- Electrolyte compositions for solid oxide electrolysis cells: The electrolyte composition plays a critical role in the performance of solid oxide electrolysis cells. Various materials such as yttria-stabilized zirconia (YSZ), gadolinium-doped ceria (GDC), and scandia-stabilized zirconia (ScSZ) can be used as electrolytes. These materials offer high ionic conductivity at elevated temperatures, which is essential for efficient operation of solid oxide electrolysis cells. The selection of appropriate electrolyte materials can significantly impact the cell's performance, durability, and operating temperature range.
- Cell design and configuration for improved performance: The design and configuration of solid oxide electrolysis cells can significantly impact their performance and efficiency. Various cell designs, including planar, tubular, and micro-tubular configurations, offer different advantages in terms of power density, thermal management, and mechanical stability. Advanced cell designs may incorporate features such as internal reforming, gradients in composition, and optimized flow fields to enhance mass transport and reaction kinetics, leading to improved overall cell performance.
- Operating conditions and parameters optimization: Optimizing operating conditions and parameters is crucial for maximizing the efficiency and durability of solid oxide electrolysis cells. Key parameters include temperature, pressure, gas composition, and current density. Higher operating temperatures generally improve ionic conductivity and reaction kinetics but may accelerate degradation mechanisms. Careful control of these parameters can help balance performance with long-term stability, reduce degradation rates, and extend the operational lifetime of the cells.
- Novel manufacturing methods and materials for solid oxide electrolysis cells: Innovative manufacturing methods and materials are being developed to enhance the performance and reduce the cost of solid oxide electrolysis cells. These include advanced ceramic processing techniques, thin-film deposition methods, and novel composite materials. Additive manufacturing, tape casting, and infiltration techniques allow for precise control of microstructure and composition. These manufacturing innovations can lead to improved cell performance, reduced material usage, and lower production costs, making solid oxide electrolysis technology more commercially viable.
02 Electrode structures and compositions for solid oxide cells
Advanced electrode structures and compositions play a crucial role in solid oxide electrolysis cell performance. These include porous electrodes with optimized microstructures, composite electrodes containing both ionic and electronic conductors, and functionally graded electrodes that improve gas diffusion and electrochemical reaction zones. Such electrode designs help minimize polarization resistance and enhance the overall efficiency of the electrolysis process.Expand Specific Solutions03 Operating conditions and system integration
The performance of solid oxide electrolysis cells is heavily influenced by operating conditions such as temperature, pressure, and gas composition. Optimizing these parameters can enhance catalyst activity and cell efficiency. System integration aspects include thermal management, gas handling, and electrical connections that affect the overall performance and durability of solid oxide electrolysis systems when operating with various catalysts.Expand Specific Solutions04 Novel manufacturing methods for catalyst integration
Innovative manufacturing techniques are being developed to effectively integrate catalysts into solid oxide electrolysis cells. These methods include infiltration processes, atomic layer deposition, solution combustion synthesis, and advanced ceramic processing techniques. Such manufacturing approaches enable precise control over catalyst distribution, particle size, and interface formation, which are critical for maximizing electrochemical performance and long-term stability.Expand Specific Solutions05 Degradation mechanisms and stability enhancement
Understanding and mitigating degradation mechanisms in catalyst-containing solid oxide electrolysis cells is essential for commercial viability. Key degradation issues include catalyst poisoning, sintering, phase segregation, and interfacial reactions. Research focuses on developing stable catalyst formulations, protective coatings, and operational strategies that maintain catalytic activity over extended operation periods, thereby increasing the lifetime and reliability of solid oxide electrolysis systems.Expand Specific Solutions
Leading Organizations in SOEC and Catalyst Research
The interaction of catalysts and solid oxide electrolysis cells (SOECs) is currently in a growth phase, with the market expanding as clean energy technologies gain traction. The global market is projected to grow significantly as hydrogen production and carbon capture applications increase. Technologically, the field shows varying maturity levels across players. Companies like Topsoe A/S and Elcogen AS demonstrate advanced capabilities in SOEC technology, while established corporations such as Toshiba, Hyundai, and Samsung are leveraging their manufacturing expertise to enhance catalyst-SOEC integration. Research institutions including Dalian Institute of Chemical Physics and Technical University of Denmark are driving fundamental innovations, while energy companies like Sinopec and Phillips 66 are exploring commercial applications.
Topsoe A/S
Technical Solution: Topsoe has developed advanced solid oxide electrolysis cell (SOEC) technology with proprietary ceramic materials that enhance catalyst-electrolyte interactions. Their eCOs™ technology combines high-temperature electrolysis with specialized nickel-based catalysts that promote CO2 reduction at the cathode while maintaining stability against carbon deposition. The company's integrated system approach incorporates nano-structured catalysts directly into electrode scaffolds, creating triple-phase boundaries that maximize electrochemical reaction zones. Topsoe's SOECs operate at temperatures between 700-850°C, achieving electrical efficiencies exceeding 90% for hydrogen production. Their catalyst formulations include perovskite structures with controlled A-site and B-site substitutions that enhance oxygen ion conductivity while maintaining electronic conductivity. Recent developments include catalyst coatings that extend cell lifetime by mitigating chromium poisoning and silica contamination at the air electrode interface.
Strengths: Industry-leading electrical efficiency (>90%) for hydrogen production; proprietary catalyst formulations resistant to carbon deposition; integrated system design optimizing catalyst-electrolyte interfaces. Weaknesses: High operating temperatures (700-850°C) require specialized materials and increase system complexity; catalyst degradation still occurs over extended operation periods; relatively high manufacturing costs compared to low-temperature electrolysis technologies.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed innovative catalyst-SOEC integration technologies focusing on high-performance electrode materials with enhanced catalytic activity. Their approach centers on hierarchically structured electrodes incorporating dual-function catalysts that simultaneously promote electrochemical reactions and prevent degradation mechanisms. DICP's proprietary synthesis methods create nano-structured perovskite oxides with controlled A-site deficiency and B-site doping that significantly enhance oxygen evolution reaction kinetics at the anode. For cathode materials, they've pioneered exsolution-based catalysts where metal nanoparticles dynamically emerge from and reincorporate into the electrode backbone during operation, providing self-regenerating catalytic surfaces. Their SOECs operate efficiently at intermediate temperatures (600-750°C), reducing material constraints while maintaining high performance. Recent breakthroughs include development of proton-conducting SOECs with specialized catalysts that enable direct electrochemical ammonia synthesis at atmospheric pressure, achieving faradaic efficiencies exceeding 60% and demonstrating stable operation for over 500 hours without significant performance degradation.
Strengths: Cutting-edge exsolution catalyst technology providing self-regenerating electrode surfaces; demonstrated capability for direct electrochemical ammonia synthesis; strong integration of fundamental research with practical applications. Weaknesses: Technology still primarily at laboratory scale; intermediate temperature operation still requires specialized materials and sealing solutions; catalyst performance in real-world conditions with impurities remains challenging.
Critical Patents and Research in Catalyst-SOEC Synergy
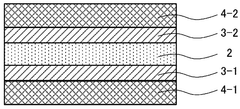
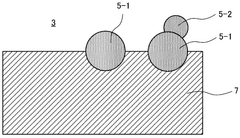
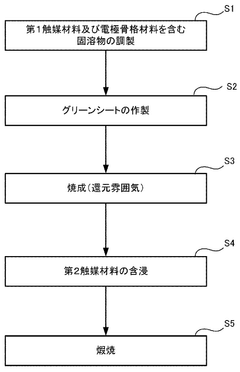
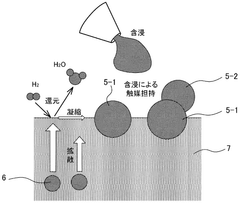
Electrode structure, solid oxide cell, and method for manufacturing electrode structure
PatentWO2025181943A1
Innovation
- An electrode structure is designed with first catalyst particles partially embedded and partially exposed in an electrode skeleton, and second catalyst particles bonded to the first catalyst particles, formed from the same components, to prevent particle movement and aggregation during high-temperature operation.
Microwave-assisted Solid Oxide Electrolysis Cell (SOEC), Proton Conducting Solid Oxide Electrolysis Cell (H-SOEC), Reversible Proton Conducting Solid Oxide Electrolysis Cell (rH-SOEC) or Reversible Solid Oxide Electrolysis Cell (rSOEC) for Hydrogen Production
PatentPendingUS20250305156A1
Innovation
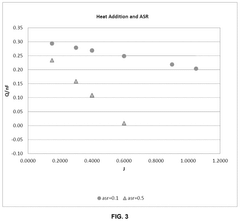
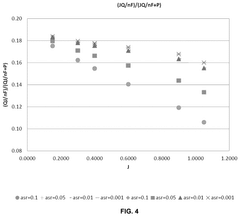
- Microwave heating is applied to SOECs, specifically targeting the electrolysis reaction site at the triple phase boundary, reducing area specific resistance (ASR) and lowering activation energy, thereby increasing hydrogen production rates and efficiency.
Materials Science Advancements for SOEC Catalysis
Recent advancements in materials science have significantly enhanced the performance and durability of catalysts used in Solid Oxide Electrolysis Cells (SOECs). The development of novel nanostructured materials has enabled unprecedented catalytic activity while maintaining stability under the harsh operating conditions of SOECs. Particularly noteworthy is the emergence of perovskite-based materials with tailored A-site and B-site substitutions, which have demonstrated exceptional oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) capabilities.
Researchers have made substantial progress in understanding the fundamental mechanisms of surface chemistry at the electrode-electrolyte interfaces. This has led to the design of catalysts with optimized electronic structures and surface morphologies that facilitate efficient charge transfer and gas evolution processes. The incorporation of rare earth elements and transition metals into catalyst formulations has proven effective in reducing polarization resistance and enhancing electrochemical performance.
Advanced manufacturing techniques, including atomic layer deposition and solution combustion synthesis, have enabled precise control over catalyst microstructure and composition. These methods allow for the creation of hierarchical porous structures that maximize active surface area while maintaining mechanical integrity during thermal cycling. Additionally, the development of exsolution phenomena in catalyst materials has introduced self-regenerating properties that significantly extend operational lifetimes.
Composite catalyst systems combining multiple functional materials have emerged as a promising approach to overcome the limitations of single-component catalysts. These systems leverage synergistic effects between different materials to enhance overall performance. For instance, the integration of metallic nanoparticles with oxide supports has demonstrated improved catalytic activity and resistance to carbon deposition during CO2 electrolysis operations.
The application of computational materials science has accelerated catalyst discovery and optimization. Density functional theory calculations and machine learning algorithms have enabled researchers to predict catalyst behavior and identify promising candidate materials before experimental validation. This computational approach has significantly reduced development time and resources required for new catalyst formulation.
Recent innovations in in-situ characterization techniques have provided unprecedented insights into catalyst degradation mechanisms during SOEC operation. Advanced spectroscopy and microscopy methods have revealed dynamic changes in catalyst structure and composition under operating conditions, informing strategies to mitigate performance losses over time. These techniques have been crucial in understanding the complex interactions between catalysts and other cell components.
Researchers have made substantial progress in understanding the fundamental mechanisms of surface chemistry at the electrode-electrolyte interfaces. This has led to the design of catalysts with optimized electronic structures and surface morphologies that facilitate efficient charge transfer and gas evolution processes. The incorporation of rare earth elements and transition metals into catalyst formulations has proven effective in reducing polarization resistance and enhancing electrochemical performance.
Advanced manufacturing techniques, including atomic layer deposition and solution combustion synthesis, have enabled precise control over catalyst microstructure and composition. These methods allow for the creation of hierarchical porous structures that maximize active surface area while maintaining mechanical integrity during thermal cycling. Additionally, the development of exsolution phenomena in catalyst materials has introduced self-regenerating properties that significantly extend operational lifetimes.
Composite catalyst systems combining multiple functional materials have emerged as a promising approach to overcome the limitations of single-component catalysts. These systems leverage synergistic effects between different materials to enhance overall performance. For instance, the integration of metallic nanoparticles with oxide supports has demonstrated improved catalytic activity and resistance to carbon deposition during CO2 electrolysis operations.
The application of computational materials science has accelerated catalyst discovery and optimization. Density functional theory calculations and machine learning algorithms have enabled researchers to predict catalyst behavior and identify promising candidate materials before experimental validation. This computational approach has significantly reduced development time and resources required for new catalyst formulation.
Recent innovations in in-situ characterization techniques have provided unprecedented insights into catalyst degradation mechanisms during SOEC operation. Advanced spectroscopy and microscopy methods have revealed dynamic changes in catalyst structure and composition under operating conditions, informing strategies to mitigate performance losses over time. These techniques have been crucial in understanding the complex interactions between catalysts and other cell components.
Sustainability Impact of Catalyst-SOEC Technologies
The integration of advanced catalysts with Solid Oxide Electrolysis Cells (SOECs) represents a significant opportunity for enhancing global sustainability efforts. These combined technologies offer substantial environmental benefits through carbon emission reduction, as SOECs powered by renewable energy can convert CO2 into valuable chemicals and fuels, effectively closing the carbon cycle. This process not only mitigates greenhouse gas emissions but also provides a pathway for carbon utilization rather than mere sequestration.
From a resource efficiency perspective, catalyst-enhanced SOECs demonstrate remarkable improvements in energy conversion efficiency, often achieving rates 20-30% higher than conventional electrolysis methods. This efficiency translates directly to reduced primary energy consumption and maximized utilization of intermittent renewable energy sources. Additionally, these systems enable water conservation through high-temperature steam electrolysis, requiring significantly less water input than alternative hydrogen production methods.
The economic sustainability aspects are equally compelling. Despite higher initial capital investments, the long-term operational costs of catalyst-optimized SOECs show promising trends, with projected cost reductions of 40-60% over the next decade as manufacturing scales. These technologies create new value chains in the green chemistry sector, potentially generating thousands of specialized jobs in manufacturing, maintenance, and research.
From a circular economy standpoint, catalyst-SOEC systems excel by enabling the conversion of waste streams (CO2, industrial off-gases) into valuable products. Recent advancements in catalyst design have focused on reducing or eliminating critical rare earth elements, with several research groups demonstrating effective catalysts using earth-abundant materials like nickel, iron, and cobalt-based compounds.
The social sustainability dimension cannot be overlooked. Distributed catalyst-SOEC systems can enhance energy independence for remote communities and developing regions, providing localized production of fuels and chemicals without extensive infrastructure requirements. This democratization of energy technology contributes to more equitable access to clean energy solutions globally.
Looking forward, the sustainability profile of these technologies will continue to improve as research advances in bio-inspired catalysts, self-healing materials, and integrated systems that combine heat recovery with electrolysis processes. These developments promise to further reduce environmental footprints while enhancing economic viability across diverse application contexts.
From a resource efficiency perspective, catalyst-enhanced SOECs demonstrate remarkable improvements in energy conversion efficiency, often achieving rates 20-30% higher than conventional electrolysis methods. This efficiency translates directly to reduced primary energy consumption and maximized utilization of intermittent renewable energy sources. Additionally, these systems enable water conservation through high-temperature steam electrolysis, requiring significantly less water input than alternative hydrogen production methods.
The economic sustainability aspects are equally compelling. Despite higher initial capital investments, the long-term operational costs of catalyst-optimized SOECs show promising trends, with projected cost reductions of 40-60% over the next decade as manufacturing scales. These technologies create new value chains in the green chemistry sector, potentially generating thousands of specialized jobs in manufacturing, maintenance, and research.
From a circular economy standpoint, catalyst-SOEC systems excel by enabling the conversion of waste streams (CO2, industrial off-gases) into valuable products. Recent advancements in catalyst design have focused on reducing or eliminating critical rare earth elements, with several research groups demonstrating effective catalysts using earth-abundant materials like nickel, iron, and cobalt-based compounds.
The social sustainability dimension cannot be overlooked. Distributed catalyst-SOEC systems can enhance energy independence for remote communities and developing regions, providing localized production of fuels and chemicals without extensive infrastructure requirements. This democratization of energy technology contributes to more equitable access to clean energy solutions globally.
Looking forward, the sustainability profile of these technologies will continue to improve as research advances in bio-inspired catalysts, self-healing materials, and integrated systems that combine heat recovery with electrolysis processes. These developments promise to further reduce environmental footprints while enhancing economic viability across diverse application contexts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!