Solid oxide electrolysis cells’ influence on sustainable energy policies
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SOEC Technology Background and Objectives
Solid oxide electrolysis cells (SOECs) represent a transformative technology in the global pursuit of sustainable energy systems. Emerging from decades of research in high-temperature electrochemical systems, SOECs have evolved from laboratory curiosities to commercially viable solutions for energy conversion and storage. The technology leverages the reverse operation of solid oxide fuel cells, utilizing electrical energy to split water or carbon dioxide into hydrogen or carbon monoxide, respectively, at high operating temperatures typically between 700-900°C.
The historical development of SOEC technology traces back to the 1980s, with significant acceleration in research intensity occurring in the early 2000s as concerns about climate change and energy security gained prominence. The technology has witnessed remarkable improvements in durability, efficiency, and cost-effectiveness over the past decade, positioning it as a critical component in the transition toward renewable energy systems.
Current technological objectives for SOEC development focus on several key areas. First, enhancing long-term stability and durability remains paramount, with researchers targeting operational lifetimes exceeding 40,000 hours for commercial viability. Second, reducing manufacturing costs through materials innovation and process optimization is essential for widespread adoption. Third, improving system integration capabilities to enable flexible operation with intermittent renewable energy sources represents a critical technical goal.
The technology aims to achieve electrical efficiency exceeding 80% for hydrogen production, substantially outperforming alternative electrolysis technologies. Additionally, SOECs offer unique capabilities for co-electrolysis of water and carbon dioxide, enabling the production of synthetic fuels through power-to-X pathways—a feature increasingly recognized as vital for sectors difficult to electrify directly.
From a policy perspective, SOEC technology aligns with multiple sustainable development objectives. It enables large-scale seasonal energy storage through hydrogen or synthetic methane production, addresses grid balancing challenges associated with variable renewable energy sources, and provides pathways for industrial decarbonization in sectors like steel, chemicals, and cement manufacturing.
The convergence of technological maturation, declining renewable electricity costs, and increasingly ambitious climate policies has created a favorable environment for SOEC deployment. Research institutions and industrial players worldwide are now focusing on scaling up the technology from laboratory demonstrations to industrial applications, with particular emphasis on integration with renewable energy systems and existing industrial processes.
The historical development of SOEC technology traces back to the 1980s, with significant acceleration in research intensity occurring in the early 2000s as concerns about climate change and energy security gained prominence. The technology has witnessed remarkable improvements in durability, efficiency, and cost-effectiveness over the past decade, positioning it as a critical component in the transition toward renewable energy systems.
Current technological objectives for SOEC development focus on several key areas. First, enhancing long-term stability and durability remains paramount, with researchers targeting operational lifetimes exceeding 40,000 hours for commercial viability. Second, reducing manufacturing costs through materials innovation and process optimization is essential for widespread adoption. Third, improving system integration capabilities to enable flexible operation with intermittent renewable energy sources represents a critical technical goal.
The technology aims to achieve electrical efficiency exceeding 80% for hydrogen production, substantially outperforming alternative electrolysis technologies. Additionally, SOECs offer unique capabilities for co-electrolysis of water and carbon dioxide, enabling the production of synthetic fuels through power-to-X pathways—a feature increasingly recognized as vital for sectors difficult to electrify directly.
From a policy perspective, SOEC technology aligns with multiple sustainable development objectives. It enables large-scale seasonal energy storage through hydrogen or synthetic methane production, addresses grid balancing challenges associated with variable renewable energy sources, and provides pathways for industrial decarbonization in sectors like steel, chemicals, and cement manufacturing.
The convergence of technological maturation, declining renewable electricity costs, and increasingly ambitious climate policies has created a favorable environment for SOEC deployment. Research institutions and industrial players worldwide are now focusing on scaling up the technology from laboratory demonstrations to industrial applications, with particular emphasis on integration with renewable energy systems and existing industrial processes.
Market Demand for Green Hydrogen Production
The global market for green hydrogen production is experiencing unprecedented growth, driven primarily by the urgent need to decarbonize energy-intensive industries and transportation sectors. Solid oxide electrolysis cells (SOECs) have emerged as a pivotal technology in this landscape, offering higher efficiency rates compared to conventional alkaline and PEM electrolyzers when operating at elevated temperatures. Current market projections indicate that green hydrogen demand could reach 530 million tons by 2050, representing a substantial portion of the global energy mix.
Industrial sectors constitute the largest demand segment for green hydrogen, particularly in hard-to-abate industries such as steel manufacturing, ammonia production, and chemical processing. These sectors collectively account for approximately 20% of global carbon emissions and are increasingly seeking viable pathways toward decarbonization. The steel industry alone could potentially consume 160 million tons of hydrogen annually by 2050 if widespread adoption occurs.
Transportation represents another significant market driver, with hydrogen fuel cell vehicles gaining traction in heavy-duty transport applications where battery electric solutions face limitations. Maritime shipping and aviation sectors are also exploring hydrogen and hydrogen-derived fuels as alternatives to conventional fossil fuels, potentially creating substantial demand for SOEC-produced hydrogen in the coming decades.
Regional market dynamics reveal varying adoption rates and policy frameworks. The European Union leads with ambitious targets under its Hydrogen Strategy, aiming to install 40GW of electrolyzer capacity by 2030. Asia-Pacific markets, particularly Japan, South Korea, and increasingly China, are developing robust hydrogen economies supported by government initiatives and industrial consortia. North America is accelerating investments following recent policy developments like the Inflation Reduction Act in the United States.
Economic factors significantly influence market development, with production costs remaining a critical barrier. Current green hydrogen production costs range between $3-8 per kilogram, substantially higher than gray hydrogen produced from natural gas. However, SOEC technology offers promising cost reduction potential through higher electrical efficiency and the ability to utilize waste heat, potentially reducing production costs by 20-30% compared to low-temperature electrolysis methods.
Market forecasts suggest the global electrolyzer market could grow at a CAGR exceeding 50% through 2030, with SOEC technology capturing an increasing market share as technological maturity improves. This growth trajectory is further supported by over $300 billion in announced investments across the hydrogen value chain, with significant portions dedicated to production technologies including advanced electrolysis systems.
Industrial sectors constitute the largest demand segment for green hydrogen, particularly in hard-to-abate industries such as steel manufacturing, ammonia production, and chemical processing. These sectors collectively account for approximately 20% of global carbon emissions and are increasingly seeking viable pathways toward decarbonization. The steel industry alone could potentially consume 160 million tons of hydrogen annually by 2050 if widespread adoption occurs.
Transportation represents another significant market driver, with hydrogen fuel cell vehicles gaining traction in heavy-duty transport applications where battery electric solutions face limitations. Maritime shipping and aviation sectors are also exploring hydrogen and hydrogen-derived fuels as alternatives to conventional fossil fuels, potentially creating substantial demand for SOEC-produced hydrogen in the coming decades.
Regional market dynamics reveal varying adoption rates and policy frameworks. The European Union leads with ambitious targets under its Hydrogen Strategy, aiming to install 40GW of electrolyzer capacity by 2030. Asia-Pacific markets, particularly Japan, South Korea, and increasingly China, are developing robust hydrogen economies supported by government initiatives and industrial consortia. North America is accelerating investments following recent policy developments like the Inflation Reduction Act in the United States.
Economic factors significantly influence market development, with production costs remaining a critical barrier. Current green hydrogen production costs range between $3-8 per kilogram, substantially higher than gray hydrogen produced from natural gas. However, SOEC technology offers promising cost reduction potential through higher electrical efficiency and the ability to utilize waste heat, potentially reducing production costs by 20-30% compared to low-temperature electrolysis methods.
Market forecasts suggest the global electrolyzer market could grow at a CAGR exceeding 50% through 2030, with SOEC technology capturing an increasing market share as technological maturity improves. This growth trajectory is further supported by over $300 billion in announced investments across the hydrogen value chain, with significant portions dedicated to production technologies including advanced electrolysis systems.
SOEC Development Status and Technical Barriers
Solid Oxide Electrolysis Cells (SOECs) have emerged as a promising technology for sustainable energy conversion, yet their widespread implementation faces significant technical barriers. Currently, SOECs operate at high temperatures (700-900°C), which necessitates expensive materials and complex thermal management systems. This temperature requirement represents one of the primary technical challenges limiting commercial viability.
Material degradation presents another substantial barrier to SOEC advancement. The harsh operating conditions lead to accelerated degradation of electrodes and electrolytes, resulting in performance losses of 1-2% per 1000 hours of operation—significantly higher than the 0.1-0.2% target needed for commercial applications. Chromium poisoning from interconnect materials and delamination at electrode-electrolyte interfaces further exacerbate durability issues.
Scalability remains problematic for SOEC technology. While laboratory demonstrations have shown promising results, scaling to industrial levels introduces challenges in maintaining uniform temperature distribution, gas flow, and electrical current across larger cell areas. Manufacturing processes for large-scale production lack standardization, contributing to inconsistent performance and high production costs.
System integration represents another critical barrier. SOECs require sophisticated balance-of-plant components including heat exchangers, gas handling systems, and power electronics. The complexity of these systems increases capital costs and reduces overall system efficiency, currently limiting total system efficiency to 70-80% compared to theoretical potentials exceeding 90%.
Economic viability continues to challenge SOEC deployment. Current capital costs range from $2,000-5,000/kW, significantly higher than the $500-800/kW target needed to compete with alternative hydrogen production methods. The high-temperature ceramic materials, precious metal catalysts, and complex manufacturing processes all contribute to these elevated costs.
Internationally, research efforts show geographic concentration. Europe leads SOEC development through initiatives like the Horizon Europe program, with significant contributions from Denmark's Technical University and Germany's Forschungszentrum Jülich. In Asia, Japan and China have established robust research programs, while North American efforts are primarily concentrated at institutions like Idaho National Laboratory and Northwestern University.
Recent technological advancements have focused on intermediate-temperature SOECs (500-700°C), proton-conducting electrolytes, and novel electrode materials to address degradation issues. However, these solutions remain largely at the laboratory scale, with technology readiness levels typically between 4-6, indicating significant development is still required before commercial readiness.
Material degradation presents another substantial barrier to SOEC advancement. The harsh operating conditions lead to accelerated degradation of electrodes and electrolytes, resulting in performance losses of 1-2% per 1000 hours of operation—significantly higher than the 0.1-0.2% target needed for commercial applications. Chromium poisoning from interconnect materials and delamination at electrode-electrolyte interfaces further exacerbate durability issues.
Scalability remains problematic for SOEC technology. While laboratory demonstrations have shown promising results, scaling to industrial levels introduces challenges in maintaining uniform temperature distribution, gas flow, and electrical current across larger cell areas. Manufacturing processes for large-scale production lack standardization, contributing to inconsistent performance and high production costs.
System integration represents another critical barrier. SOECs require sophisticated balance-of-plant components including heat exchangers, gas handling systems, and power electronics. The complexity of these systems increases capital costs and reduces overall system efficiency, currently limiting total system efficiency to 70-80% compared to theoretical potentials exceeding 90%.
Economic viability continues to challenge SOEC deployment. Current capital costs range from $2,000-5,000/kW, significantly higher than the $500-800/kW target needed to compete with alternative hydrogen production methods. The high-temperature ceramic materials, precious metal catalysts, and complex manufacturing processes all contribute to these elevated costs.
Internationally, research efforts show geographic concentration. Europe leads SOEC development through initiatives like the Horizon Europe program, with significant contributions from Denmark's Technical University and Germany's Forschungszentrum Jülich. In Asia, Japan and China have established robust research programs, while North American efforts are primarily concentrated at institutions like Idaho National Laboratory and Northwestern University.
Recent technological advancements have focused on intermediate-temperature SOECs (500-700°C), proton-conducting electrolytes, and novel electrode materials to address degradation issues. However, these solutions remain largely at the laboratory scale, with technology readiness levels typically between 4-6, indicating significant development is still required before commercial readiness.
Current SOEC System Designs and Implementations
01 Electrode materials and structures for SOECs
Various electrode materials and structures are used in solid oxide electrolysis cells to improve performance and durability. These include specialized cathode and anode materials that enhance electrochemical reactions, reduce degradation, and improve conductivity. Advanced electrode structures may incorporate composite materials, nanostructures, or gradient compositions to optimize the triple-phase boundary where electrolyte, electrode, and gas phases meet, which is crucial for efficient operation.- Electrode materials and structures for solid oxide electrolysis cells: Various electrode materials and structures are used in solid oxide electrolysis cells to improve performance and durability. These include specialized cathode and anode materials that enhance electrochemical reactions, reduce degradation, and improve efficiency. Advanced electrode structures may incorporate composite materials, nanostructures, or gradient compositions to optimize ion transport and catalytic activity at operating temperatures.
- Electrolyte compositions for high-temperature operation: Specialized electrolyte materials are developed for solid oxide electrolysis cells that can withstand high operating temperatures while maintaining excellent ionic conductivity. These electrolytes typically include yttria-stabilized zirconia (YSZ), gadolinium-doped ceria (GDC), or other ceramic materials that facilitate oxygen ion transport. Innovations in electrolyte composition focus on reducing operating temperatures while maintaining performance and extending cell lifetime.
- System integration and stack design for solid oxide electrolysis: Advanced stack designs and system integration approaches are crucial for efficient solid oxide electrolysis operation. These include innovative cell stacking methods, sealing technologies, and interconnect designs that minimize electrical resistance and thermal stress. Complete systems incorporate thermal management, gas handling, and control systems to optimize hydrogen or syngas production while ensuring long-term stability and safety during high-temperature operation.
- Co-electrolysis of steam and carbon dioxide: Solid oxide electrolysis cells can be used for co-electrolysis of steam and carbon dioxide to produce syngas (a mixture of hydrogen and carbon monoxide). This approach enables efficient conversion of CO2 and H2O into valuable chemical feedstocks that can be further processed into synthetic fuels or chemicals. The technology leverages the high operating temperature of solid oxide cells to facilitate both electrochemical reactions simultaneously, offering a pathway for carbon utilization and renewable fuel production.
- Degradation mechanisms and durability enhancement: Understanding and mitigating degradation mechanisms is essential for improving the durability of solid oxide electrolysis cells. Research focuses on addressing issues such as chromium poisoning, electrode delamination, and microstructural changes during long-term operation. Protective coatings, modified compositions, and optimized operating protocols are developed to extend cell lifetime and maintain performance under various operating conditions, including thermal cycling and load variations.
02 Electrolyte development for high-temperature operation
Electrolyte materials for solid oxide electrolysis cells are designed to withstand high operating temperatures while maintaining high ionic conductivity and low electronic conductivity. Common materials include yttria-stabilized zirconia (YSZ), gadolinium-doped ceria (GDC), and lanthanum gallate-based compounds. Research focuses on reducing electrolyte thickness to minimize ohmic resistance and developing materials that can operate at intermediate temperatures (600-800°C) to reduce system costs and improve longevity.Expand Specific Solutions03 System integration and stack design
The integration of solid oxide electrolysis cells into complete systems involves careful stack design to maximize efficiency and durability. This includes considerations for gas flow distribution, thermal management, sealing technologies, and electrical connections between cells. Advanced stack designs incorporate features to minimize thermal gradients, manage pressure differentials, and facilitate maintenance. System-level integration also addresses balance-of-plant components such as heat exchangers, gas processing units, and control systems.Expand Specific Solutions04 High-efficiency hydrogen and syngas production
Solid oxide electrolysis cells are utilized for efficient production of hydrogen and synthesis gas (syngas) through high-temperature electrolysis. These systems can achieve higher electrical efficiency compared to low-temperature electrolyzers due to favorable thermodynamics at elevated temperatures. Co-electrolysis of water and carbon dioxide can produce syngas directly, which serves as a precursor for synthetic fuels. Research focuses on optimizing operating conditions, catalyst formulations, and cell configurations to maximize production rates while minimizing energy consumption.Expand Specific Solutions05 Degradation mechanisms and durability enhancement
Understanding and mitigating degradation mechanisms is crucial for improving the long-term durability of solid oxide electrolysis cells. Common degradation issues include electrode poisoning, chromium poisoning from interconnects, delamination at interfaces, and microstructural changes during thermal cycling. Research focuses on developing protective coatings, implementing redox-stable materials, optimizing operating protocols, and introducing self-healing mechanisms to extend cell lifetime under various operating conditions.Expand Specific Solutions
Leading Organizations in SOEC Research and Commercialization
The solid oxide electrolysis cell (SOEC) market is in an early growth phase, with increasing momentum driven by sustainable energy transitions. The global market size is projected to expand significantly as countries pursue decarbonization strategies, though current commercial deployment remains limited. Technologically, SOECs are advancing from laboratory to commercial readiness, with varying maturity levels among key players. Companies like DynElectro ApS are pioneering extended cell lifespans (up to 10 years), while established industrial firms including Air Liquide, Kyocera, and Samsung Electro-Mechanics are leveraging their manufacturing expertise. Academic institutions (Technical University of Denmark, Northwestern University, Tsinghua University) are driving fundamental research, while energy corporations (State Grid Corp. of China, Sinopec, Phillips 66) are exploring integration opportunities for green hydrogen production and carbon utilization applications.
DynElectro ApS
Technical Solution: DynElectro has pioneered a dynamic electrolysis technology platform specifically optimized for solid oxide electrolysis cells (SOECs). Their proprietary DynElectro system employs advanced ceramic composite electrodes with nano-engineered interfaces that significantly enhance electrochemical performance while reducing degradation rates. The company's SOECs operate at temperatures between 650-800°C, achieving hydrogen production rates of 3-4 Nm³/h per stack with electrical efficiency exceeding 85% when coupled with waste heat integration. Their modular stack design incorporates innovative sealing solutions that address thermal cycling challenges, enabling rapid start-up capabilities (under 30 minutes) compared to conventional SOECs. DynElectro's technology platform includes intelligent control systems that dynamically adjust operating parameters based on available renewable energy inputs, making their solution particularly valuable for grid balancing applications. The company has demonstrated successful integration with wind and solar power sources, providing a comprehensive solution for converting excess renewable electricity into storable hydrogen.
Strengths: Exceptional dynamic operation capability allowing rapid response to fluctuating renewable energy inputs; advanced materials science expertise resulting in reduced degradation rates; modular design enabling scalable deployment. Weaknesses: Limited commercial-scale installations compared to industry leaders; higher initial capital costs than alternative electrolysis technologies; technology still requires specialized expertise for maintenance and operation.
Air Liquide SA
Technical Solution: Air Liquide has developed an integrated SOEC technology platform focused on industrial-scale hydrogen and syngas production. Their system utilizes proprietary ceramic materials with enhanced ionic conductivity, operating at temperatures between 700-850°C to achieve electricity-to-hydrogen conversion efficiencies of up to 84%. Air Liquide's SOEC stacks incorporate advanced interconnect designs with protective coatings that significantly reduce chromium poisoning and extend operational lifetimes to over 20,000 hours. The company has successfully demonstrated co-electrolysis capabilities, simultaneously converting steam and CO2 into syngas (H2 + CO) with precise composition control, enabling direct integration with downstream synthetic fuel production processes. Their modular system architecture allows for scalable installations from 1 MW to 100+ MW, with integrated heat recovery systems that utilize waste heat from adjacent industrial processes to provide the thermal energy required for high-temperature electrolysis. Air Liquide has implemented advanced manufacturing techniques that reduce production costs while maintaining high performance standards, positioning their SOEC technology as economically competitive with conventional hydrogen production methods when operated with renewable electricity.
Strengths: Extensive industrial gas expertise enabling seamless integration with existing hydrogen infrastructure; proven co-electrolysis capabilities for syngas production; robust supply chain and manufacturing capabilities supporting commercial deployment. Weaknesses: Higher capital expenditure compared to alkaline electrolyzers; complex system integration requirements; technology performance heavily dependent on stable high-temperature operation.
Key Innovations in SOEC Materials and Efficiency
Solid oxide cell systems
PatentPendingEP4611079A1
Innovation
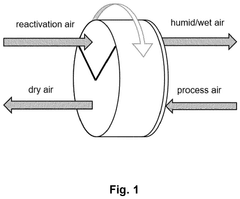
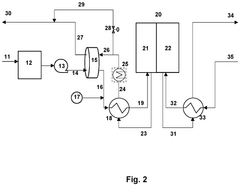
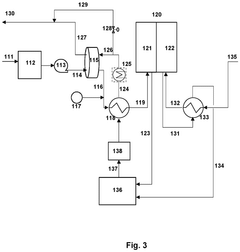
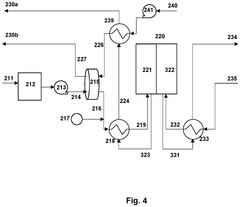
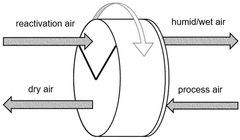
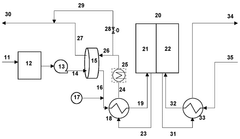
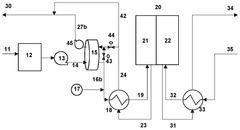
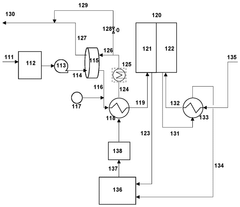
- Integration of a low-pressure desiccant dehumidifier and air recuperator in the SOC system, utilizing waste heat for desiccant regeneration and eliminating the need for separate heaters or blowers, with optimized thermal management to dehumidify process air and prevent chromium migration.
Solid oxide cell systems
PatentWO2025181156A1
Innovation
- Integration of a low-pressure desiccant dehumidifier and air recuperator in the SOC system, utilizing waste heat for desiccant regeneration and eliminating the need for separate heaters or blowers, optimizing thermal management and reducing cell/stack degradation.
Policy Framework Impact on SOEC Deployment
The policy landscape surrounding Solid Oxide Electrolysis Cells (SOECs) significantly influences their deployment trajectory and market penetration. Current regulatory frameworks in leading regions demonstrate varying approaches to SOEC integration. The European Union has established comprehensive support mechanisms through its Hydrogen Strategy, incorporating SOECs within broader renewable energy directives and providing substantial funding through programs like Horizon Europe and the Innovation Fund. These policies create market certainty through feed-in tariffs for green hydrogen and investment subsidies for SOEC installations, effectively reducing capital barriers.
In contrast, the United States has adopted a more market-driven approach with the Inflation Reduction Act offering production tax credits for clean hydrogen, though lacking SOEC-specific provisions. This creates a technology-agnostic competitive environment where SOECs must demonstrate economic advantages against alternative hydrogen production methods. Japan and South Korea have implemented targeted industrial policies that position SOECs within their national hydrogen roadmaps, providing direct research funding and demonstration project support.
Policy stability represents a critical factor affecting SOEC deployment. Historical analysis reveals that fluctuating renewable energy policies have disrupted investment cycles in adjacent technologies. Long-term policy commitments with clear decarbonization targets provide the investment security necessary for SOEC manufacturing scale-up and infrastructure development. The experience of Denmark, where consistent policy support has fostered a robust SOEC innovation ecosystem, demonstrates this principle effectively.
Regulatory barriers persist across jurisdictions, particularly regarding grid connection requirements, hydrogen purity standards, and safety protocols. These technical regulations often fail to account for the unique operational characteristics of SOECs, creating compliance challenges that increase deployment costs. Progressive policy frameworks in Germany and Australia have addressed these barriers by implementing regulatory sandboxes that allow controlled SOEC deployment with adaptive compliance pathways.
Cross-sectoral policy coordination emerges as another critical factor, as SOECs operate at the intersection of electricity, gas, and industrial sectors. Policies that harmonize regulations across these domains—such as integrated energy planning frameworks in Denmark and the Netherlands—have proven most effective at enabling SOEC deployment. These approaches align electricity market rules, gas infrastructure standards, and industrial decarbonization incentives to create coherent market signals for SOEC investors and operators.
In contrast, the United States has adopted a more market-driven approach with the Inflation Reduction Act offering production tax credits for clean hydrogen, though lacking SOEC-specific provisions. This creates a technology-agnostic competitive environment where SOECs must demonstrate economic advantages against alternative hydrogen production methods. Japan and South Korea have implemented targeted industrial policies that position SOECs within their national hydrogen roadmaps, providing direct research funding and demonstration project support.
Policy stability represents a critical factor affecting SOEC deployment. Historical analysis reveals that fluctuating renewable energy policies have disrupted investment cycles in adjacent technologies. Long-term policy commitments with clear decarbonization targets provide the investment security necessary for SOEC manufacturing scale-up and infrastructure development. The experience of Denmark, where consistent policy support has fostered a robust SOEC innovation ecosystem, demonstrates this principle effectively.
Regulatory barriers persist across jurisdictions, particularly regarding grid connection requirements, hydrogen purity standards, and safety protocols. These technical regulations often fail to account for the unique operational characteristics of SOECs, creating compliance challenges that increase deployment costs. Progressive policy frameworks in Germany and Australia have addressed these barriers by implementing regulatory sandboxes that allow controlled SOEC deployment with adaptive compliance pathways.
Cross-sectoral policy coordination emerges as another critical factor, as SOECs operate at the intersection of electricity, gas, and industrial sectors. Policies that harmonize regulations across these domains—such as integrated energy planning frameworks in Denmark and the Netherlands—have proven most effective at enabling SOEC deployment. These approaches align electricity market rules, gas infrastructure standards, and industrial decarbonization incentives to create coherent market signals for SOEC investors and operators.
Economic Viability and Cost Reduction Strategies
The economic viability of Solid Oxide Electrolysis Cells (SOECs) remains a significant challenge for widespread commercial adoption. Current capital costs for SOEC systems range between $800-1,500/kW, substantially higher than competing hydrogen production technologies. This cost barrier primarily stems from expensive materials such as yttria-stabilized zirconia electrolytes, nickel-based electrodes, and specialized interconnect materials that must withstand high operating temperatures (700-900°C).
Manufacturing complexity further compounds economic challenges, with precision requirements for cell fabrication and stack assembly driving up production costs. The specialized nature of these components limits economies of scale, while high-temperature operation necessitates expensive heat management systems and thermal insulation materials, adding to overall system costs.
Several promising cost reduction strategies have emerged in recent research and development efforts. Material innovation represents a critical pathway, with researchers developing lower-cost ceramic composites and exploring alternative catalyst materials to reduce dependency on precious metals. Advanced manufacturing techniques, including 3D printing and roll-to-roll processing, show potential to streamline production while improving quality consistency and reducing labor costs.
System integration improvements offer additional cost reduction opportunities. Co-electrolysis approaches that simultaneously produce hydrogen and carbon monoxide can improve overall system efficiency, while waste heat recovery systems can significantly enhance energy utilization. Integration with renewable energy sources provides pathways to leverage otherwise curtailed electricity at favorable rates.
Economic modeling suggests that with continued technological advancement and scaled production, SOEC costs could decrease by 40-60% by 2030. This projection assumes manufacturing volumes increasing from current pilot scales to industrial production levels exceeding 100 MW annually. Such cost reductions would position SOECs as economically competitive with conventional hydrogen production methods, particularly in regions with favorable renewable electricity pricing.
Policy support mechanisms remain essential for bridging the current economic gap. Carbon pricing, renewable energy subsidies, and dedicated hydrogen production incentives can significantly improve the business case for SOEC deployment. Several countries have implemented such policies, with Germany's H2Global initiative and Japan's Green Innovation Fund providing examples of frameworks that enhance SOEC economic viability through direct subsidies and market creation mechanisms.
Manufacturing complexity further compounds economic challenges, with precision requirements for cell fabrication and stack assembly driving up production costs. The specialized nature of these components limits economies of scale, while high-temperature operation necessitates expensive heat management systems and thermal insulation materials, adding to overall system costs.
Several promising cost reduction strategies have emerged in recent research and development efforts. Material innovation represents a critical pathway, with researchers developing lower-cost ceramic composites and exploring alternative catalyst materials to reduce dependency on precious metals. Advanced manufacturing techniques, including 3D printing and roll-to-roll processing, show potential to streamline production while improving quality consistency and reducing labor costs.
System integration improvements offer additional cost reduction opportunities. Co-electrolysis approaches that simultaneously produce hydrogen and carbon monoxide can improve overall system efficiency, while waste heat recovery systems can significantly enhance energy utilization. Integration with renewable energy sources provides pathways to leverage otherwise curtailed electricity at favorable rates.
Economic modeling suggests that with continued technological advancement and scaled production, SOEC costs could decrease by 40-60% by 2030. This projection assumes manufacturing volumes increasing from current pilot scales to industrial production levels exceeding 100 MW annually. Such cost reductions would position SOECs as economically competitive with conventional hydrogen production methods, particularly in regions with favorable renewable electricity pricing.
Policy support mechanisms remain essential for bridging the current economic gap. Carbon pricing, renewable energy subsidies, and dedicated hydrogen production incentives can significantly improve the business case for SOEC deployment. Several countries have implemented such policies, with Germany's H2Global initiative and Japan's Green Innovation Fund providing examples of frameworks that enhance SOEC economic viability through direct subsidies and market creation mechanisms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!