Mechanisms driving solid oxide electrolysis cells in energy storage
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SOEC Technology Background and Objectives
Solid Oxide Electrolysis Cells (SOECs) represent a transformative technology in the realm of energy storage, with roots dating back to the early 20th century. The fundamental principle of SOECs involves the electrochemical conversion of water and carbon dioxide into hydrogen, carbon monoxide, and oxygen using solid oxide electrolytes at elevated temperatures (700-900°C). This technology has evolved significantly over the past decades, transitioning from laboratory curiosity to a promising solution for large-scale energy storage applications.
The evolution of SOEC technology has been marked by several key milestones, including the development of more stable and efficient electrode materials, improvements in electrolyte conductivity, and advancements in cell design and manufacturing processes. Recent breakthroughs in materials science, particularly in the development of mixed ionic-electronic conductors and perovskite-structured electrodes, have substantially enhanced SOEC performance and durability.
Current technological trends in SOEC development focus on addressing critical challenges such as degradation mechanisms, thermal cycling stability, and cost reduction. Research is increasingly directed toward understanding the complex electrochemical reactions at the electrode-electrolyte interfaces, optimizing microstructural properties, and developing novel materials with enhanced catalytic activity and stability under operating conditions.
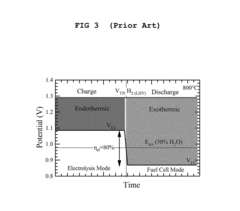
The primary technical objectives for SOEC advancement include achieving higher current densities while maintaining cell integrity, extending operational lifetimes beyond 40,000 hours, reducing manufacturing costs to make the technology economically viable, and integrating SOECs into comprehensive energy storage systems. Additionally, there is growing interest in reversible operation, allowing the same device to function as both an electrolysis cell and a fuel cell, thereby enhancing system flexibility and efficiency.
From an energy storage perspective, SOECs offer unique advantages due to their ability to convert electrical energy into chemical energy carriers with high efficiency. The produced hydrogen or syngas can be stored for extended periods and reconverted to electricity when needed, addressing the intermittency challenges associated with renewable energy sources. Furthermore, SOECs can utilize waste heat from industrial processes, potentially achieving system efficiencies exceeding 90%.
The long-term technological trajectory for SOECs points toward their integration into multi-vector energy systems, where they serve as critical components in sector coupling between electricity, heat, and chemical production. This evolution aligns with global efforts to decarbonize energy systems and establish hydrogen as a key element in future energy landscapes, positioning SOEC technology as a cornerstone of sustainable energy infrastructure.
The evolution of SOEC technology has been marked by several key milestones, including the development of more stable and efficient electrode materials, improvements in electrolyte conductivity, and advancements in cell design and manufacturing processes. Recent breakthroughs in materials science, particularly in the development of mixed ionic-electronic conductors and perovskite-structured electrodes, have substantially enhanced SOEC performance and durability.
Current technological trends in SOEC development focus on addressing critical challenges such as degradation mechanisms, thermal cycling stability, and cost reduction. Research is increasingly directed toward understanding the complex electrochemical reactions at the electrode-electrolyte interfaces, optimizing microstructural properties, and developing novel materials with enhanced catalytic activity and stability under operating conditions.
The primary technical objectives for SOEC advancement include achieving higher current densities while maintaining cell integrity, extending operational lifetimes beyond 40,000 hours, reducing manufacturing costs to make the technology economically viable, and integrating SOECs into comprehensive energy storage systems. Additionally, there is growing interest in reversible operation, allowing the same device to function as both an electrolysis cell and a fuel cell, thereby enhancing system flexibility and efficiency.
From an energy storage perspective, SOECs offer unique advantages due to their ability to convert electrical energy into chemical energy carriers with high efficiency. The produced hydrogen or syngas can be stored for extended periods and reconverted to electricity when needed, addressing the intermittency challenges associated with renewable energy sources. Furthermore, SOECs can utilize waste heat from industrial processes, potentially achieving system efficiencies exceeding 90%.
The long-term technological trajectory for SOECs points toward their integration into multi-vector energy systems, where they serve as critical components in sector coupling between electricity, heat, and chemical production. This evolution aligns with global efforts to decarbonize energy systems and establish hydrogen as a key element in future energy landscapes, positioning SOEC technology as a cornerstone of sustainable energy infrastructure.
Market Analysis for SOEC Energy Storage Applications
The global market for Solid Oxide Electrolysis Cell (SOEC) energy storage applications is experiencing significant growth, driven by increasing demand for efficient and sustainable energy storage solutions. The market is primarily segmented into power-to-gas, power-to-liquid, and grid balancing applications, with power-to-gas currently dominating due to its versatility in producing hydrogen and synthetic natural gas.
Europe leads the SOEC market with approximately 40% market share, followed by North America and Asia-Pacific regions. This dominance stems from Europe's aggressive renewable energy targets and substantial investments in hydrogen infrastructure. Germany, Denmark, and France are particularly active in deploying SOEC technologies for energy storage applications.
Market analysis indicates that the industrial sector represents the largest end-user segment for SOEC energy storage, particularly in chemical production, refining, and steel manufacturing. The utility sector follows closely, implementing SOEC systems for grid stabilization and renewable energy integration. The transportation sector is emerging as a promising market as hydrogen fuel cell vehicles gain traction.
Key market drivers include the declining costs of renewable electricity, which improves the economics of electrolysis-based energy storage. Government policies supporting decarbonization and renewable energy integration are creating favorable market conditions. Additionally, the growing need for long-duration energy storage solutions is expanding market opportunities for SOEC technologies.
Market challenges persist, including high capital costs compared to alternative technologies. Current SOEC systems require significant upfront investment, although costs are projected to decrease by 30-50% over the next decade as manufacturing scales up. Technical barriers related to durability and degradation rates under dynamic operation conditions also impact market adoption.
The competitive landscape features established industrial players like Sunfire, Bloom Energy, and Haldor Topsoe, alongside emerging technology companies. Strategic partnerships between technology providers and energy utilities are becoming increasingly common to accelerate commercialization and market penetration.
Market forecasts suggest compound annual growth rates of 20-25% for SOEC energy storage applications over the next five years. The power-to-gas segment is expected to maintain its leading position, while power-to-liquid applications will see accelerated growth due to increasing demand for sustainable aviation fuels and chemical feedstocks.
Regional analysis reveals that while Europe maintains market leadership, China is rapidly expanding its SOEC deployment capabilities, supported by national hydrogen strategy initiatives and substantial government funding for demonstration projects.
Europe leads the SOEC market with approximately 40% market share, followed by North America and Asia-Pacific regions. This dominance stems from Europe's aggressive renewable energy targets and substantial investments in hydrogen infrastructure. Germany, Denmark, and France are particularly active in deploying SOEC technologies for energy storage applications.
Market analysis indicates that the industrial sector represents the largest end-user segment for SOEC energy storage, particularly in chemical production, refining, and steel manufacturing. The utility sector follows closely, implementing SOEC systems for grid stabilization and renewable energy integration. The transportation sector is emerging as a promising market as hydrogen fuel cell vehicles gain traction.
Key market drivers include the declining costs of renewable electricity, which improves the economics of electrolysis-based energy storage. Government policies supporting decarbonization and renewable energy integration are creating favorable market conditions. Additionally, the growing need for long-duration energy storage solutions is expanding market opportunities for SOEC technologies.
Market challenges persist, including high capital costs compared to alternative technologies. Current SOEC systems require significant upfront investment, although costs are projected to decrease by 30-50% over the next decade as manufacturing scales up. Technical barriers related to durability and degradation rates under dynamic operation conditions also impact market adoption.
The competitive landscape features established industrial players like Sunfire, Bloom Energy, and Haldor Topsoe, alongside emerging technology companies. Strategic partnerships between technology providers and energy utilities are becoming increasingly common to accelerate commercialization and market penetration.
Market forecasts suggest compound annual growth rates of 20-25% for SOEC energy storage applications over the next five years. The power-to-gas segment is expected to maintain its leading position, while power-to-liquid applications will see accelerated growth due to increasing demand for sustainable aviation fuels and chemical feedstocks.
Regional analysis reveals that while Europe maintains market leadership, China is rapidly expanding its SOEC deployment capabilities, supported by national hydrogen strategy initiatives and substantial government funding for demonstration projects.
Current SOEC Technology Challenges
Despite significant advancements in Solid Oxide Electrolysis Cell (SOEC) technology, several critical challenges continue to impede widespread commercial deployment in energy storage applications. The most pressing issue remains the limited durability and operational lifetime of SOEC systems. Current cells typically demonstrate degradation rates of 1-2% per 1000 hours of operation, which falls short of the commercial target of less than 0.25% per 1000 hours. This degradation is primarily attributed to microstructural changes at high operating temperatures (700-850°C), including electrode delamination, chromium poisoning, and nickel agglomeration.
Material stability presents another significant hurdle, particularly at the oxygen electrode where delamination frequently occurs due to oxygen pressure buildup at the electrode-electrolyte interface. The hydrogen electrode faces challenges with nickel coarsening and carbon deposition when operating in co-electrolysis mode with CO2. Additionally, interconnect materials suffer from chromium volatilization, which poisons electrodes and accelerates performance degradation.
Thermal cycling resistance remains inadequate for practical applications. Current SOEC systems experience mechanical failures during startup and shutdown procedures due to thermal expansion mismatches between cell components. This limitation severely restricts the operational flexibility required for integration with intermittent renewable energy sources.
Cost factors continue to constrain market penetration, with current SOEC systems priced at approximately $2,000-3,000/kW, significantly higher than the $500/kW target needed for commercial viability. This cost barrier is exacerbated by the requirement for expensive high-temperature materials and complex balance-of-plant components, including heat exchangers and gas handling systems.
Scalability challenges persist in manufacturing processes. While laboratory-scale cells demonstrate promising performance, translating these results to industrial-scale production while maintaining quality and performance consistency remains problematic. Current manufacturing techniques struggle with reproducibility issues and high rejection rates during scale-up.
System integration complexities further complicate SOEC deployment. The high-temperature operation necessitates sophisticated thermal management systems and creates challenges for integration with downstream processes such as hydrogen compression, storage, or synthesis gas conversion. The balance between system efficiency and complexity has not yet reached optimal levels for commercial implementation.
Addressing these technical challenges requires coordinated research efforts focused on advanced materials development, innovative cell architectures, improved manufacturing processes, and system-level optimization. Recent progress in perovskite-based electrodes and scandia-stabilized zirconia electrolytes shows promise, but significant work remains to overcome these barriers and realize the full potential of SOEC technology for large-scale energy storage applications.
Material stability presents another significant hurdle, particularly at the oxygen electrode where delamination frequently occurs due to oxygen pressure buildup at the electrode-electrolyte interface. The hydrogen electrode faces challenges with nickel coarsening and carbon deposition when operating in co-electrolysis mode with CO2. Additionally, interconnect materials suffer from chromium volatilization, which poisons electrodes and accelerates performance degradation.
Thermal cycling resistance remains inadequate for practical applications. Current SOEC systems experience mechanical failures during startup and shutdown procedures due to thermal expansion mismatches between cell components. This limitation severely restricts the operational flexibility required for integration with intermittent renewable energy sources.
Cost factors continue to constrain market penetration, with current SOEC systems priced at approximately $2,000-3,000/kW, significantly higher than the $500/kW target needed for commercial viability. This cost barrier is exacerbated by the requirement for expensive high-temperature materials and complex balance-of-plant components, including heat exchangers and gas handling systems.
Scalability challenges persist in manufacturing processes. While laboratory-scale cells demonstrate promising performance, translating these results to industrial-scale production while maintaining quality and performance consistency remains problematic. Current manufacturing techniques struggle with reproducibility issues and high rejection rates during scale-up.
System integration complexities further complicate SOEC deployment. The high-temperature operation necessitates sophisticated thermal management systems and creates challenges for integration with downstream processes such as hydrogen compression, storage, or synthesis gas conversion. The balance between system efficiency and complexity has not yet reached optimal levels for commercial implementation.
Addressing these technical challenges requires coordinated research efforts focused on advanced materials development, innovative cell architectures, improved manufacturing processes, and system-level optimization. Recent progress in perovskite-based electrodes and scandia-stabilized zirconia electrolytes shows promise, but significant work remains to overcome these barriers and realize the full potential of SOEC technology for large-scale energy storage applications.
Current SOEC Mechanism Solutions
01 Electrochemical reaction mechanisms in SOECs
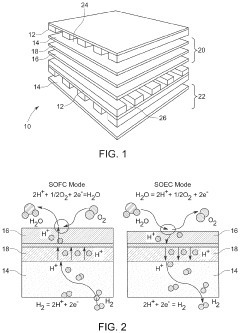
Solid oxide electrolysis cells operate through specific electrochemical reaction mechanisms where oxygen ions are transported through a solid electrolyte at high temperatures. The key reactions involve water splitting at the cathode to produce hydrogen and oxygen ions, which then migrate through the electrolyte to the anode where they combine to form oxygen gas. These fundamental mechanisms determine the efficiency and performance of the cells, with factors such as temperature, pressure, and electrode materials significantly influencing the reaction kinetics.- Electrochemical reaction mechanisms in SOECs: Solid oxide electrolysis cells operate through specific electrochemical reaction mechanisms where oxygen ions are transported through a solid electrolyte at high temperatures. The process involves the reduction of water or carbon dioxide at the cathode, releasing electrons that flow through an external circuit to the anode where oxygen ions combine to form oxygen gas. Understanding these fundamental reaction mechanisms is crucial for optimizing cell performance and efficiency in hydrogen or syngas production.
- Materials and microstructure for SOEC components: The performance of solid oxide electrolysis cells heavily depends on the materials and microstructure of their key components: electrolyte, cathode, and anode. Advanced ceramic materials like yttria-stabilized zirconia (YSZ) for electrolytes and perovskite-structured materials for electrodes are commonly used. The microstructural design of these components affects ion conductivity, catalytic activity, and long-term stability. Innovations in material composition and microstructure engineering are essential for enhancing cell durability and efficiency.
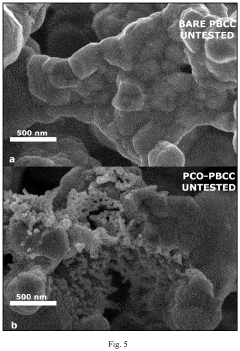
- Degradation mechanisms and durability enhancement: Solid oxide electrolysis cells face various degradation mechanisms during operation, including electrode delamination, material phase changes, and impurity poisoning. These degradation processes are accelerated by high operating temperatures and current densities. Understanding these mechanisms is crucial for developing strategies to enhance cell durability, such as protective coatings, compositional modifications, and optimized operating conditions that can significantly extend cell lifetime while maintaining performance.
- High-temperature operation and thermal management: Solid oxide electrolysis cells typically operate at temperatures between 600-900°C to achieve sufficient ionic conductivity in the solid electrolyte. This high-temperature operation presents unique challenges and advantages, including thermal cycling stress, heat management requirements, and enhanced reaction kinetics. Effective thermal management strategies, such as gradual heating/cooling protocols and thermally integrated system designs, are essential for maintaining cell integrity and optimizing energy efficiency during operation.
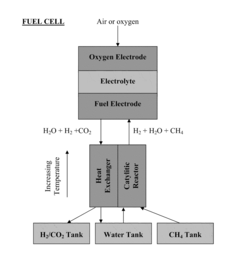
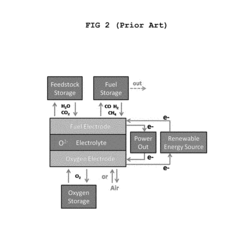
- Novel cell configurations and system integration: Innovative cell configurations and system integration approaches are being developed to enhance the performance and applicability of solid oxide electrolysis cells. These include reversible operation (SOEC/SOFC), pressurized operation, co-electrolysis of H2O and CO2, and integration with renewable energy sources. Advanced designs such as tubular cells, planar cells with metal supports, and segmented-in-series arrangements offer various advantages in terms of thermal management, mechanical stability, and scalability for industrial applications.
02 Electrode materials and microstructure optimization
The performance of solid oxide electrolysis cells heavily depends on electrode materials and their microstructure. Advanced electrode materials with optimized porosity, particle size distribution, and catalytic activity can significantly enhance the electrochemical reactions. Composite electrodes containing mixed ionic-electronic conductors reduce polarization resistance and improve cell efficiency. The microstructural design of electrodes affects gas diffusion pathways, active reaction sites, and long-term stability, making it a critical factor in SOEC development.Expand Specific Solutions03 Degradation mechanisms and durability enhancement
Understanding degradation mechanisms is crucial for improving the durability of solid oxide electrolysis cells. Common degradation phenomena include electrode delamination, chromium poisoning, nickel agglomeration, and electrolyte cracking. These issues are often caused by thermal cycling, high current densities, impurities in feed gases, and mechanical stresses. Various approaches to enhance durability include protective coatings, modified electrode compositions, controlled operating conditions, and novel cell architectures that minimize thermal and mechanical stresses during operation.Expand Specific Solutions04 High-temperature electrolysis process optimization
High-temperature operation is a defining characteristic of solid oxide electrolysis cells that significantly affects their performance. Operating at temperatures between 700-900°C enhances ionic conductivity and reaction kinetics, reducing cell resistance and improving efficiency. Process parameters such as steam concentration, current density, and system pressure must be carefully optimized to maximize hydrogen production while minimizing degradation. Thermal management strategies, including heat recovery systems and gradual heating/cooling protocols, are essential for maintaining cell integrity and extending operational lifetime.Expand Specific Solutions05 Novel cell configurations and system integration
Innovative cell configurations and system integration approaches are being developed to enhance the performance and applicability of solid oxide electrolysis technology. These include reversible solid oxide cells capable of both electrolysis and fuel cell operation, tubular designs that improve thermal cycling resistance, and planar configurations optimized for mass production. Advanced system integration focuses on efficient thermal management, steam generation, product separation, and control systems. Co-electrolysis of water and carbon dioxide to produce syngas represents another frontier, enabling the production of valuable chemicals and synthetic fuels from renewable electricity.Expand Specific Solutions
Key Industry Players in SOEC Development
The solid oxide electrolysis cell (SOEC) energy storage market is in its early growth phase, characterized by increasing R&D investments and emerging commercial applications. The global market is projected to expand significantly as renewable energy integration accelerates, with estimates suggesting multi-billion dollar potential by 2030. Technologically, SOECs are advancing from laboratory to commercial deployment, with varying maturity levels among key players. Industry leaders include Bloom Energy and Siemens Energy, who have developed commercial-scale systems, while academic institutions like Tsinghua University, Northwestern University, and MIT are driving fundamental innovations. DynElectro and Proof Energy represent emerging specialized players with proprietary technologies addressing key challenges such as durability and efficiency. Traditional energy companies including Saudi Aramco, Phillips 66, and Toshiba are increasingly investing in this space, recognizing its strategic importance in energy transition.
Bloom Energy Corp.
Technical Solution: Bloom Energy has developed a proprietary solid oxide electrolyzer cell (SOEC) technology that operates at high temperatures (700-900°C) for efficient hydrogen production. Their system utilizes a ceramic electrolyte made of yttria-stabilized zirconia (YSZ) with specialized nickel-based anodes and lanthanum strontium manganite cathodes. The company's reversible solid oxide cells can function in both fuel cell and electrolysis modes, enabling a flexible energy storage solution. Their SOEC technology achieves electricity-to-hydrogen conversion efficiencies exceeding 85% when utilizing waste heat integration[1]. Bloom's systems incorporate advanced thermal management to maintain optimal operating temperatures and utilize proprietary sealing technologies to ensure long-term durability despite thermal cycling. Their modular design allows for scalable deployment from distributed to utility-scale applications, with integrated power electronics for grid integration.
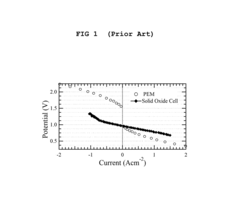
Strengths: High electrical efficiency (>85%) compared to PEM electrolyzers (60-70%); ability to operate in reversible mode; excellent thermal integration capabilities; scalable modular design. Weaknesses: High operating temperatures require specialized materials and longer startup times; degradation challenges from thermal cycling; higher capital costs compared to alkaline electrolyzers; requires high-purity water input.
Siemens AG
Technical Solution: Siemens has developed advanced Solid Oxide Electrolysis Cell (SOEC) technology under their SILYZER platform, focusing on high-temperature electrolysis for energy storage applications. Their system operates at temperatures between 700-850°C, utilizing a scandium-stabilized zirconia electrolyte with specialized nickel-based cermet anodes and lanthanum strontium cobalt ferrite cathodes. Siemens' SOEC technology achieves electrical efficiency up to 84% (HHV) with system efficiency reaching 95% when thermal integration is optimized[2]. Their cells feature a planar design with metallic interconnects and glass-ceramic sealing technology to withstand thermal cycling. Siemens has implemented advanced thermal management systems that recover waste heat from exothermic reactions to support the endothermic electrolysis process. Their SOEC stacks incorporate proprietary degradation mitigation strategies, including controlled operating parameters and specialized protective coatings on electrodes to extend operational lifetime beyond 20,000 hours[3].
Strengths: Superior electrical efficiency compared to low-temperature electrolysis; excellent thermal integration with industrial processes; ability to utilize various energy sources including renewables; scalable from industrial to grid-scale applications. Weaknesses: High capital costs; material degradation challenges at elevated temperatures; complex system integration requirements; longer startup times compared to PEM technology; requires specialized balance-of-plant components.
Critical SOEC Materials and Electrochemical Processes
Solid Oxide Cell
PatentPendingUS20240088402A1
Innovation
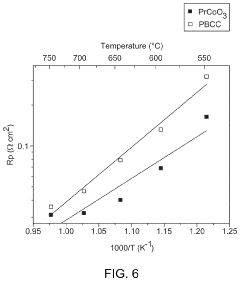
- Incorporating a perovskite oxide material coated with a Pr1−xCo1−yO3 catalyst into the oxygen electrode and using a proton conducting electrolyte, such as BaZr0.1Ce0.7Y0.1Yb0.1O3−δ, to enhance reaction rates and resistance to degradation, with a method involving infiltration of the catalyst into the electrode to form a stable and efficient solid oxide cell.
Method for improving the efficiency and durability of electrical energy storage using solid oxide electrolysis cell
PatentActiveUS9945039B2
Innovation
- Operating SOECs with specific C—H—O gas mixtures at reduced temperatures and elevated pressures to lower thermal-neutral voltage, maintaining thermal balance, and integrating a thermally-integrated catalytic reactor for efficient fuel production and storage.
Environmental Impact and Sustainability Assessment
Solid oxide electrolysis cells (SOECs) represent a promising technology for energy storage through hydrogen production and carbon dioxide conversion. When evaluating their environmental impact and sustainability, it is essential to consider their entire lifecycle from manufacturing to decommissioning.
The production phase of SOECs involves energy-intensive processes and rare earth materials, particularly for ceramic electrolytes and specialized catalysts. Current manufacturing methods require high temperatures (1400-1600°C) for sintering ceramic components, resulting in significant carbon emissions. However, compared to conventional hydrogen production through steam methane reforming, SOECs powered by renewable energy can reduce greenhouse gas emissions by 90-95% over their operational lifetime.
Water consumption presents another critical environmental consideration. While SOECs require water as a feedstock for hydrogen production, they consume approximately 9 liters of water per kilogram of hydrogen produced—significantly less than the 18-24 liters required by competing electrolysis technologies. This reduced water footprint becomes particularly valuable in water-stressed regions where energy storage solutions are increasingly deployed.
Land use impacts vary considerably depending on implementation scale. Large-scale SOEC installations integrated with renewable energy sources require substantial land area, though this impact can be mitigated through strategic siting on brownfield sites or integration with existing industrial facilities. The high energy density of hydrogen storage (33.3 kWh/kg) compared to batteries (0.1-0.5 kWh/kg) means that SOEC-based energy storage systems ultimately require less physical space per unit of energy stored.
Material sustainability represents both a challenge and opportunity. Current SOECs rely on critical materials including yttrium, zirconium, and sometimes rare earth elements. Recent research indicates potential for reducing rare earth content by 40-60% through advanced material engineering without compromising performance. Additionally, end-of-life recycling protocols are being developed to recover up to 85% of these valuable materials, significantly improving lifecycle sustainability.
Carbon footprint analyses demonstrate that SOEC systems achieve carbon payback within 1-3 years when powered by renewable energy, compared to 5-8 years for comparable energy storage technologies. This favorable carbon balance is further enhanced when SOECs are utilized for CO2 conversion to synthetic fuels, effectively creating carbon-neutral energy carriers from captured carbon dioxide.
The resilience of SOEC systems to climate change impacts must also be considered. Their high operating temperatures (700-850°C) make cooling requirements minimal compared to low-temperature electrolysis, reducing vulnerability to increasing ambient temperatures in warming climates. However, this advantage must be balanced against the higher initial energy investment required to reach operating temperature.
The production phase of SOECs involves energy-intensive processes and rare earth materials, particularly for ceramic electrolytes and specialized catalysts. Current manufacturing methods require high temperatures (1400-1600°C) for sintering ceramic components, resulting in significant carbon emissions. However, compared to conventional hydrogen production through steam methane reforming, SOECs powered by renewable energy can reduce greenhouse gas emissions by 90-95% over their operational lifetime.
Water consumption presents another critical environmental consideration. While SOECs require water as a feedstock for hydrogen production, they consume approximately 9 liters of water per kilogram of hydrogen produced—significantly less than the 18-24 liters required by competing electrolysis technologies. This reduced water footprint becomes particularly valuable in water-stressed regions where energy storage solutions are increasingly deployed.
Land use impacts vary considerably depending on implementation scale. Large-scale SOEC installations integrated with renewable energy sources require substantial land area, though this impact can be mitigated through strategic siting on brownfield sites or integration with existing industrial facilities. The high energy density of hydrogen storage (33.3 kWh/kg) compared to batteries (0.1-0.5 kWh/kg) means that SOEC-based energy storage systems ultimately require less physical space per unit of energy stored.
Material sustainability represents both a challenge and opportunity. Current SOECs rely on critical materials including yttrium, zirconium, and sometimes rare earth elements. Recent research indicates potential for reducing rare earth content by 40-60% through advanced material engineering without compromising performance. Additionally, end-of-life recycling protocols are being developed to recover up to 85% of these valuable materials, significantly improving lifecycle sustainability.
Carbon footprint analyses demonstrate that SOEC systems achieve carbon payback within 1-3 years when powered by renewable energy, compared to 5-8 years for comparable energy storage technologies. This favorable carbon balance is further enhanced when SOECs are utilized for CO2 conversion to synthetic fuels, effectively creating carbon-neutral energy carriers from captured carbon dioxide.
The resilience of SOEC systems to climate change impacts must also be considered. Their high operating temperatures (700-850°C) make cooling requirements minimal compared to low-temperature electrolysis, reducing vulnerability to increasing ambient temperatures in warming climates. However, this advantage must be balanced against the higher initial energy investment required to reach operating temperature.
Economic Viability and Commercialization Roadmap
The economic viability of Solid Oxide Electrolysis Cells (SOECs) for energy storage is currently at a critical juncture. Production costs remain a significant barrier, with current estimates ranging from $800-1,500/kW, substantially higher than competing technologies. However, cost projections indicate potential reductions to $500-700/kW by 2030 through economies of scale and manufacturing innovations, particularly in electrode materials and cell fabrication processes.
System efficiency represents a key economic driver, with SOECs demonstrating electricity-to-hydrogen conversion efficiencies of 70-90%, significantly outperforming alternative electrolysis technologies. This efficiency advantage translates to lower operational costs over the system lifetime, partially offsetting higher initial capital expenditures.
Durability improvements present another crucial economic factor. Current SOEC systems typically demonstrate degradation rates of 1-2% per 1,000 operating hours, necessitating replacement after 20,000-30,000 hours. Research breakthroughs in materials science are progressively extending operational lifetimes, with next-generation systems targeting degradation rates below 0.5% per 1,000 hours.
The commercialization roadmap for SOEC technology follows a multi-phase approach. The initial phase (2023-2025) focuses on demonstration projects and niche applications where high-temperature operation provides unique advantages, such as industrial settings with waste heat availability. These early deployments primarily target green hydrogen production for industrial processes.
The mid-term phase (2026-2030) will likely see expanded deployment in grid-scale energy storage applications, particularly in regions with high renewable penetration. This phase requires policy support through carbon pricing mechanisms and renewable energy incentives to achieve market competitiveness.
Long-term commercialization (2031-2040) depends on achieving manufacturing scale and establishing standardized designs. Industry consortia are forming to address these challenges, with significant investments from both private sector and government sources. The European Hydrogen Backbone initiative and similar programs in Asia represent coordinated efforts to develop the necessary infrastructure for widespread SOEC deployment.
Regulatory frameworks will significantly impact commercialization timelines. Countries implementing strong decarbonization policies, particularly in the EU, Japan, and increasingly in the US, are creating favorable conditions for SOEC adoption through targeted subsidies and carbon pricing mechanisms that improve the technology's economic proposition relative to fossil fuel alternatives.
System efficiency represents a key economic driver, with SOECs demonstrating electricity-to-hydrogen conversion efficiencies of 70-90%, significantly outperforming alternative electrolysis technologies. This efficiency advantage translates to lower operational costs over the system lifetime, partially offsetting higher initial capital expenditures.
Durability improvements present another crucial economic factor. Current SOEC systems typically demonstrate degradation rates of 1-2% per 1,000 operating hours, necessitating replacement after 20,000-30,000 hours. Research breakthroughs in materials science are progressively extending operational lifetimes, with next-generation systems targeting degradation rates below 0.5% per 1,000 hours.
The commercialization roadmap for SOEC technology follows a multi-phase approach. The initial phase (2023-2025) focuses on demonstration projects and niche applications where high-temperature operation provides unique advantages, such as industrial settings with waste heat availability. These early deployments primarily target green hydrogen production for industrial processes.
The mid-term phase (2026-2030) will likely see expanded deployment in grid-scale energy storage applications, particularly in regions with high renewable penetration. This phase requires policy support through carbon pricing mechanisms and renewable energy incentives to achieve market competitiveness.
Long-term commercialization (2031-2040) depends on achieving manufacturing scale and establishing standardized designs. Industry consortia are forming to address these challenges, with significant investments from both private sector and government sources. The European Hydrogen Backbone initiative and similar programs in Asia represent coordinated efforts to develop the necessary infrastructure for widespread SOEC deployment.
Regulatory frameworks will significantly impact commercialization timelines. Countries implementing strong decarbonization policies, particularly in the EU, Japan, and increasingly in the US, are creating favorable conditions for SOEC adoption through targeted subsidies and carbon pricing mechanisms that improve the technology's economic proposition relative to fossil fuel alternatives.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!