Sodium Acetate in Heat Absorption Technologies: Emerging Trends
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Heat Tech Evolution and Objectives
Sodium acetate has emerged as a promising material for heat absorption technologies, marking a significant evolution in thermal energy storage systems. The journey of sodium acetate in this field began in the late 20th century when researchers identified its potential as a phase change material (PCM) for thermal energy storage. Its unique properties, including high latent heat of fusion and suitable melting point, made it an attractive candidate for various heat absorption applications.
The evolution of sodium acetate in heat absorption technologies has been driven by the growing demand for efficient and sustainable energy storage solutions. Initially, the focus was on understanding the fundamental thermodynamic properties of sodium acetate trihydrate (SAT) and its behavior during phase transitions. Researchers explored various methods to enhance its performance, such as improving nucleation and reducing supercooling effects.
As the technology progressed, the objectives shifted towards developing practical applications and overcoming implementation challenges. One of the primary goals has been to enhance the thermal conductivity of sodium acetate-based PCMs, as their inherently low thermal conductivity limits heat transfer rates. This led to the development of composite materials, incorporating high-conductivity additives like graphite or metal particles to improve overall thermal performance.
Another significant objective in the evolution of sodium acetate heat absorption technologies has been to address the issue of phase segregation and improve long-term cycling stability. Researchers have explored various techniques, including the use of thickening agents and nucleating additives, to maintain the homogeneity of the material over multiple heating and cooling cycles.
In recent years, the focus has expanded to integrate sodium acetate-based PCMs into diverse applications, ranging from building materials for passive temperature regulation to thermal management systems in electronics and renewable energy storage. The objectives now include optimizing the encapsulation methods for sodium acetate, developing smart triggering mechanisms for controlled heat release, and exploring hybrid systems that combine sodium acetate with other PCMs or energy storage technologies.
Looking ahead, the evolution of sodium acetate in heat absorption technologies aims to address global challenges in energy efficiency and sustainability. Objectives include scaling up production processes to reduce costs, improving the environmental footprint of sodium acetate-based systems, and developing advanced control strategies for more efficient heat absorption and release cycles. Additionally, there is a growing interest in exploring the potential of sodium acetate in novel applications, such as wearable thermal management devices and space heating systems.
The evolution of sodium acetate in heat absorption technologies has been driven by the growing demand for efficient and sustainable energy storage solutions. Initially, the focus was on understanding the fundamental thermodynamic properties of sodium acetate trihydrate (SAT) and its behavior during phase transitions. Researchers explored various methods to enhance its performance, such as improving nucleation and reducing supercooling effects.
As the technology progressed, the objectives shifted towards developing practical applications and overcoming implementation challenges. One of the primary goals has been to enhance the thermal conductivity of sodium acetate-based PCMs, as their inherently low thermal conductivity limits heat transfer rates. This led to the development of composite materials, incorporating high-conductivity additives like graphite or metal particles to improve overall thermal performance.
Another significant objective in the evolution of sodium acetate heat absorption technologies has been to address the issue of phase segregation and improve long-term cycling stability. Researchers have explored various techniques, including the use of thickening agents and nucleating additives, to maintain the homogeneity of the material over multiple heating and cooling cycles.
In recent years, the focus has expanded to integrate sodium acetate-based PCMs into diverse applications, ranging from building materials for passive temperature regulation to thermal management systems in electronics and renewable energy storage. The objectives now include optimizing the encapsulation methods for sodium acetate, developing smart triggering mechanisms for controlled heat release, and exploring hybrid systems that combine sodium acetate with other PCMs or energy storage technologies.
Looking ahead, the evolution of sodium acetate in heat absorption technologies aims to address global challenges in energy efficiency and sustainability. Objectives include scaling up production processes to reduce costs, improving the environmental footprint of sodium acetate-based systems, and developing advanced control strategies for more efficient heat absorption and release cycles. Additionally, there is a growing interest in exploring the potential of sodium acetate in novel applications, such as wearable thermal management devices and space heating systems.
Market Analysis for Thermal Energy Storage
The thermal energy storage (TES) market is experiencing significant growth, driven by the increasing demand for efficient and sustainable energy solutions. As renewable energy sources become more prevalent, the need for effective energy storage technologies has become paramount. The global TES market is projected to expand at a compound annual growth rate (CAGR) of over 10% in the coming years, with the market value expected to reach several billion dollars by 2025.
Sodium acetate, a phase change material (PCM), is emerging as a promising solution for heat absorption technologies within the TES sector. Its unique properties, including high latent heat capacity and relatively low cost, make it an attractive option for various applications. The market for sodium acetate-based TES systems is showing strong potential, particularly in industries such as building heating and cooling, industrial process heat recovery, and solar thermal energy storage.
The residential and commercial building sector represents a significant portion of the market for sodium acetate TES systems. With increasing emphasis on energy-efficient buildings and smart energy management, the demand for innovative heating and cooling solutions is rising. Sodium acetate-based TES systems offer the advantage of reducing peak energy demand and improving overall energy efficiency in buildings.
In the industrial sector, sodium acetate TES systems are gaining traction for waste heat recovery applications. Industries with high thermal energy requirements, such as food processing, chemical manufacturing, and metalworking, are exploring sodium acetate-based solutions to capture and reuse waste heat, thereby reducing energy costs and improving overall process efficiency.
The renewable energy sector, particularly solar thermal power generation, is another key market for sodium acetate TES systems. As the intermittency of solar energy remains a challenge, efficient energy storage solutions are crucial for ensuring a stable power supply. Sodium acetate-based TES systems offer a cost-effective method for storing excess thermal energy during peak production periods and releasing it during periods of low solar irradiation.
Geographically, Europe and North America are currently leading the market for sodium acetate TES systems, driven by stringent energy efficiency regulations and government incentives for sustainable energy solutions. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, fueled by rapid industrialization, urbanization, and increasing investments in renewable energy infrastructure.
Despite the promising outlook, challenges such as the need for further technological advancements, scalability issues, and competition from other TES technologies may impact market growth. Ongoing research and development efforts are focused on enhancing the performance and cost-effectiveness of sodium acetate-based TES systems to address these challenges and expand their market penetration.
Sodium acetate, a phase change material (PCM), is emerging as a promising solution for heat absorption technologies within the TES sector. Its unique properties, including high latent heat capacity and relatively low cost, make it an attractive option for various applications. The market for sodium acetate-based TES systems is showing strong potential, particularly in industries such as building heating and cooling, industrial process heat recovery, and solar thermal energy storage.
The residential and commercial building sector represents a significant portion of the market for sodium acetate TES systems. With increasing emphasis on energy-efficient buildings and smart energy management, the demand for innovative heating and cooling solutions is rising. Sodium acetate-based TES systems offer the advantage of reducing peak energy demand and improving overall energy efficiency in buildings.
In the industrial sector, sodium acetate TES systems are gaining traction for waste heat recovery applications. Industries with high thermal energy requirements, such as food processing, chemical manufacturing, and metalworking, are exploring sodium acetate-based solutions to capture and reuse waste heat, thereby reducing energy costs and improving overall process efficiency.
The renewable energy sector, particularly solar thermal power generation, is another key market for sodium acetate TES systems. As the intermittency of solar energy remains a challenge, efficient energy storage solutions are crucial for ensuring a stable power supply. Sodium acetate-based TES systems offer a cost-effective method for storing excess thermal energy during peak production periods and releasing it during periods of low solar irradiation.
Geographically, Europe and North America are currently leading the market for sodium acetate TES systems, driven by stringent energy efficiency regulations and government incentives for sustainable energy solutions. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, fueled by rapid industrialization, urbanization, and increasing investments in renewable energy infrastructure.
Despite the promising outlook, challenges such as the need for further technological advancements, scalability issues, and competition from other TES technologies may impact market growth. Ongoing research and development efforts are focused on enhancing the performance and cost-effectiveness of sodium acetate-based TES systems to address these challenges and expand their market penetration.
Current Challenges in Sodium Acetate Heat Absorption
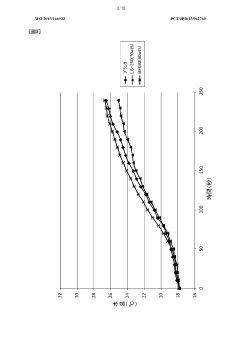
Despite the promising potential of sodium acetate in heat absorption technologies, several challenges currently hinder its widespread adoption and optimal performance. One of the primary issues is the supercooling effect, which can lead to unpredictable crystallization and reduced efficiency in heat release. This phenomenon occurs when the sodium acetate solution remains in a liquid state below its melting point, requiring external triggers to initiate crystallization.
Another significant challenge is the corrosive nature of sodium acetate, particularly in its aqueous form. This corrosivity can lead to degradation of containment materials and heat exchange surfaces, potentially compromising the longevity and reliability of heat storage systems. Addressing this issue requires careful selection of compatible materials or the development of protective coatings, which can increase overall system costs.
The thermal conductivity of sodium acetate hydrate presents an additional hurdle. While it exhibits excellent heat storage capacity, its relatively low thermal conductivity can limit the rate of heat transfer during both charging and discharging processes. This limitation can result in reduced system efficiency and longer cycle times, particularly in applications requiring rapid heat absorption or release.
Stability and cycling performance pose further challenges in sodium acetate-based heat absorption systems. Over multiple charge-discharge cycles, the material may experience phase segregation, where the salt and water components separate, leading to reduced heat storage capacity and overall system performance degradation. This issue necessitates the development of strategies to maintain homogeneity and prevent component separation during repeated thermal cycling.
The precise control of the phase change process in sodium acetate systems remains a complex task. Factors such as impurities, container geometry, and thermal gradients can influence nucleation and crystal growth, affecting the consistency and predictability of heat absorption and release. Achieving reliable and reproducible phase change behavior is crucial for the practical implementation of sodium acetate in heat storage applications.
Scalability and cost-effectiveness present additional challenges in the commercialization of sodium acetate heat absorption technologies. While sodium acetate is relatively inexpensive, the development of large-scale, efficient heat storage systems requires significant engineering efforts and may involve substantial initial investments. Balancing performance improvements with cost considerations remains a key challenge in making these technologies economically viable for widespread adoption.
Another significant challenge is the corrosive nature of sodium acetate, particularly in its aqueous form. This corrosivity can lead to degradation of containment materials and heat exchange surfaces, potentially compromising the longevity and reliability of heat storage systems. Addressing this issue requires careful selection of compatible materials or the development of protective coatings, which can increase overall system costs.
The thermal conductivity of sodium acetate hydrate presents an additional hurdle. While it exhibits excellent heat storage capacity, its relatively low thermal conductivity can limit the rate of heat transfer during both charging and discharging processes. This limitation can result in reduced system efficiency and longer cycle times, particularly in applications requiring rapid heat absorption or release.
Stability and cycling performance pose further challenges in sodium acetate-based heat absorption systems. Over multiple charge-discharge cycles, the material may experience phase segregation, where the salt and water components separate, leading to reduced heat storage capacity and overall system performance degradation. This issue necessitates the development of strategies to maintain homogeneity and prevent component separation during repeated thermal cycling.
The precise control of the phase change process in sodium acetate systems remains a complex task. Factors such as impurities, container geometry, and thermal gradients can influence nucleation and crystal growth, affecting the consistency and predictability of heat absorption and release. Achieving reliable and reproducible phase change behavior is crucial for the practical implementation of sodium acetate in heat storage applications.
Scalability and cost-effectiveness present additional challenges in the commercialization of sodium acetate heat absorption technologies. While sodium acetate is relatively inexpensive, the development of large-scale, efficient heat storage systems requires significant engineering efforts and may involve substantial initial investments. Balancing performance improvements with cost considerations remains a key challenge in making these technologies economically viable for widespread adoption.
Existing Sodium Acetate Heat Absorption Solutions
01 Heat absorption and storage properties of sodium acetate
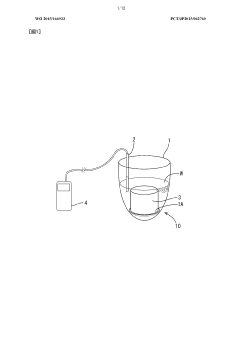
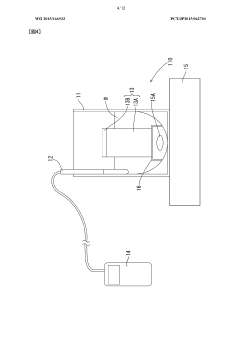
Sodium acetate exhibits excellent heat absorption and storage properties, making it suitable for use in thermal energy storage systems. When sodium acetate trihydrate undergoes a phase change from solid to liquid, it absorbs a significant amount of heat, which can be released later when the material solidifies. This property makes it useful in various applications, including heat packs and thermal management systems.- Heat absorption and storage properties of sodium acetate: Sodium acetate exhibits excellent heat absorption and storage properties, making it suitable for use in thermal energy storage systems. When sodium acetate trihydrate undergoes a phase change from solid to liquid, it absorbs a significant amount of heat, which can be released later when the material solidifies. This property makes it useful in various applications requiring heat storage and release.
- Application in heat packs and warming devices: Sodium acetate is commonly used in reusable heat packs and warming devices. These products utilize the supercooling property of sodium acetate, where it remains in a liquid state below its melting point. When triggered, the solution crystallizes, releasing heat. This technology is applied in various products such as hand warmers, body warmers, and therapeutic heat packs.
- Integration in thermal management systems: Sodium acetate-based heat absorption systems are integrated into thermal management solutions for various applications. These systems can be used in buildings, vehicles, and industrial processes to regulate temperature and improve energy efficiency. The heat absorption properties of sodium acetate help in maintaining stable temperatures and reducing energy consumption in cooling and heating systems.
- Enhancement of heat absorption efficiency: Research focuses on improving the heat absorption efficiency of sodium acetate-based systems. This includes developing composite materials, modifying the crystal structure, and incorporating additives to enhance thermal conductivity and heat transfer rates. These advancements aim to increase the overall performance and effectiveness of sodium acetate in heat absorption applications.
- Novel applications in energy storage and conversion: Innovative applications of sodium acetate's heat absorption properties are being explored in energy storage and conversion technologies. This includes its use in solar thermal energy storage, waste heat recovery systems, and phase change materials for building insulation. These applications leverage the material's ability to absorb, store, and release thermal energy efficiently.
02 Sodium acetate in phase change materials (PCMs)
Sodium acetate is widely used as a phase change material due to its ability to store and release latent heat during phase transitions. PCMs containing sodium acetate can be incorporated into various products and systems to regulate temperature and improve energy efficiency. These materials can absorb excess heat during warm periods and release it when temperatures drop, helping to maintain stable temperatures in different applications.Expand Specific Solutions03 Applications in heating devices and systems
Sodium acetate's heat absorption properties make it suitable for use in various heating devices and systems. It can be utilized in portable heat packs, thermal cushions, and other personal warming products. Additionally, sodium acetate-based materials can be integrated into larger-scale heating systems for buildings or industrial processes, providing efficient and controllable heat storage and release mechanisms.Expand Specific Solutions04 Enhancing thermal performance in construction materials
Sodium acetate can be incorporated into construction materials to improve their thermal performance. By adding sodium acetate-based phase change materials to building components such as walls, floors, or ceilings, the overall thermal mass of the structure can be increased. This helps to regulate indoor temperatures, reduce energy consumption for heating and cooling, and enhance occupant comfort.Expand Specific Solutions05 Sodium acetate in energy storage and heat transfer fluids
The heat absorption properties of sodium acetate make it valuable in energy storage applications and heat transfer fluids. It can be used in thermal energy storage systems to capture and store excess heat from renewable energy sources or industrial processes. Sodium acetate-based heat transfer fluids can efficiently transport thermal energy in various applications, such as solar thermal systems or waste heat recovery processes.Expand Specific Solutions
Key Players in Thermal Energy Storage Industry
The emerging trends in Sodium Acetate for heat absorption technologies are shaping a competitive landscape characterized by rapid growth and increasing market potential. The industry is in an early expansion phase, with a growing market size driven by the rising demand for sustainable energy storage solutions. While the technology is still evolving, it has reached a moderate level of maturity, attracting interest from both established players and innovative startups. Companies like Air Products & Chemicals, BASF Corp., and Air Liquide SA are leveraging their expertise in chemical manufacturing to develop advanced Sodium Acetate-based heat absorption systems. Meanwhile, research institutions such as Fraunhofer-Gesellschaft and Worcester Polytechnic Institute are contributing to technological advancements, pushing the boundaries of efficiency and application scope in this promising field.
Air Products & Chemicals, Inc.
Technical Solution: Air Products & Chemicals, Inc. has focused on developing innovative heat transfer fluids and thermal management solutions incorporating sodium acetate. Their research has led to the creation of advanced heat absorption systems that utilize the unique properties of sodium acetate trihydrate. The company has engineered specialized additives and stabilizers to enhance the performance and reliability of sodium acetate-based PCMs [4]. Air Products has also developed novel heat exchanger designs optimized for use with these materials, improving overall system efficiency and heat transfer rates [5]. Their solutions find applications in industrial processes, HVAC systems, and renewable energy storage.
Strengths: Expertise in chemical engineering and process optimization, wide range of industrial applications. Weaknesses: May face competition from more specialized PCM manufacturers.
Fraunhofer-Gesellschaft eV
Technical Solution: Fraunhofer-Gesellschaft eV has conducted extensive research on sodium acetate-based heat absorption technologies, focusing on improving their efficiency and applicability. Their scientists have developed advanced composite materials that combine sodium acetate with other PCMs and supporting matrices to enhance thermal conductivity and stability [6]. Fraunhofer has also pioneered the use of nanoparticles and carbon-based additives to improve the heat transfer characteristics of sodium acetate PCMs [7]. Additionally, they have explored novel applications such as integrating these materials into building components for passive temperature regulation and energy-efficient construction [8].
Strengths: Strong research capabilities, interdisciplinary approach to material development. Weaknesses: May face challenges in scaling up laboratory innovations to commercial production.
Innovative Patents in Sodium Acetate Heat Tech
Heat device having a latent-heat storage means
PatentActiveEP3096723A2
Innovation
- Incorporating a latent heat storage medium, specifically a phase change material like sodium acetate, which absorbs and releases heat during endothermic and exothermic state changes, to regulate the temperature of the heating device, ensuring safer and more comfortable heat delivery.
Heat storage material
PatentWO2015166933A1
Innovation
- Incorporating silicon carbide into sodium acetate-based heat storage materials, with a preferred silicon carbide content of 50% to 70% by mass, to enhance heat transfer and warming efficiency.
Environmental Impact of Sodium Acetate Technologies
The environmental impact of sodium acetate technologies in heat absorption applications is a crucial consideration as these systems gain prominence. Sodium acetate, while offering significant advantages in thermal energy storage, also presents certain environmental challenges that must be addressed.
One of the primary environmental benefits of sodium acetate-based heat absorption technologies is their potential to reduce energy consumption and greenhouse gas emissions. By efficiently storing and releasing thermal energy, these systems can decrease the reliance on conventional heating and cooling methods, which often rely on fossil fuels. This reduction in energy demand can lead to a substantial decrease in carbon dioxide emissions, contributing to climate change mitigation efforts.
However, the production of sodium acetate itself requires careful environmental management. The manufacturing process involves the reaction of sodium hydroxide with acetic acid, both of which are industrial chemicals with potential environmental impacts if not properly handled. Ensuring responsible production practices, including proper waste management and emissions control, is essential to minimize the environmental footprint of sodium acetate production.
The disposal and recycling of sodium acetate-based heat absorption systems also warrant attention. While sodium acetate is generally considered non-toxic and biodegradable, improper disposal of large quantities could potentially affect local ecosystems. Developing efficient recycling processes for these systems at the end of their lifecycle is crucial to minimize waste and promote a circular economy approach.
Water usage is another environmental factor to consider in sodium acetate technologies. Some heat absorption systems may require significant amounts of water for their operation, particularly in industrial applications. Implementing water-efficient designs and exploring alternative cooling methods can help mitigate this impact and conserve water resources.
The long-term environmental effects of widespread adoption of sodium acetate heat absorption technologies must also be evaluated. This includes assessing the potential impacts on local microclimates, particularly in urban areas where these systems might be extensively deployed. While the overall effect is likely to be positive due to reduced energy consumption, localized changes in heat distribution patterns should be monitored and managed.
Lastly, the environmental impact of sodium acetate technologies extends to the sourcing of raw materials. Ensuring sustainable and ethical sourcing practices for the components used in these systems is essential to minimize ecological disruption and social impacts in resource-rich regions.
One of the primary environmental benefits of sodium acetate-based heat absorption technologies is their potential to reduce energy consumption and greenhouse gas emissions. By efficiently storing and releasing thermal energy, these systems can decrease the reliance on conventional heating and cooling methods, which often rely on fossil fuels. This reduction in energy demand can lead to a substantial decrease in carbon dioxide emissions, contributing to climate change mitigation efforts.
However, the production of sodium acetate itself requires careful environmental management. The manufacturing process involves the reaction of sodium hydroxide with acetic acid, both of which are industrial chemicals with potential environmental impacts if not properly handled. Ensuring responsible production practices, including proper waste management and emissions control, is essential to minimize the environmental footprint of sodium acetate production.
The disposal and recycling of sodium acetate-based heat absorption systems also warrant attention. While sodium acetate is generally considered non-toxic and biodegradable, improper disposal of large quantities could potentially affect local ecosystems. Developing efficient recycling processes for these systems at the end of their lifecycle is crucial to minimize waste and promote a circular economy approach.
Water usage is another environmental factor to consider in sodium acetate technologies. Some heat absorption systems may require significant amounts of water for their operation, particularly in industrial applications. Implementing water-efficient designs and exploring alternative cooling methods can help mitigate this impact and conserve water resources.
The long-term environmental effects of widespread adoption of sodium acetate heat absorption technologies must also be evaluated. This includes assessing the potential impacts on local microclimates, particularly in urban areas where these systems might be extensively deployed. While the overall effect is likely to be positive due to reduced energy consumption, localized changes in heat distribution patterns should be monitored and managed.
Lastly, the environmental impact of sodium acetate technologies extends to the sourcing of raw materials. Ensuring sustainable and ethical sourcing practices for the components used in these systems is essential to minimize ecological disruption and social impacts in resource-rich regions.
Cost-Benefit Analysis of Sodium Acetate Heat Systems
The cost-benefit analysis of sodium acetate heat systems reveals a compelling case for their adoption in various applications. Initial investment costs for sodium acetate-based thermal energy storage systems are generally higher than traditional alternatives due to the specialized materials and manufacturing processes involved. However, these systems offer significant long-term economic benefits that often outweigh the upfront expenses.
One of the primary advantages of sodium acetate heat systems is their high energy density, which allows for more compact and efficient storage solutions. This translates to reduced space requirements and potentially lower installation costs compared to other thermal storage technologies. Additionally, the phase change properties of sodium acetate enable it to store and release large amounts of heat at a constant temperature, improving overall system efficiency.
Operational costs for sodium acetate heat systems are generally lower than conventional heating methods. The ability to store excess heat during off-peak hours and release it during peak demand periods can lead to substantial energy savings and reduced utility bills. This load-shifting capability is particularly valuable in regions with time-of-use electricity pricing, allowing users to capitalize on lower rates.
Maintenance requirements for sodium acetate systems are relatively minimal, contributing to reduced long-term operational expenses. The stable chemical composition of sodium acetate ensures a long lifespan for the storage medium, with many systems capable of withstanding thousands of charge-discharge cycles without significant degradation in performance.
From an environmental perspective, sodium acetate heat systems offer notable benefits. By enabling more efficient use of renewable energy sources and reducing reliance on fossil fuels for heating, these systems can significantly lower carbon emissions. This aligns with increasingly stringent environmental regulations and can potentially qualify users for various green energy incentives or carbon credits, further improving the economic proposition.
However, it is important to consider potential drawbacks. The corrosive nature of sodium acetate solutions necessitates the use of specialized containment materials, which can increase initial costs. Additionally, the technology's effectiveness may vary depending on specific application requirements and local climate conditions, impacting the overall cost-benefit ratio.
In conclusion, while sodium acetate heat systems may require a higher initial investment, their long-term economic and environmental benefits often justify the costs. As the technology continues to mature and production scales up, it is likely that initial costs will decrease, further improving the cost-benefit analysis in favor of these innovative thermal storage solutions.
One of the primary advantages of sodium acetate heat systems is their high energy density, which allows for more compact and efficient storage solutions. This translates to reduced space requirements and potentially lower installation costs compared to other thermal storage technologies. Additionally, the phase change properties of sodium acetate enable it to store and release large amounts of heat at a constant temperature, improving overall system efficiency.
Operational costs for sodium acetate heat systems are generally lower than conventional heating methods. The ability to store excess heat during off-peak hours and release it during peak demand periods can lead to substantial energy savings and reduced utility bills. This load-shifting capability is particularly valuable in regions with time-of-use electricity pricing, allowing users to capitalize on lower rates.
Maintenance requirements for sodium acetate systems are relatively minimal, contributing to reduced long-term operational expenses. The stable chemical composition of sodium acetate ensures a long lifespan for the storage medium, with many systems capable of withstanding thousands of charge-discharge cycles without significant degradation in performance.
From an environmental perspective, sodium acetate heat systems offer notable benefits. By enabling more efficient use of renewable energy sources and reducing reliance on fossil fuels for heating, these systems can significantly lower carbon emissions. This aligns with increasingly stringent environmental regulations and can potentially qualify users for various green energy incentives or carbon credits, further improving the economic proposition.
However, it is important to consider potential drawbacks. The corrosive nature of sodium acetate solutions necessitates the use of specialized containment materials, which can increase initial costs. Additionally, the technology's effectiveness may vary depending on specific application requirements and local climate conditions, impacting the overall cost-benefit ratio.
In conclusion, while sodium acetate heat systems may require a higher initial investment, their long-term economic and environmental benefits often justify the costs. As the technology continues to mature and production scales up, it is likely that initial costs will decrease, further improving the cost-benefit analysis in favor of these innovative thermal storage solutions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!