Evaluating ICP-MS Performance Through Systematic Robustness Testing
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Technology Evolution and Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the early 1980s. This analytical technique combines the high-temperature ICP source with a mass spectrometer to detect and quantify trace elements at concentrations as low as one part per trillion. The evolution of ICP-MS technology has been driven by the increasing demand for more sensitive, accurate, and reliable elemental analysis across various industries including environmental monitoring, pharmaceutical research, food safety, and semiconductor manufacturing.
The initial ICP-MS systems faced significant challenges including spectral interferences, matrix effects, and limited dynamic range. Throughout the 1990s, technological advancements led to the development of collision/reaction cell technology, which revolutionized the field by effectively reducing polyatomic interferences. This innovation marked a critical milestone in ICP-MS evolution, substantially improving detection limits and analytical accuracy for problematic elements.
The early 2000s witnessed the emergence of high-resolution ICP-MS systems, capable of resolving spectral interferences through superior mass separation capabilities. Simultaneously, improvements in sample introduction systems, including desolvation nebulizers and laser ablation techniques, expanded the application scope of ICP-MS to solid samples and specialized matrices, further enhancing its versatility as an analytical tool.
Recent technological developments have focused on enhancing instrument robustness and stability under challenging analytical conditions. Modern ICP-MS systems incorporate advanced plasma interface designs, improved ion optics, and sophisticated software algorithms for interference correction. These innovations have collectively contributed to more reliable performance in routine analysis, particularly when dealing with complex sample matrices or during extended analytical runs.
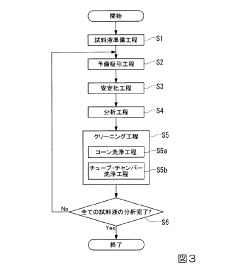
The primary objective of systematic robustness testing in ICP-MS is to evaluate and ensure consistent analytical performance under varying operational conditions. This includes assessing stability during prolonged operation, tolerance to matrix-heavy samples, resistance to drift, and reproducibility across different sample types. Such testing is crucial for validating instrument specifications and establishing optimal operating parameters for specific analytical applications.
Future technological objectives in ICP-MS development include further miniaturization for field-deployable systems, enhanced integration with separation techniques, improved multi-element capabilities, and the development of more sophisticated interference management strategies. Additionally, there is growing interest in developing more environmentally sustainable ICP-MS systems with reduced argon consumption and lower power requirements, aligning with broader industry trends toward greener analytical technologies.
The initial ICP-MS systems faced significant challenges including spectral interferences, matrix effects, and limited dynamic range. Throughout the 1990s, technological advancements led to the development of collision/reaction cell technology, which revolutionized the field by effectively reducing polyatomic interferences. This innovation marked a critical milestone in ICP-MS evolution, substantially improving detection limits and analytical accuracy for problematic elements.
The early 2000s witnessed the emergence of high-resolution ICP-MS systems, capable of resolving spectral interferences through superior mass separation capabilities. Simultaneously, improvements in sample introduction systems, including desolvation nebulizers and laser ablation techniques, expanded the application scope of ICP-MS to solid samples and specialized matrices, further enhancing its versatility as an analytical tool.
Recent technological developments have focused on enhancing instrument robustness and stability under challenging analytical conditions. Modern ICP-MS systems incorporate advanced plasma interface designs, improved ion optics, and sophisticated software algorithms for interference correction. These innovations have collectively contributed to more reliable performance in routine analysis, particularly when dealing with complex sample matrices or during extended analytical runs.
The primary objective of systematic robustness testing in ICP-MS is to evaluate and ensure consistent analytical performance under varying operational conditions. This includes assessing stability during prolonged operation, tolerance to matrix-heavy samples, resistance to drift, and reproducibility across different sample types. Such testing is crucial for validating instrument specifications and establishing optimal operating parameters for specific analytical applications.
Future technological objectives in ICP-MS development include further miniaturization for field-deployable systems, enhanced integration with separation techniques, improved multi-element capabilities, and the development of more sophisticated interference management strategies. Additionally, there is growing interest in developing more environmentally sustainable ICP-MS systems with reduced argon consumption and lower power requirements, aligning with broader industry trends toward greener analytical technologies.
Market Applications and Analytical Demands
The ICP-MS (Inductively Coupled Plasma Mass Spectrometry) market has experienced significant growth across diverse sectors, driven by increasing demands for precise elemental analysis. The global ICP-MS market was valued at approximately 1.2 billion USD in 2022, with projections indicating a compound annual growth rate of 7.8% through 2028, reflecting the expanding applications and analytical requirements.
Environmental monitoring represents a substantial market segment, where regulatory agencies worldwide require robust testing methodologies for detecting trace elements in water, soil, and air samples. The implementation of stringent environmental protection policies in developed regions has accelerated the adoption of ICP-MS technology, particularly for monitoring heavy metal contamination in drinking water and industrial effluents.
The pharmaceutical and biotechnology sectors constitute rapidly growing markets for ICP-MS applications, particularly in drug development and quality control processes. These industries demand increasingly sensitive analytical methods capable of detecting elemental impurities at parts-per-trillion levels, in compliance with ICH Q3D guidelines and pharmacopeia standards. The need for validated, robust testing protocols has become paramount as regulatory scrutiny intensifies.
Food safety testing represents another critical application area, with growing consumer awareness and regulatory requirements driving demand for comprehensive elemental analysis. The ability to detect contaminants and authenticate food origins through multi-elemental fingerprinting has positioned ICP-MS as an essential analytical tool in this sector.
Clinical diagnostics and biomedical research have emerged as high-growth segments, utilizing ICP-MS for trace element analysis in biological samples. The increasing focus on personalized medicine and biomarker discovery has created demand for highly sensitive and reproducible analytical methods capable of handling complex biological matrices.
Semiconductor and electronics manufacturing rely heavily on ICP-MS for quality control and process monitoring, where ultra-trace detection capabilities are essential for maintaining product integrity. As device miniaturization continues, the analytical demands for lower detection limits and higher precision have intensified.
These diverse market applications share common analytical demands: increased sensitivity, improved interference management, enhanced matrix tolerance, and greater operational robustness. End-users across all sectors require analytical systems that maintain performance stability under varying sample loads and matrix complexities, with minimal recalibration and maintenance requirements.
Environmental monitoring represents a substantial market segment, where regulatory agencies worldwide require robust testing methodologies for detecting trace elements in water, soil, and air samples. The implementation of stringent environmental protection policies in developed regions has accelerated the adoption of ICP-MS technology, particularly for monitoring heavy metal contamination in drinking water and industrial effluents.
The pharmaceutical and biotechnology sectors constitute rapidly growing markets for ICP-MS applications, particularly in drug development and quality control processes. These industries demand increasingly sensitive analytical methods capable of detecting elemental impurities at parts-per-trillion levels, in compliance with ICH Q3D guidelines and pharmacopeia standards. The need for validated, robust testing protocols has become paramount as regulatory scrutiny intensifies.
Food safety testing represents another critical application area, with growing consumer awareness and regulatory requirements driving demand for comprehensive elemental analysis. The ability to detect contaminants and authenticate food origins through multi-elemental fingerprinting has positioned ICP-MS as an essential analytical tool in this sector.
Clinical diagnostics and biomedical research have emerged as high-growth segments, utilizing ICP-MS for trace element analysis in biological samples. The increasing focus on personalized medicine and biomarker discovery has created demand for highly sensitive and reproducible analytical methods capable of handling complex biological matrices.
Semiconductor and electronics manufacturing rely heavily on ICP-MS for quality control and process monitoring, where ultra-trace detection capabilities are essential for maintaining product integrity. As device miniaturization continues, the analytical demands for lower detection limits and higher precision have intensified.
These diverse market applications share common analytical demands: increased sensitivity, improved interference management, enhanced matrix tolerance, and greater operational robustness. End-users across all sectors require analytical systems that maintain performance stability under varying sample loads and matrix complexities, with minimal recalibration and maintenance requirements.
Current Challenges in ICP-MS Robustness
Despite significant advancements in ICP-MS technology, several persistent challenges continue to impact the robustness and reliability of these systems during routine and specialized analytical applications. The most prominent issue remains matrix effects, where complex sample compositions can cause signal suppression or enhancement, leading to inaccurate quantification. High dissolved solid content in samples (exceeding 0.2%) frequently causes cone orifice clogging, resulting in signal drift and necessitating system maintenance that interrupts analytical workflows.
Polyatomic interferences continue to challenge accurate trace element determination, particularly for elements like arsenic, selenium, and iron where molecular species formed in the plasma can overlap with analyte signals. While collision/reaction cell technologies have mitigated many of these issues, they introduce additional complexity to method development and validation processes.
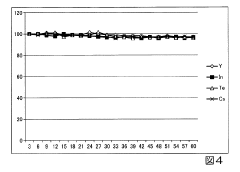
Long-term signal stability remains problematic, especially during extended analytical runs spanning multiple days. Gradual sensitivity changes due to component aging, deposit accumulation, and plasma fluctuations necessitate frequent recalibration and internal standard correction, increasing analysis time and complexity. This instability is particularly challenging for laboratories processing large sample batches or conducting longitudinal studies requiring consistent performance over time.
Memory effects present another significant challenge, particularly when analyzing elements like mercury, boron, and iodine that tend to persist in the sample introduction system. These carryover effects can compromise detection limits and accuracy for subsequent samples, requiring extended washout procedures that reduce sample throughput.
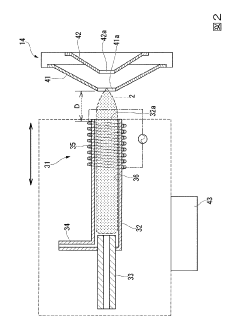
The sample introduction system itself remains a critical vulnerability point. Nebulizer clogging, spray chamber temperature fluctuations, and peristaltic pump tubing degradation all contribute to signal instability. Modern systems have improved but still require regular maintenance and optimization to maintain performance specifications.
Instrument-to-instrument variability poses challenges for method transfer between laboratories and complicates multi-instrument studies. Different ICP-MS platforms, even from the same manufacturer, may exhibit varying susceptibilities to interferences and matrix effects, necessitating laboratory-specific method validation and optimization.
Finally, the increasing demand for ultra-trace analysis (sub-ppt levels) pushes ICP-MS systems to their fundamental limits, where contamination from reagents, labware, and the laboratory environment becomes the limiting factor rather than instrumental capabilities. Maintaining clean laboratory conditions and developing appropriate blank correction procedures remain significant challenges for achieving reliable ultra-trace measurements.
Polyatomic interferences continue to challenge accurate trace element determination, particularly for elements like arsenic, selenium, and iron where molecular species formed in the plasma can overlap with analyte signals. While collision/reaction cell technologies have mitigated many of these issues, they introduce additional complexity to method development and validation processes.
Long-term signal stability remains problematic, especially during extended analytical runs spanning multiple days. Gradual sensitivity changes due to component aging, deposit accumulation, and plasma fluctuations necessitate frequent recalibration and internal standard correction, increasing analysis time and complexity. This instability is particularly challenging for laboratories processing large sample batches or conducting longitudinal studies requiring consistent performance over time.
Memory effects present another significant challenge, particularly when analyzing elements like mercury, boron, and iodine that tend to persist in the sample introduction system. These carryover effects can compromise detection limits and accuracy for subsequent samples, requiring extended washout procedures that reduce sample throughput.
The sample introduction system itself remains a critical vulnerability point. Nebulizer clogging, spray chamber temperature fluctuations, and peristaltic pump tubing degradation all contribute to signal instability. Modern systems have improved but still require regular maintenance and optimization to maintain performance specifications.
Instrument-to-instrument variability poses challenges for method transfer between laboratories and complicates multi-instrument studies. Different ICP-MS platforms, even from the same manufacturer, may exhibit varying susceptibilities to interferences and matrix effects, necessitating laboratory-specific method validation and optimization.
Finally, the increasing demand for ultra-trace analysis (sub-ppt levels) pushes ICP-MS systems to their fundamental limits, where contamination from reagents, labware, and the laboratory environment becomes the limiting factor rather than instrumental capabilities. Maintaining clean laboratory conditions and developing appropriate blank correction procedures remain significant challenges for achieving reliable ultra-trace measurements.
Established Protocols for ICP-MS Performance Validation
01 ICP-MS System Design and Components
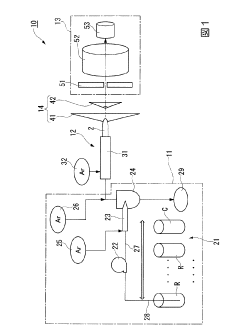
Innovations in the design and components of ICP-MS systems to enhance performance, including improved ion source configurations, interface designs, and detector systems. These advancements focus on optimizing plasma generation, ion extraction, and detection sensitivity to achieve better analytical results with lower detection limits and higher precision.- ICP-MS Instrumentation Improvements: Advancements in ICP-MS hardware components including plasma torches, ion optics, mass analyzers, and detectors that enhance overall performance. These improvements focus on increasing sensitivity, reducing interference, improving resolution, and enhancing stability during analysis. Innovations in instrument design allow for better detection limits, wider dynamic range, and more reliable quantification of trace elements.
- Sample Introduction and Preparation Techniques: Novel methods for sample introduction and preparation that improve ICP-MS performance. These include specialized nebulizers, spray chambers, and sample preparation protocols designed to minimize matrix effects and enhance ionization efficiency. Techniques for handling complex matrices, reducing memory effects, and improving sample throughput while maintaining analytical quality are emphasized.
- Interference Reduction and Elimination Strategies: Methods to reduce or eliminate spectral and non-spectral interferences in ICP-MS analysis. These include collision/reaction cell technologies, mathematical correction models, and specialized sample preparation techniques. Approaches for dealing with polyatomic interferences, isobaric overlaps, and matrix-induced suppression or enhancement effects are covered to improve measurement accuracy.
- Calibration and Quantification Methods: Advanced calibration strategies and quantification methods for improving ICP-MS analytical performance. These include internal standardization techniques, isotope dilution methods, standard addition approaches, and matrix-matched calibration. Innovations in calibration software, automated calibration procedures, and quality control protocols that enhance measurement accuracy and precision are featured.
- Specialized Applications and Hyphenated Techniques: Integration of ICP-MS with other analytical techniques to enhance performance for specialized applications. These include coupling with chromatographic methods (LC-ICP-MS, GC-ICP-MS), laser ablation systems (LA-ICP-MS), and other sample introduction techniques. Specialized methods for isotope ratio measurements, nanoparticle analysis, speciation studies, and ultra-trace element determination are described.
02 Sample Introduction and Preparation Techniques
Methods and devices for sample introduction and preparation that improve ICP-MS performance. These include specialized nebulizers, desolvation systems, and sample preparation protocols designed to minimize interferences, reduce matrix effects, and enhance ionization efficiency, resulting in more accurate and reliable analytical measurements.Expand Specific Solutions03 Interference Reduction and Elimination Strategies
Techniques for reducing or eliminating spectral and non-spectral interferences in ICP-MS analysis. These include collision/reaction cell technologies, mathematical correction algorithms, and specialized sample introduction systems that minimize polyatomic interferences, matrix effects, and isobaric overlaps to improve measurement accuracy and detection limits.Expand Specific Solutions04 Calibration and Quantification Methods
Advanced calibration and quantification approaches for ICP-MS that enhance analytical performance. These include internal standardization techniques, isotope dilution methods, standard addition procedures, and multi-element calibration strategies designed to improve accuracy, precision, and reliability of quantitative measurements across a wide dynamic range.Expand Specific Solutions05 Hyphenated Techniques and Specialized Applications
Coupling of ICP-MS with other analytical techniques and specialized applications to extend analytical capabilities. These include hyphenated systems like LC-ICP-MS, GC-ICP-MS, and laser ablation ICP-MS, as well as specialized applications for nanoparticle analysis, speciation studies, and isotope ratio measurements that leverage the high sensitivity and multi-element capabilities of ICP-MS.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The ICP-MS robustness testing market is currently in a growth phase, with increasing adoption across pharmaceutical, environmental, and industrial sectors. The global market size for ICP-MS technology is expanding at approximately 7-8% annually, driven by stringent regulatory requirements and growing demand for trace element analysis. Technologically, the field shows varying maturity levels, with established players like Agilent Technologies, PerkinElmer (Revvity), and Shimadzu leading innovation through advanced hardware and software solutions for systematic performance evaluation. Emerging competitors include FUJIFILM and Applied Materials, who are integrating ICP-MS capabilities into broader analytical platforms. Academic institutions like Guangdong University of Technology and Southwest Jiaotong University are contributing significant research advancements, while corporate laboratories at IBM and Siemens are developing automated robustness testing protocols to enhance reproducibility and reliability.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed comprehensive ICP-MS robustness testing protocols centered around their ICP-MS MassHunter software and 7900 ICP-MS systems. Their approach includes automated daily performance checks that evaluate sensitivity, oxide ratios, doubly charged ion formation, and background signals across the mass range. The company has implemented an intelligent diagnostic system that continuously monitors over 150 instrument parameters during analysis, providing real-time feedback on instrument health. Agilent's High Matrix Introduction (HMI) technology specifically addresses matrix tolerance by aerosol dilution, allowing for direct analysis of samples containing up to 3% total dissolved solids without physical dilution. Their collision/reaction cell technology (ORS4) effectively removes polyatomic interferences while maintaining sensitivity, with helium mode operation demonstrating exceptional robustness across varying matrix compositions.
Strengths: Industry-leading matrix tolerance with HMI technology; comprehensive automated diagnostics; proven long-term stability with minimal drift. Weaknesses: Higher initial investment cost compared to some competitors; complex software interface requires significant training for new users.
Shimadzu Corp.
Technical Solution: Shimadzu has engineered a systematic robustness testing approach for their ICPMS-2030 platform focused on maintaining performance under challenging analytical conditions. Their proprietary Development Assistant software incorporates automated method development tools that optimize collision cell parameters based on sample type, ensuring consistent performance across diverse matrices. Shimadzu's Eco mode technology reduces argon gas consumption by up to 30% while maintaining analytical performance, addressing both operational costs and environmental concerns. Their robustness testing protocol includes a unique "stress test" where the system is deliberately challenged with high-salt matrices over extended periods to evaluate long-term stability. The ICPMS-2030's octopole collision cell employs kinetic energy discrimination with helium gas to effectively remove polyatomic interferences while maintaining sensitivity for target analytes across the mass range.
Strengths: Exceptional energy efficiency with Eco mode technology; intuitive software interface reduces training requirements; excellent price-to-performance ratio. Weaknesses: Somewhat lower sensitivity for certain elements compared to top-tier competitors; more limited mass range than some high-end systems.
Critical Parameters and Interference Management Techniques
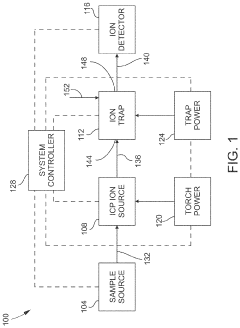
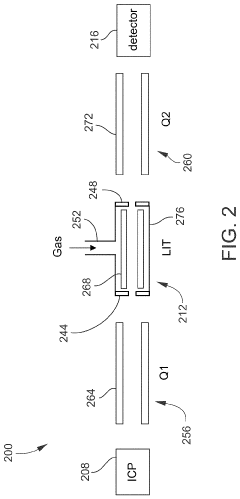
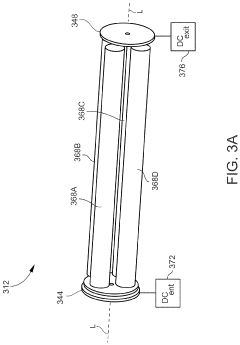
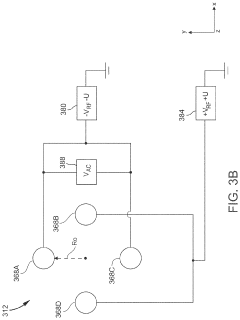
Inductively coupled plasma mass spectrometry (ICP-MS) with ion trapping
PatentActiveUS11443933B1
Innovation
- Incorporating an ion trap, such as a linear ion trap, into the ICP-MS system to confine and mass-selectively eject ions, allowing for the simultaneous analysis of multiple elements from transient signals by preventing ion exit and entry during a confinement period and transmitting selected ions to a detector for measurement.
Inductively coupled plasma mass spectrometry method
PatentInactiveJP2016008918A
Innovation
- An inductively coupled plasma mass spectrometry method that allows undiluted high-salt sample analysis, incorporating a cleaning step to remove deposits on the sampler using plasma exposure and a cleaning liquid, maintaining detection sensitivity and accuracy.
Quality Assurance Standards and Compliance
Quality assurance in ICP-MS testing requires adherence to rigorous international and regional standards that govern analytical procedures and performance criteria. The primary regulatory frameworks include ISO/IEC 17025 for testing laboratories, which establishes general requirements for competence, impartiality, and consistent operation. This standard serves as the foundation for laboratory accreditation and ensures that ICP-MS robustness testing follows internationally recognized protocols.
For environmental applications, the US EPA Method 6020 and Method 200.8 provide specific guidelines for ICP-MS analysis of trace elements in environmental samples, detailing quality control procedures and performance specifications that laboratories must meet. Similarly, the European Union's Water Framework Directive establishes compliance requirements for water quality monitoring using ICP-MS technology.
In pharmaceutical contexts, USP <232> and <233> standards govern elemental impurity analysis, while ICH Q3D guidelines provide a global framework for controlling metal contaminants in drug products. These standards specify acceptance criteria for system suitability tests that directly relate to instrument robustness.
Clinical laboratories utilizing ICP-MS must comply with CLIA regulations in the US and equivalent standards internationally, which mandate regular performance verification and proficiency testing. The implementation of these standards requires comprehensive quality management systems that include documentation of instrument qualification, method validation, and ongoing performance verification.
Proficiency testing programs, such as those offered by NIST, LGC Standards, and ERA, provide external validation of laboratory performance and are essential components of compliance frameworks. Participation in these programs allows laboratories to demonstrate their analytical capabilities and identify potential areas for improvement in their robustness testing protocols.
Traceability to certified reference materials (CRMs) is another critical aspect of quality assurance in ICP-MS testing. Organizations such as NIST, IRMM, and NRC produce matrix-matched reference materials that enable laboratories to validate their analytical methods and establish measurement traceability chains.
The evolution of quality standards has increasingly emphasized risk-based approaches to analytical testing, requiring laboratories to identify critical performance parameters and establish appropriate control strategies. This trend is reflected in updated versions of ISO/IEC 17025 and pharmaceutical quality guidelines, which now incorporate risk management principles into compliance frameworks.
Laboratories must also consider regional variations in compliance requirements, as different jurisdictions may impose additional or modified standards based on local regulatory priorities. Maintaining awareness of these differences is essential for organizations operating across multiple regions or serving diverse markets with their analytical services.
For environmental applications, the US EPA Method 6020 and Method 200.8 provide specific guidelines for ICP-MS analysis of trace elements in environmental samples, detailing quality control procedures and performance specifications that laboratories must meet. Similarly, the European Union's Water Framework Directive establishes compliance requirements for water quality monitoring using ICP-MS technology.
In pharmaceutical contexts, USP <232> and <233> standards govern elemental impurity analysis, while ICH Q3D guidelines provide a global framework for controlling metal contaminants in drug products. These standards specify acceptance criteria for system suitability tests that directly relate to instrument robustness.
Clinical laboratories utilizing ICP-MS must comply with CLIA regulations in the US and equivalent standards internationally, which mandate regular performance verification and proficiency testing. The implementation of these standards requires comprehensive quality management systems that include documentation of instrument qualification, method validation, and ongoing performance verification.
Proficiency testing programs, such as those offered by NIST, LGC Standards, and ERA, provide external validation of laboratory performance and are essential components of compliance frameworks. Participation in these programs allows laboratories to demonstrate their analytical capabilities and identify potential areas for improvement in their robustness testing protocols.
Traceability to certified reference materials (CRMs) is another critical aspect of quality assurance in ICP-MS testing. Organizations such as NIST, IRMM, and NRC produce matrix-matched reference materials that enable laboratories to validate their analytical methods and establish measurement traceability chains.
The evolution of quality standards has increasingly emphasized risk-based approaches to analytical testing, requiring laboratories to identify critical performance parameters and establish appropriate control strategies. This trend is reflected in updated versions of ISO/IEC 17025 and pharmaceutical quality guidelines, which now incorporate risk management principles into compliance frameworks.
Laboratories must also consider regional variations in compliance requirements, as different jurisdictions may impose additional or modified standards based on local regulatory priorities. Maintaining awareness of these differences is essential for organizations operating across multiple regions or serving diverse markets with their analytical services.
Environmental Impact and Sustainable Practices
The environmental impact of ICP-MS (Inductively Coupled Plasma Mass Spectrometry) operations extends beyond laboratory walls, necessitating careful consideration of sustainable practices throughout the testing lifecycle. Systematic robustness testing of ICP-MS systems generates significant waste streams, including acidic solutions, heavy metal standards, and argon gas emissions that require proper management protocols.
Laboratory facilities conducting extensive ICP-MS robustness testing typically consume substantial quantities of ultra-pure water, with advanced systems requiring 5-10 liters per hour during operation. This water usage represents a considerable environmental footprint, particularly in regions facing water scarcity challenges. Additionally, the high energy consumption of plasma generation—often requiring 1-2 kW of continuous power—contributes to carbon emissions when sourced from non-renewable energy supplies.
Waste management presents another critical environmental consideration. The acidic solutions and calibration standards containing heavy metals must undergo specialized treatment before disposal to prevent contamination of water systems. Progressive laboratories have implemented closed-loop recycling systems for certain reagents, reducing waste volumes by up to 40% while maintaining analytical integrity during robustness testing protocols.
Argon gas consumption represents both an environmental and economic concern in ICP-MS operations. Standard robustness testing procedures may consume 15-20 liters of argon per minute, with comprehensive testing regimes potentially utilizing several hundred liters daily. Innovative approaches to gas recycling and plasma efficiency optimization have emerged, with some systems now incorporating gas recirculation technologies that reduce consumption by 25-30%.
The electronic components and consumables associated with ICP-MS systems also present end-of-life disposal challenges. Sustainable procurement practices increasingly emphasize selecting instruments with longer operational lifespans and modular designs that facilitate component replacement rather than complete system disposal. Several manufacturers have instituted take-back programs for electronic components and consumable items, ensuring proper recycling of precious metals and reduction of electronic waste.
Forward-thinking laboratories have begun implementing comprehensive environmental management systems specifically addressing ICP-MS operations. These frameworks include regular environmental impact assessments, energy efficiency audits, and waste reduction targets. Certification programs such as ISO 14001 provide structured approaches to minimizing environmental impacts while maintaining analytical excellence during robustness testing procedures.
Laboratory facilities conducting extensive ICP-MS robustness testing typically consume substantial quantities of ultra-pure water, with advanced systems requiring 5-10 liters per hour during operation. This water usage represents a considerable environmental footprint, particularly in regions facing water scarcity challenges. Additionally, the high energy consumption of plasma generation—often requiring 1-2 kW of continuous power—contributes to carbon emissions when sourced from non-renewable energy supplies.
Waste management presents another critical environmental consideration. The acidic solutions and calibration standards containing heavy metals must undergo specialized treatment before disposal to prevent contamination of water systems. Progressive laboratories have implemented closed-loop recycling systems for certain reagents, reducing waste volumes by up to 40% while maintaining analytical integrity during robustness testing protocols.
Argon gas consumption represents both an environmental and economic concern in ICP-MS operations. Standard robustness testing procedures may consume 15-20 liters of argon per minute, with comprehensive testing regimes potentially utilizing several hundred liters daily. Innovative approaches to gas recycling and plasma efficiency optimization have emerged, with some systems now incorporating gas recirculation technologies that reduce consumption by 25-30%.
The electronic components and consumables associated with ICP-MS systems also present end-of-life disposal challenges. Sustainable procurement practices increasingly emphasize selecting instruments with longer operational lifespans and modular designs that facilitate component replacement rather than complete system disposal. Several manufacturers have instituted take-back programs for electronic components and consumable items, ensuring proper recycling of precious metals and reduction of electronic waste.
Forward-thinking laboratories have begun implementing comprehensive environmental management systems specifically addressing ICP-MS operations. These frameworks include regular environmental impact assessments, energy efficiency audits, and waste reduction targets. Certification programs such as ISO 14001 provide structured approaches to minimizing environmental impacts while maintaining analytical excellence during robustness testing procedures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!