Future Trends in Sodium Acetate Research & Applications
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Research Evolution and Objectives
Sodium acetate, a versatile compound with a rich history, has undergone significant evolution in research and applications over the past century. Initially recognized for its role in textile manufacturing and food preservation, sodium acetate has since expanded its reach into diverse fields, including energy storage, pharmaceuticals, and materials science.
The research trajectory of sodium acetate has been marked by several key milestones. In the early 20th century, studies focused primarily on its chemical properties and basic industrial applications. The mid-century saw an increased interest in its potential as a buffer solution in biochemical research, leading to its widespread use in laboratory settings.
The 1970s and 1980s witnessed a surge in research exploring sodium acetate's phase change properties, particularly its ability to release heat upon crystallization. This discovery paved the way for its application in thermal energy storage systems and hand warmers, sparking a new wave of innovation in sustainable energy solutions.
Recent decades have seen a shift towards more sophisticated applications of sodium acetate. In the field of materials science, researchers have been investigating its potential as a precursor for advanced carbon materials and as a component in novel composite materials. The pharmaceutical industry has also shown growing interest in sodium acetate, exploring its use as an excipient in drug formulations and as a potential therapeutic agent in certain medical conditions.
Looking ahead, the objectives of sodium acetate research are multifaceted and ambitious. One primary goal is to optimize its performance in thermal energy storage systems, aiming to enhance efficiency and expand its applicability in renewable energy technologies. Researchers are also focusing on developing new, sustainable production methods for sodium acetate, aligning with global efforts towards greener chemical processes.
Another key objective is to unlock novel applications in emerging fields such as nanotechnology and bioengineering. Scientists are exploring the potential of sodium acetate-based nanostructures for applications in drug delivery, environmental remediation, and advanced sensing technologies.
Furthermore, there is a growing emphasis on understanding the fundamental properties of sodium acetate at the molecular level. This includes investigating its behavior under extreme conditions and its interactions with various materials, which could lead to unforeseen applications in fields ranging from space technology to advanced manufacturing.
As research continues to evolve, the overarching aim is to position sodium acetate as a key player in addressing global challenges, particularly in areas of sustainability, energy efficiency, and healthcare. The future of sodium acetate research promises to be dynamic and impactful, with potential breakthroughs that could revolutionize multiple industries and contribute to a more sustainable future.
The research trajectory of sodium acetate has been marked by several key milestones. In the early 20th century, studies focused primarily on its chemical properties and basic industrial applications. The mid-century saw an increased interest in its potential as a buffer solution in biochemical research, leading to its widespread use in laboratory settings.
The 1970s and 1980s witnessed a surge in research exploring sodium acetate's phase change properties, particularly its ability to release heat upon crystallization. This discovery paved the way for its application in thermal energy storage systems and hand warmers, sparking a new wave of innovation in sustainable energy solutions.
Recent decades have seen a shift towards more sophisticated applications of sodium acetate. In the field of materials science, researchers have been investigating its potential as a precursor for advanced carbon materials and as a component in novel composite materials. The pharmaceutical industry has also shown growing interest in sodium acetate, exploring its use as an excipient in drug formulations and as a potential therapeutic agent in certain medical conditions.
Looking ahead, the objectives of sodium acetate research are multifaceted and ambitious. One primary goal is to optimize its performance in thermal energy storage systems, aiming to enhance efficiency and expand its applicability in renewable energy technologies. Researchers are also focusing on developing new, sustainable production methods for sodium acetate, aligning with global efforts towards greener chemical processes.
Another key objective is to unlock novel applications in emerging fields such as nanotechnology and bioengineering. Scientists are exploring the potential of sodium acetate-based nanostructures for applications in drug delivery, environmental remediation, and advanced sensing technologies.
Furthermore, there is a growing emphasis on understanding the fundamental properties of sodium acetate at the molecular level. This includes investigating its behavior under extreme conditions and its interactions with various materials, which could lead to unforeseen applications in fields ranging from space technology to advanced manufacturing.
As research continues to evolve, the overarching aim is to position sodium acetate as a key player in addressing global challenges, particularly in areas of sustainability, energy efficiency, and healthcare. The future of sodium acetate research promises to be dynamic and impactful, with potential breakthroughs that could revolutionize multiple industries and contribute to a more sustainable future.
Market Analysis for Sodium Acetate Applications
The sodium acetate market has shown significant growth in recent years, driven by its versatile applications across various industries. The global sodium acetate market size was valued at approximately $180 million in 2020 and is projected to reach $250 million by 2027, growing at a CAGR of around 5% during the forecast period. This growth is primarily attributed to the increasing demand from end-use industries such as textiles, pharmaceuticals, and food and beverages.
In the textile industry, sodium acetate is widely used as a dyeing auxiliary and pH buffer. The growing textile industry, particularly in developing countries like China, India, and Bangladesh, is expected to drive the demand for sodium acetate. The pharmaceutical sector is another key market for sodium acetate, where it is used in the production of various medications and as a buffering agent in hemodialysis solutions. The increasing prevalence of chronic diseases and the expanding pharmaceutical industry are likely to boost the demand for sodium acetate in this sector.
The food and beverage industry represents a significant market for sodium acetate, where it is used as a preservative and flavoring agent. With the rising consumer demand for processed and convenience foods, the use of sodium acetate in this sector is expected to grow. Additionally, the increasing awareness of food safety and the need for extended shelf life of products are driving the adoption of sodium acetate in food preservation applications.
Geographically, Asia Pacific is expected to dominate the sodium acetate market due to the rapid industrialization and growing end-use industries in countries like China and India. North America and Europe are also significant markets, driven by the pharmaceutical and food industries. The Middle East and Africa region is anticipated to witness substantial growth in the coming years, primarily due to the expanding textile and pharmaceutical sectors.
However, the market faces challenges such as the availability of substitutes and environmental concerns associated with the production process. Manufacturers are focusing on developing eco-friendly production methods and exploring new applications to overcome these challenges and capitalize on emerging opportunities. The increasing research and development activities in sodium acetate applications, particularly in the fields of energy storage and phase change materials, are expected to create new growth avenues for the market in the future.
In the textile industry, sodium acetate is widely used as a dyeing auxiliary and pH buffer. The growing textile industry, particularly in developing countries like China, India, and Bangladesh, is expected to drive the demand for sodium acetate. The pharmaceutical sector is another key market for sodium acetate, where it is used in the production of various medications and as a buffering agent in hemodialysis solutions. The increasing prevalence of chronic diseases and the expanding pharmaceutical industry are likely to boost the demand for sodium acetate in this sector.
The food and beverage industry represents a significant market for sodium acetate, where it is used as a preservative and flavoring agent. With the rising consumer demand for processed and convenience foods, the use of sodium acetate in this sector is expected to grow. Additionally, the increasing awareness of food safety and the need for extended shelf life of products are driving the adoption of sodium acetate in food preservation applications.
Geographically, Asia Pacific is expected to dominate the sodium acetate market due to the rapid industrialization and growing end-use industries in countries like China and India. North America and Europe are also significant markets, driven by the pharmaceutical and food industries. The Middle East and Africa region is anticipated to witness substantial growth in the coming years, primarily due to the expanding textile and pharmaceutical sectors.
However, the market faces challenges such as the availability of substitutes and environmental concerns associated with the production process. Manufacturers are focusing on developing eco-friendly production methods and exploring new applications to overcome these challenges and capitalize on emerging opportunities. The increasing research and development activities in sodium acetate applications, particularly in the fields of energy storage and phase change materials, are expected to create new growth avenues for the market in the future.
Current Challenges in Sodium Acetate Technology
Despite the widespread use of sodium acetate in various industries, several challenges persist in its technology, hindering its full potential and broader application. One of the primary issues is the energy-intensive production process, which often relies on fossil fuels, contributing to high carbon emissions and environmental concerns. This challenge is particularly pressing in the face of increasing global pressure to reduce carbon footprints and adopt more sustainable manufacturing practices.
Another significant hurdle is the limited purity levels achievable in large-scale production. While high-purity sodium acetate is crucial for certain applications, such as in the pharmaceutical and food industries, current manufacturing processes struggle to consistently produce ultra-pure grades without significant cost increases. This limitation restricts its use in sensitive applications and hampers market expansion in high-value sectors.
The hygroscopic nature of sodium acetate presents storage and handling challenges, especially in humid environments. This property can lead to caking and reduced shelf life, necessitating special packaging and storage conditions that increase overall costs and logistical complexities. Moreover, the development of more efficient and cost-effective moisture-resistant formulations remains an ongoing challenge for researchers and manufacturers alike.
In the realm of thermal energy storage applications, while sodium acetate trihydrate shows promise as a phase change material, issues with supercooling and phase separation during repeated thermal cycles limit its long-term stability and efficiency. Overcoming these limitations to create more reliable and durable thermal storage solutions is a key focus area for current research and development efforts.
The scalability of sodium acetate production for emerging applications, such as in advanced materials and green chemistry, also poses significant challenges. Current production methods may not be sufficiently flexible or cost-effective to meet the diverse and specialized needs of these new markets, potentially limiting innovation and market growth.
Lastly, there is a growing need for more sustainable and bio-based production methods for sodium acetate. While some progress has been made in utilizing renewable resources and waste streams as feedstocks, scaling these processes to industrial levels while maintaining economic viability remains a significant challenge. This aspect is crucial for aligning sodium acetate production with circular economy principles and meeting the increasing demand for environmentally friendly chemical products.
Another significant hurdle is the limited purity levels achievable in large-scale production. While high-purity sodium acetate is crucial for certain applications, such as in the pharmaceutical and food industries, current manufacturing processes struggle to consistently produce ultra-pure grades without significant cost increases. This limitation restricts its use in sensitive applications and hampers market expansion in high-value sectors.
The hygroscopic nature of sodium acetate presents storage and handling challenges, especially in humid environments. This property can lead to caking and reduced shelf life, necessitating special packaging and storage conditions that increase overall costs and logistical complexities. Moreover, the development of more efficient and cost-effective moisture-resistant formulations remains an ongoing challenge for researchers and manufacturers alike.
In the realm of thermal energy storage applications, while sodium acetate trihydrate shows promise as a phase change material, issues with supercooling and phase separation during repeated thermal cycles limit its long-term stability and efficiency. Overcoming these limitations to create more reliable and durable thermal storage solutions is a key focus area for current research and development efforts.
The scalability of sodium acetate production for emerging applications, such as in advanced materials and green chemistry, also poses significant challenges. Current production methods may not be sufficiently flexible or cost-effective to meet the diverse and specialized needs of these new markets, potentially limiting innovation and market growth.
Lastly, there is a growing need for more sustainable and bio-based production methods for sodium acetate. While some progress has been made in utilizing renewable resources and waste streams as feedstocks, scaling these processes to industrial levels while maintaining economic viability remains a significant challenge. This aspect is crucial for aligning sodium acetate production with circular economy principles and meeting the increasing demand for environmentally friendly chemical products.
Existing Sodium Acetate Production Methods
01 Use of sodium acetate in heat storage materials
Sodium acetate is utilized in heat storage materials due to its phase change properties. It can absorb and release heat during phase transitions, making it suitable for thermal energy storage applications. These materials can be used in various industries for temperature regulation and energy conservation.- Use of sodium acetate in chemical processes: Sodium acetate is widely used in various chemical processes as a reagent, catalyst, or buffer. It plays a role in reactions such as acetylation, esterification, and pH control. Its properties make it valuable in industrial applications, including the production of pharmaceuticals, textiles, and other chemical compounds.
- Application in heat storage and thermal management: Sodium acetate trihydrate is utilized in heat storage systems and thermal management applications. It undergoes a phase change at specific temperatures, allowing it to store and release latent heat. This property is exploited in heat packs, building materials for temperature regulation, and energy storage systems.
- Use in food and beverage industry: Sodium acetate serves as a food additive, functioning as a preservative, acidity regulator, and flavoring agent. It is used in various food products to enhance shelf life, control pH, and impart a mild salty taste. Its application extends to beverages, condiments, and processed foods.
- Application in wastewater treatment: Sodium acetate is employed in wastewater treatment processes, particularly in biological treatment systems. It serves as a carbon source for microorganisms involved in denitrification and other biodegradation processes. This application helps in reducing nitrogen compounds and organic pollutants in wastewater.
- Use in textile and leather industries: Sodium acetate finds applications in textile and leather processing. It is used as a dyeing auxiliary, helping to improve color fastness and dye penetration. In leather tanning, it serves as a buffering agent and helps in the fixation of certain tanning agents. Its use contributes to improved product quality in these industries.
02 Sodium acetate in chemical synthesis processes
Sodium acetate serves as a reagent or intermediate in various chemical synthesis processes. It is used in the production of organic compounds, pharmaceuticals, and other industrial chemicals. Its properties as a weak base and acetate source make it valuable in numerous chemical reactions.Expand Specific Solutions03 Application of sodium acetate in food preservation
Sodium acetate is employed as a food preservative and pH regulator in the food industry. It helps extend the shelf life of various food products by inhibiting microbial growth and maintaining acidity levels. This application is particularly useful in processed foods and beverages.Expand Specific Solutions04 Sodium acetate in textile and leather processing
The textile and leather industries utilize sodium acetate in various processes. It serves as a buffering agent, pH regulator, and dyeing auxiliary. Sodium acetate helps improve the quality and durability of textiles and leather products during manufacturing.Expand Specific Solutions05 Use of sodium acetate in environmental applications
Sodium acetate finds applications in environmental remediation and wastewater treatment processes. It can be used as a carbon source for denitrification in wastewater treatment plants and as a de-icing agent with reduced environmental impact compared to traditional salt-based de-icers.Expand Specific Solutions
Key Industry Players in Sodium Acetate Field
The research and applications of sodium acetate are entering a mature phase, with a growing market size driven by diverse industrial applications. The technology's maturity is evident from the involvement of established players like Novartis AG and BASF Corp., alongside specialized companies such as Sunamp Ltd. and Purac Biochem BV. Academic institutions, including Beijing University of Chemical Technology and Zhejiang University, are contributing to advancing the field. The competitive landscape is characterized by a mix of large corporations, specialized chemical companies, and research institutions, indicating a well-developed ecosystem. As the market expands, we can expect increased focus on innovative applications and improved production methods to meet growing demand across various sectors.
Sunamp Ltd.
Technical Solution: Sunamp Ltd. is pioneering the use of sodium acetate in advanced thermal energy storage systems. Their technology utilizes the phase change properties of sodium acetate trihydrate to store and release heat efficiently. The company has developed a range of thermal batteries that can store heat from various sources, including renewable energy and waste heat from industrial processes. These batteries can provide on-demand heating and cooling for residential and commercial applications. Sunamp's thermal batteries using sodium acetate have shown to be up to four times more energy-dense than water-based systems[1], allowing for compact and efficient energy storage solutions.
Strengths: High energy density, compact design, and versatility in heat storage applications. Weaknesses: Potential for supercooling and crystallization issues in sodium acetate systems.
Altris AB
Technical Solution: Altris AB is at the forefront of sodium-ion battery technology, utilizing sodium acetate as a precursor for cathode materials. The company has developed a proprietary Prussian Blue analogue cathode material called Fennac®, which is produced using a low-temperature, water-based process with sodium acetate as a key ingredient. This innovative approach allows for the production of high-performance cathode materials without the need for lithium, cobalt, or nickel. Altris' technology has demonstrated energy densities comparable to lithium-ion phosphate batteries[2], with the potential for further improvements. The company is scaling up production to meet the growing demand for sustainable energy storage solutions.
Strengths: Sustainable and cost-effective cathode production, lithium-free technology. Weaknesses: Lower energy density compared to some advanced lithium-ion chemistries, relatively new technology in the market.
Innovative Sodium Acetate Research Breakthroughs
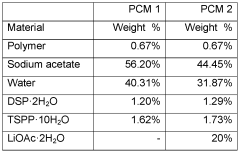
Improved phase change compositions
PatentActiveIN11003DELNP2015A
Innovation
- Aqueous compositions containing sodium acetate trihydrate, an alkali soluble polymer to inhibit anhydrous crystal formation, and a nucleation promoter to promote stable phase changes, ensuring resistance to sodium acetate crystallization and maintaining thermodynamic stability across repeated heating and cooling cycles.
Improved phase change compositions
PatentWO2014195691A1
Innovation
- Incorporating an alkali soluble polymer and a nucleation promoter into sodium acetate trihydrate compositions to inhibit the formation of sodium acetate anhydrous crystals and promote nucleation, thereby maintaining thermodynamic stability and homogeneity during heating and cooling cycles.
Environmental Impact of Sodium Acetate Use
The environmental impact of sodium acetate use is a critical consideration as its applications continue to expand across various industries. One of the primary concerns is the potential for increased sodium levels in aquatic ecosystems when sodium acetate is used as a de-icing agent on roads and runways. While it is generally considered less harmful than traditional rock salt, prolonged use can still lead to elevated salinity in nearby water bodies, affecting aquatic life and vegetation.
However, sodium acetate offers some environmental benefits compared to alternative compounds. Its biodegradability reduces long-term accumulation in the environment, and it has a lower corrosive effect on infrastructure and vehicles. This can indirectly contribute to reduced environmental impact by extending the lifespan of materials and decreasing the need for frequent replacements.
In industrial applications, such as textile manufacturing and oil drilling, the use of sodium acetate can have mixed environmental consequences. While it may help reduce the use of more harmful chemicals in certain processes, improper disposal of sodium acetate-containing waste can still lead to localized environmental issues. Proper waste management and treatment protocols are essential to mitigate these risks.
The production of sodium acetate itself also carries environmental implications. The manufacturing process typically involves the reaction of acetic acid with sodium hydroxide or sodium carbonate. While these processes are well-established, they still require energy inputs and may generate waste products. Ongoing research is focused on developing more sustainable production methods, including the use of renewable feedstocks and energy-efficient processes.
As sodium acetate finds increasing use in energy storage applications, particularly in phase change materials for thermal energy storage, its environmental profile becomes more complex. On one hand, these applications can contribute to more efficient energy use and reduced reliance on fossil fuels. On the other hand, the large-scale production and eventual disposal of these materials present new environmental challenges that need to be carefully managed.
Future trends in sodium acetate research and applications are likely to focus on enhancing its environmental performance. This may include developing more eco-friendly production methods, improving its efficiency in various applications to reduce overall consumption, and exploring new uses that leverage its biodegradability. Additionally, research into the long-term ecological effects of sodium acetate use in different contexts will be crucial for informed decision-making and sustainable practices.
However, sodium acetate offers some environmental benefits compared to alternative compounds. Its biodegradability reduces long-term accumulation in the environment, and it has a lower corrosive effect on infrastructure and vehicles. This can indirectly contribute to reduced environmental impact by extending the lifespan of materials and decreasing the need for frequent replacements.
In industrial applications, such as textile manufacturing and oil drilling, the use of sodium acetate can have mixed environmental consequences. While it may help reduce the use of more harmful chemicals in certain processes, improper disposal of sodium acetate-containing waste can still lead to localized environmental issues. Proper waste management and treatment protocols are essential to mitigate these risks.
The production of sodium acetate itself also carries environmental implications. The manufacturing process typically involves the reaction of acetic acid with sodium hydroxide or sodium carbonate. While these processes are well-established, they still require energy inputs and may generate waste products. Ongoing research is focused on developing more sustainable production methods, including the use of renewable feedstocks and energy-efficient processes.
As sodium acetate finds increasing use in energy storage applications, particularly in phase change materials for thermal energy storage, its environmental profile becomes more complex. On one hand, these applications can contribute to more efficient energy use and reduced reliance on fossil fuels. On the other hand, the large-scale production and eventual disposal of these materials present new environmental challenges that need to be carefully managed.
Future trends in sodium acetate research and applications are likely to focus on enhancing its environmental performance. This may include developing more eco-friendly production methods, improving its efficiency in various applications to reduce overall consumption, and exploring new uses that leverage its biodegradability. Additionally, research into the long-term ecological effects of sodium acetate use in different contexts will be crucial for informed decision-making and sustainable practices.
Regulatory Framework for Sodium Acetate Products
The regulatory framework for sodium acetate products is evolving to address the growing applications and potential environmental impacts of this versatile compound. In the United States, the Food and Drug Administration (FDA) regulates sodium acetate as a generally recognized as safe (GRAS) substance for use in food products. However, as new applications emerge, particularly in pharmaceuticals and industrial processes, regulatory bodies are reassessing their guidelines.
The Environmental Protection Agency (EPA) is considering stricter regulations on the industrial use of sodium acetate, particularly in wastewater treatment and de-icing applications. These potential changes aim to mitigate environmental concerns related to water quality and aquatic ecosystems. Manufacturers and users of sodium acetate may need to adapt their processes to comply with more stringent discharge limits and handling requirements.
In the European Union, the European Chemicals Agency (ECHA) is reviewing the registration, evaluation, authorization, and restriction of chemicals (REACH) dossier for sodium acetate. This review could lead to updated safety assessments and potentially new restrictions on certain applications. Companies operating in the EU market should closely monitor these developments to ensure continued compliance.
The pharmaceutical industry is experiencing increased scrutiny regarding the use of sodium acetate in drug formulations. Regulatory agencies, including the FDA and the European Medicines Agency (EMA), are implementing more rigorous testing and documentation requirements for sodium acetate as an excipient. This trend is likely to continue, with a focus on ensuring product safety and efficacy across various drug delivery systems.
Globally, there is a push towards harmonizing regulations for chemical substances, including sodium acetate. The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) is being adopted by more countries, which will impact the labeling and safety data sheet requirements for sodium acetate products. This harmonization effort aims to facilitate international trade while maintaining high safety standards.
As sustainability becomes a key focus in regulatory frameworks, future regulations may incentivize the development of more environmentally friendly production methods for sodium acetate. This could include requirements for reduced carbon footprints, increased use of renewable energy in manufacturing processes, and the implementation of circular economy principles in product lifecycle management.
The Environmental Protection Agency (EPA) is considering stricter regulations on the industrial use of sodium acetate, particularly in wastewater treatment and de-icing applications. These potential changes aim to mitigate environmental concerns related to water quality and aquatic ecosystems. Manufacturers and users of sodium acetate may need to adapt their processes to comply with more stringent discharge limits and handling requirements.
In the European Union, the European Chemicals Agency (ECHA) is reviewing the registration, evaluation, authorization, and restriction of chemicals (REACH) dossier for sodium acetate. This review could lead to updated safety assessments and potentially new restrictions on certain applications. Companies operating in the EU market should closely monitor these developments to ensure continued compliance.
The pharmaceutical industry is experiencing increased scrutiny regarding the use of sodium acetate in drug formulations. Regulatory agencies, including the FDA and the European Medicines Agency (EMA), are implementing more rigorous testing and documentation requirements for sodium acetate as an excipient. This trend is likely to continue, with a focus on ensuring product safety and efficacy across various drug delivery systems.
Globally, there is a push towards harmonizing regulations for chemical substances, including sodium acetate. The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) is being adopted by more countries, which will impact the labeling and safety data sheet requirements for sodium acetate products. This harmonization effort aims to facilitate international trade while maintaining high safety standards.
As sustainability becomes a key focus in regulatory frameworks, future regulations may incentivize the development of more environmentally friendly production methods for sodium acetate. This could include requirements for reduced carbon footprints, increased use of renewable energy in manufacturing processes, and the implementation of circular economy principles in product lifecycle management.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!