Guidance on ICP-MS Calibration Curve Handling for Precision
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Technology Evolution and Calibration Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the early 1980s. Initially developed as an analytical technique for elemental analysis, ICP-MS has transformed from a specialized research tool into a mainstream analytical method across various industries including environmental monitoring, pharmaceuticals, semiconductor manufacturing, and clinical diagnostics. The technology's evolution has been characterized by continuous improvements in sensitivity, precision, interference reduction, and sample throughput capabilities.
Early ICP-MS systems faced significant challenges with spectral interferences and limited dynamic range. The introduction of collision/reaction cell technology in the late 1990s marked a pivotal advancement, enabling more accurate analysis of complex matrices by reducing polyatomic interferences. Subsequently, high-resolution mass analyzers and triple quadrupole systems further enhanced the technology's capabilities for trace element analysis.
The calibration methodologies for ICP-MS have similarly evolved from simple external calibration approaches to more sophisticated techniques including isotope dilution, standard addition, and internal standardization. These developments reflect the growing demand for higher accuracy and precision across increasingly diverse analytical applications.
Current technological trends in ICP-MS focus on enhancing calibration stability, expanding dynamic range, and improving robustness for challenging sample types. The integration of automated quality control procedures and advanced data processing algorithms has become essential for maintaining calibration integrity across extended analytical sequences.
The primary objective in modern ICP-MS calibration is achieving optimal precision while maintaining practical operational efficiency. This requires balancing theoretical analytical performance with real-world constraints such as sample throughput requirements, matrix complexity, and regulatory compliance needs. Specifically for calibration curve handling, the goal is to establish protocols that ensure consistent performance across diverse analytical scenarios while minimizing systematic errors.
Key technical objectives include developing standardized approaches for calibration curve evaluation, establishing robust criteria for linearity assessment, and implementing effective strategies for handling non-linear responses at extreme concentration ranges. Additionally, there is growing emphasis on quantifying and minimizing uncertainty contributions from calibration processes to overall measurement uncertainty.
As ICP-MS applications continue to expand into new fields requiring ultra-trace analysis and enhanced specificity, calibration methodologies must evolve accordingly. Future developments will likely focus on intelligent calibration systems that can adapt to changing sample conditions and provide real-time quality assurance through advanced statistical monitoring and machine learning algorithms.
Early ICP-MS systems faced significant challenges with spectral interferences and limited dynamic range. The introduction of collision/reaction cell technology in the late 1990s marked a pivotal advancement, enabling more accurate analysis of complex matrices by reducing polyatomic interferences. Subsequently, high-resolution mass analyzers and triple quadrupole systems further enhanced the technology's capabilities for trace element analysis.
The calibration methodologies for ICP-MS have similarly evolved from simple external calibration approaches to more sophisticated techniques including isotope dilution, standard addition, and internal standardization. These developments reflect the growing demand for higher accuracy and precision across increasingly diverse analytical applications.
Current technological trends in ICP-MS focus on enhancing calibration stability, expanding dynamic range, and improving robustness for challenging sample types. The integration of automated quality control procedures and advanced data processing algorithms has become essential for maintaining calibration integrity across extended analytical sequences.
The primary objective in modern ICP-MS calibration is achieving optimal precision while maintaining practical operational efficiency. This requires balancing theoretical analytical performance with real-world constraints such as sample throughput requirements, matrix complexity, and regulatory compliance needs. Specifically for calibration curve handling, the goal is to establish protocols that ensure consistent performance across diverse analytical scenarios while minimizing systematic errors.
Key technical objectives include developing standardized approaches for calibration curve evaluation, establishing robust criteria for linearity assessment, and implementing effective strategies for handling non-linear responses at extreme concentration ranges. Additionally, there is growing emphasis on quantifying and minimizing uncertainty contributions from calibration processes to overall measurement uncertainty.
As ICP-MS applications continue to expand into new fields requiring ultra-trace analysis and enhanced specificity, calibration methodologies must evolve accordingly. Future developments will likely focus on intelligent calibration systems that can adapt to changing sample conditions and provide real-time quality assurance through advanced statistical monitoring and machine learning algorithms.
Market Demand for High-Precision Elemental Analysis
The global market for high-precision elemental analysis has experienced substantial growth in recent years, driven by increasing demands across multiple industries for accurate trace element detection and quantification. ICP-MS (Inductively Coupled Plasma Mass Spectrometry) technology has emerged as the gold standard for these applications due to its exceptional sensitivity, multi-element capabilities, and wide dynamic range.
The pharmaceutical sector represents one of the largest market segments, with stringent regulatory requirements necessitating precise elemental analysis for quality control and safety assurance. According to market research, pharmaceutical companies are increasingly investing in advanced analytical technologies to comply with regulations such as ICH Q3D guidelines for elemental impurities, creating a steady demand for improved calibration methodologies.
Environmental monitoring constitutes another significant market driver, with government agencies and private organizations requiring highly accurate elemental analysis for water, soil, and air quality assessment. The growing public concern over environmental contamination has led to more stringent monitoring protocols, further stimulating market growth for precision analytical instruments and calibration solutions.
The food and beverage industry has also become a major consumer of high-precision elemental analysis, particularly as consumer awareness regarding food safety increases. Manufacturers must ensure their products meet regulatory standards for toxic elements while also verifying nutritional content claims, creating substantial demand for reliable calibration procedures that can deliver consistent results across testing facilities.
Semiconductor and electronics manufacturing represents a rapidly expanding market segment where ultra-trace elemental analysis is critical for quality control. As device miniaturization continues, even minute elemental contamination can significantly impact performance, driving demand for increasingly sensitive and precise analytical methods with robust calibration protocols.
Academic and research institutions form a stable market base, consistently requiring advanced analytical capabilities for various scientific investigations. The push toward reproducible research has heightened focus on standardized calibration methodologies that can ensure comparable results across different laboratories and studies.
Market analysis indicates that organizations are increasingly seeking integrated solutions that combine advanced instrumentation with sophisticated calibration software to minimize human error and improve analytical precision. This trend is particularly evident in regulated industries where audit trails and data integrity are paramount concerns, creating opportunities for comprehensive calibration management systems.
The pharmaceutical sector represents one of the largest market segments, with stringent regulatory requirements necessitating precise elemental analysis for quality control and safety assurance. According to market research, pharmaceutical companies are increasingly investing in advanced analytical technologies to comply with regulations such as ICH Q3D guidelines for elemental impurities, creating a steady demand for improved calibration methodologies.
Environmental monitoring constitutes another significant market driver, with government agencies and private organizations requiring highly accurate elemental analysis for water, soil, and air quality assessment. The growing public concern over environmental contamination has led to more stringent monitoring protocols, further stimulating market growth for precision analytical instruments and calibration solutions.
The food and beverage industry has also become a major consumer of high-precision elemental analysis, particularly as consumer awareness regarding food safety increases. Manufacturers must ensure their products meet regulatory standards for toxic elements while also verifying nutritional content claims, creating substantial demand for reliable calibration procedures that can deliver consistent results across testing facilities.
Semiconductor and electronics manufacturing represents a rapidly expanding market segment where ultra-trace elemental analysis is critical for quality control. As device miniaturization continues, even minute elemental contamination can significantly impact performance, driving demand for increasingly sensitive and precise analytical methods with robust calibration protocols.
Academic and research institutions form a stable market base, consistently requiring advanced analytical capabilities for various scientific investigations. The push toward reproducible research has heightened focus on standardized calibration methodologies that can ensure comparable results across different laboratories and studies.
Market analysis indicates that organizations are increasingly seeking integrated solutions that combine advanced instrumentation with sophisticated calibration software to minimize human error and improve analytical precision. This trend is particularly evident in regulated industries where audit trails and data integrity are paramount concerns, creating opportunities for comprehensive calibration management systems.
Current Challenges in ICP-MS Calibration Methodology
Despite significant advancements in ICP-MS technology, several persistent challenges continue to affect calibration methodology and ultimately impact measurement precision. The most fundamental issue remains matrix effects, where sample composition interferes with analyte ionization efficiency, causing signal suppression or enhancement that distorts calibration curves. These effects vary unpredictably across different sample types, making standardized calibration approaches difficult to implement across diverse analytical scenarios.
Spectral interferences present another significant challenge, with polyatomic ions and isobaric overlaps creating false signals that compromise calibration accuracy. While collision/reaction cell technologies have improved this situation, complete elimination of all interferences remains elusive, particularly in complex matrices where multiple interference mechanisms may operate simultaneously.
Instrument drift continues to plague long analytical sequences, with sensitivity changes over time leading to calibration curve instability. Current drift correction methods using internal standards are imperfect, as they assume uniform drift across the mass range—an assumption often violated in practice, especially for multi-element analyses spanning wide mass ranges.
The linear dynamic range limitations of ICP-MS systems create additional calibration complexities. While modern instruments claim linear responses across 8-9 orders of magnitude, practical working ranges are often more restricted. This necessitates multiple calibration approaches for samples containing both trace and major elements, increasing analytical complexity and potential error sources.
Calibration standard quality and stability issues further complicate accurate curve generation. Even certified reference materials can contain impurities or experience concentration changes during storage. The preparation of dilution series introduces cumulative errors that propagate through the calibration process, particularly affecting low-concentration standards critical for trace analysis.
Data processing algorithms for calibration curve fitting represent another challenge area. While weighted regression methods have improved quantification at lower concentrations, optimal weighting factors vary by element and concentration range. Additionally, automated outlier rejection algorithms may sometimes eliminate valid data points, introducing subtle biases into calibration functions.
Finally, there remains significant inconsistency in calibration verification protocols across laboratories. The frequency of verification, acceptance criteria, and corrective actions vary widely, leading to different levels of confidence in analytical results between facilities using nominally identical methods.
Spectral interferences present another significant challenge, with polyatomic ions and isobaric overlaps creating false signals that compromise calibration accuracy. While collision/reaction cell technologies have improved this situation, complete elimination of all interferences remains elusive, particularly in complex matrices where multiple interference mechanisms may operate simultaneously.
Instrument drift continues to plague long analytical sequences, with sensitivity changes over time leading to calibration curve instability. Current drift correction methods using internal standards are imperfect, as they assume uniform drift across the mass range—an assumption often violated in practice, especially for multi-element analyses spanning wide mass ranges.
The linear dynamic range limitations of ICP-MS systems create additional calibration complexities. While modern instruments claim linear responses across 8-9 orders of magnitude, practical working ranges are often more restricted. This necessitates multiple calibration approaches for samples containing both trace and major elements, increasing analytical complexity and potential error sources.
Calibration standard quality and stability issues further complicate accurate curve generation. Even certified reference materials can contain impurities or experience concentration changes during storage. The preparation of dilution series introduces cumulative errors that propagate through the calibration process, particularly affecting low-concentration standards critical for trace analysis.
Data processing algorithms for calibration curve fitting represent another challenge area. While weighted regression methods have improved quantification at lower concentrations, optimal weighting factors vary by element and concentration range. Additionally, automated outlier rejection algorithms may sometimes eliminate valid data points, introducing subtle biases into calibration functions.
Finally, there remains significant inconsistency in calibration verification protocols across laboratories. The frequency of verification, acceptance criteria, and corrective actions vary widely, leading to different levels of confidence in analytical results between facilities using nominally identical methods.
Standard Calibration Curve Approaches and Best Practices
01 Calibration methods for ICP-MS precision improvement
Various calibration methods can be employed to enhance the precision of ICP-MS measurements. These include internal standardization, standard addition methods, and matrix-matched calibration approaches. These techniques compensate for matrix effects and instrumental drift, leading to more reliable calibration curves and improved analytical precision. Proper calibration method selection depends on sample complexity and the specific elements being analyzed.- Calibration methods for ICP-MS analysis: Various calibration methods are employed to enhance the precision of ICP-MS measurements. These include external calibration using standard solutions, internal standardization to compensate for matrix effects and instrument drift, isotope dilution techniques for high-accuracy quantification, and standard addition methods for complex matrices. These calibration approaches are critical for establishing reliable calibration curves with improved precision across different concentration ranges.
- Matrix effect compensation techniques: Matrix effects can significantly impact the precision of ICP-MS calibration curves. Techniques to compensate for these effects include using matrix-matched calibration standards, applying mathematical correction algorithms, employing collision/reaction cell technology, and optimizing sample preparation methods. These approaches help minimize signal suppression or enhancement caused by sample matrix components, resulting in more accurate and precise calibration curves.
- Instrument optimization for improved precision: Optimizing ICP-MS instrument parameters is essential for achieving high precision in calibration curves. This includes tuning of plasma conditions, optimizing ion optics, selecting appropriate detector modes for different concentration ranges, and implementing automated quality control procedures. Regular performance checks and maintenance routines ensure consistent instrument response, which is crucial for maintaining calibration curve precision over time.
- Statistical methods for calibration curve evaluation: Advanced statistical approaches are applied to evaluate and improve ICP-MS calibration curve precision. These include weighted regression analysis, robust statistical methods for outlier detection, uncertainty estimation techniques, and validation protocols to assess linearity, range, and detection limits. Statistical quality control measures help monitor and maintain calibration performance during routine analysis, ensuring reliable quantitative results.
- Automated calibration systems and software solutions: Automated calibration systems and specialized software solutions enhance the precision of ICP-MS calibration curves. These include intelligent calibration algorithms, automated standard preparation systems, real-time drift correction software, and integrated data processing tools. These technologies reduce human error, improve reproducibility, and enable more sophisticated calibration models that can adapt to changing instrument conditions, ultimately leading to more precise analytical results.
02 Sample preparation techniques for enhanced calibration curve precision
Effective sample preparation is crucial for achieving high precision in ICP-MS calibration curves. Techniques such as microwave digestion, acid dissolution, and dilution protocols help minimize matrix interferences and contamination. Proper sample homogenization and stabilization prior to analysis ensure representative measurements and improved calibration curve linearity, directly impacting the precision of analytical results.Expand Specific Solutions03 Advanced data processing algorithms for calibration curve optimization
Sophisticated data processing algorithms can significantly improve ICP-MS calibration curve precision. These include weighted regression models, outlier detection systems, and drift correction algorithms. Machine learning approaches can also be applied to optimize calibration models and compensate for non-linear responses. These computational methods enhance the reliability of calibration curves, particularly for complex sample matrices and wide concentration ranges.Expand Specific Solutions04 Hardware modifications and instrumental parameters for precision enhancement
Hardware configurations and instrumental parameter optimization play vital roles in achieving high-precision ICP-MS calibration curves. Innovations include improved nebulizer designs, collision/reaction cell technologies, and detector sensitivity enhancements. Optimizing parameters such as plasma power, gas flows, and integration times can significantly reduce signal fluctuations and improve measurement stability, resulting in more precise calibration curves.Expand Specific Solutions05 Quality control protocols for maintaining calibration curve precision
Implementing robust quality control protocols is essential for maintaining high precision in ICP-MS calibration curves. These include regular system performance checks, certified reference material analysis, and control chart monitoring. Periodic recalibration, blank measurements, and duplicate sample analysis help identify and correct for instrumental drift and other sources of error, ensuring consistent calibration curve precision over extended analytical sequences.Expand Specific Solutions
Leading Manufacturers and Research Institutions in ICP-MS
The ICP-MS calibration curve handling market is in a mature growth phase, with an estimated global market size of $1.5-2 billion and steady annual growth of 5-7%. Leading players include established analytical instrumentation companies like Thermo Fisher Scientific, Agilent Technologies, and Revvity Health Sciences (formerly PerkinElmer), who dominate with comprehensive solutions offering high precision and reliability. Emerging competitors such as Elemental Scientific and Shimazu KK are gaining market share through specialized calibration solutions. The technology has reached high maturity with standardized protocols, though innovation continues in automation, machine learning for curve fitting, and integration with laboratory information management systems to enhance precision and reproducibility in diverse analytical applications.
Thermo Fisher Scientific (Bremen) GmbH
Technical Solution: Thermo Fisher Scientific has developed comprehensive ICP-MS calibration solutions centered around their iCAP series instruments. Their approach incorporates multi-level calibration curves with internal standardization to compensate for matrix effects and instrument drift. The company's proprietary Qtegra Intelligent Scientific Data Solution (ISDS) software features automated calibration curve handling with dynamic weighting factors that adjust based on concentration ranges[1]. Their technology implements automatic detection of non-linear responses at high concentrations and applies appropriate mathematical models (quadratic or cubic functions) when necessary. For ultra-trace analysis, Thermo Fisher employs collision/reaction cell technology in conjunction with optimized calibration protocols to achieve detection limits in the sub-ppt range while maintaining linearity across 9-10 orders of magnitude[3].
Strengths: Industry-leading software integration with automated calibration verification and quality control monitoring. Exceptional dynamic range handling with intelligent curve fitting algorithms. Weaknesses: Proprietary software ecosystem may create vendor lock-in, and the advanced calibration features require significant user training to fully utilize.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has pioneered the High Matrix Introduction (HMI) system for ICP-MS calibration, specifically designed to handle complex sample matrices without compromising precision. Their ICP-MS MassHunter software implements intelligent calibration curve handling with automatic outlier detection and removal capabilities[2]. Agilent's approach incorporates multi-internal standard correction algorithms that dynamically select the optimal internal standard based on ionization potential and mass proximity to analytes. The company's calibration technology features weighted regression models that apply greater statistical weight to lower concentration standards, improving accuracy at trace levels. Additionally, Agilent has developed the Ultra High Matrix Introduction (UHMI) system that allows direct analysis of samples containing up to 25% total dissolved solids while maintaining calibration integrity across multiple orders of magnitude[4].
Strengths: Superior matrix tolerance with HMI/UHMI technology enables accurate calibration even with challenging sample types. Advanced internal standardization algorithms compensate for complex matrix effects. Weaknesses: Higher initial investment compared to some competitors, and calibration optimization for specific applications can require specialized expertise.
Advanced Algorithms for Calibration Curve Optimization
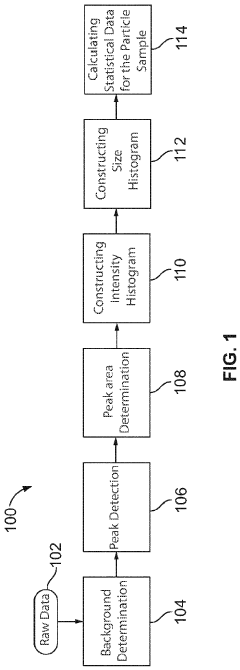
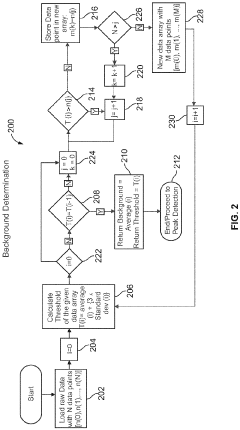
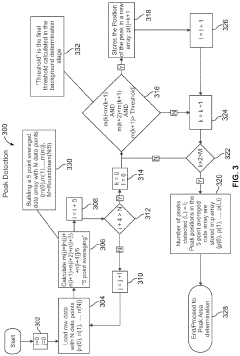
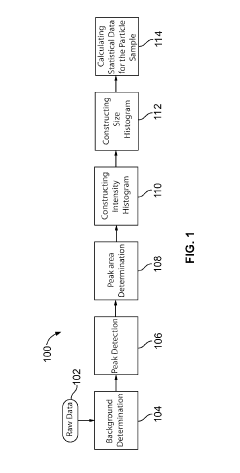
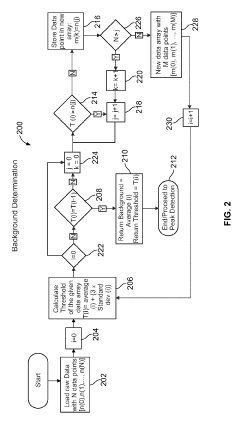
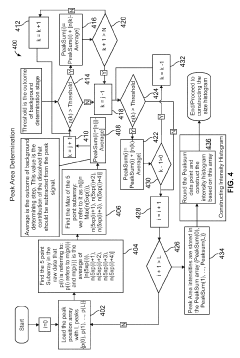
System and method for automated analysis of output in single particle inductively coupled plasma mass spectrometry and similar data sets
PatentInactiveEP3937207A1
Innovation
- An automated method for analyzing spectrometry data that involves accessing pulse count values from ICP-MS systems, determining a threshold to differentiate peak signals from background noise, and constructing histograms to calculate particle mass, size distribution, and concentration, reducing the need for extensive sample dilutions and minimizing human error.
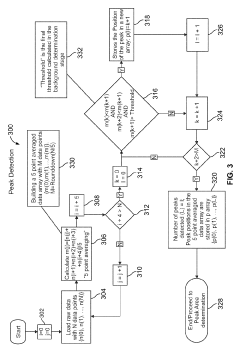
Systems and methods for automated analysis of output in single particle inductively coupled plasma mass spectrometry and similar data sets
PatentActiveUS10431444B2
Innovation
- An automated method for analyzing SP-ICP-MS data sets that involves accessing multiple pulse count values for each detected particle, determining a threshold to differentiate peak signals from background signals, and constructing histograms for particle mass, size, and concentration, allowing for efficient and precise determination of particle characteristics without extensive dilutions.
Validation Protocols and Quality Assurance Standards
Validation protocols for ICP-MS calibration curve handling must adhere to stringent quality assurance standards to ensure analytical precision and reliability. The foundation of these protocols begins with the establishment of comprehensive Standard Operating Procedures (SOPs) that detail calibration methodologies, acceptance criteria, and corrective actions. These SOPs should incorporate guidelines from regulatory bodies such as ISO/IEC 17025, FDA, EPA, and USP, which provide frameworks for analytical method validation in various industries.
Critical to validation is the implementation of multi-point calibration curves spanning the expected concentration range of analytes, typically requiring a minimum of five concentration levels. The linearity assessment must demonstrate correlation coefficients (r²) exceeding 0.995, with particular attention to the lower limit of quantification (LLOQ) where signal-to-noise ratios should be maintained above 10:1. Regular verification of these calibration curves through quality control samples is essential, with acceptance criteria typically set at ±10% of nominal values.
Robust quality assurance standards necessitate the inclusion of certified reference materials (CRMs) traceable to national or international standards. These CRMs serve as independent verification points and should be analyzed at frequencies determined by risk assessment and regulatory requirements. Internal standards must be carefully selected based on ionization potential and mass proximity to target analytes, with recovery rates monitored to fall within 80-120% of expected values.
Statistical process control tools play a vital role in ongoing monitoring of calibration performance. Control charts tracking calibration slope, intercept, and correlation coefficient over time enable early detection of instrumental drift or methodological issues. Establishing warning and action limits (typically at ±2σ and ±3σ respectively) provides objective criteria for intervention when calibration parameters deviate from historical norms.
Inter-laboratory proficiency testing represents another essential component of validation protocols, allowing comparison of calibration methodologies across different facilities and instruments. Participation in such programs should occur at least annually, with z-scores within ±2 considered acceptable performance. Documentation of all calibration activities, including raw data, calculations, and deviation investigations, must be maintained in compliance with data integrity principles.
System suitability tests conducted prior to analytical runs verify that the ICP-MS system meets minimum performance requirements for sensitivity, mass resolution, and oxide formation rates. These tests establish baseline performance metrics against which calibration curve quality can be evaluated, ensuring that instrumental variations do not compromise analytical precision.
Critical to validation is the implementation of multi-point calibration curves spanning the expected concentration range of analytes, typically requiring a minimum of five concentration levels. The linearity assessment must demonstrate correlation coefficients (r²) exceeding 0.995, with particular attention to the lower limit of quantification (LLOQ) where signal-to-noise ratios should be maintained above 10:1. Regular verification of these calibration curves through quality control samples is essential, with acceptance criteria typically set at ±10% of nominal values.
Robust quality assurance standards necessitate the inclusion of certified reference materials (CRMs) traceable to national or international standards. These CRMs serve as independent verification points and should be analyzed at frequencies determined by risk assessment and regulatory requirements. Internal standards must be carefully selected based on ionization potential and mass proximity to target analytes, with recovery rates monitored to fall within 80-120% of expected values.
Statistical process control tools play a vital role in ongoing monitoring of calibration performance. Control charts tracking calibration slope, intercept, and correlation coefficient over time enable early detection of instrumental drift or methodological issues. Establishing warning and action limits (typically at ±2σ and ±3σ respectively) provides objective criteria for intervention when calibration parameters deviate from historical norms.
Inter-laboratory proficiency testing represents another essential component of validation protocols, allowing comparison of calibration methodologies across different facilities and instruments. Participation in such programs should occur at least annually, with z-scores within ±2 considered acceptable performance. Documentation of all calibration activities, including raw data, calculations, and deviation investigations, must be maintained in compliance with data integrity principles.
System suitability tests conducted prior to analytical runs verify that the ICP-MS system meets minimum performance requirements for sensitivity, mass resolution, and oxide formation rates. These tests establish baseline performance metrics against which calibration curve quality can be evaluated, ensuring that instrumental variations do not compromise analytical precision.
Matrix Effect Mitigation Strategies
Matrix effects represent one of the most significant challenges in ICP-MS analysis, potentially causing substantial measurement bias if not properly addressed. These effects occur when components in the sample matrix interfere with analyte ionization, leading to signal enhancement or suppression. For precise calibration curve handling, implementing effective matrix effect mitigation strategies is essential. Internal standardization remains the cornerstone approach, where elements with similar mass and ionization potential to the analytes are added to all samples and standards at constant concentrations. This technique compensates for both matrix effects and instrument drift, with elements such as Indium (In), Rhodium (Rh), and Yttrium (Y) commonly employed for different mass ranges.
Matrix matching represents another fundamental strategy, where calibration standards are prepared in a matrix similar to the samples being analyzed. This approach ensures that matrix effects impact both standards and samples equivalently, thereby minimizing differential response. For complex or variable matrices, the standard addition method offers superior accuracy by adding known quantities of analytes directly to sample aliquots, effectively creating a calibration curve within the actual sample matrix.
Advanced instrumental techniques also play crucial roles in mitigating matrix effects. Collision/reaction cell technology effectively reduces polyatomic interferences by introducing collision gases (helium) or reaction gases (hydrogen, oxygen) that selectively interact with interfering species. High-resolution ICP-MS instruments can separate analytes from interferences based on slight mass differences, though at the cost of reduced sensitivity.
Sample preparation strategies constitute another critical dimension of matrix effect management. Dilution, while straightforward, can effectively reduce matrix effects when analyte concentrations remain sufficiently high. More sophisticated approaches include matrix separation techniques such as solid-phase extraction, co-precipitation, or chromatographic methods that physically separate analytes from interfering matrix components.
Mathematical correction models have gained prominence with advances in computational capabilities. These include multivariate calibration methods like partial least squares (PLS) and artificial neural networks (ANN) that can model complex matrix-analyte interactions. Such approaches are particularly valuable when dealing with highly variable or unpredictable sample matrices where physical mitigation strategies alone prove insufficient.
For optimal precision in ICP-MS calibration curves, laboratories typically implement a combination of these strategies tailored to specific analytical requirements. The selection depends on factors including sample complexity, required detection limits, available instrumentation, and throughput considerations. Regular validation using certified reference materials remains essential to verify the effectiveness of the chosen matrix effect mitigation approach.
Matrix matching represents another fundamental strategy, where calibration standards are prepared in a matrix similar to the samples being analyzed. This approach ensures that matrix effects impact both standards and samples equivalently, thereby minimizing differential response. For complex or variable matrices, the standard addition method offers superior accuracy by adding known quantities of analytes directly to sample aliquots, effectively creating a calibration curve within the actual sample matrix.
Advanced instrumental techniques also play crucial roles in mitigating matrix effects. Collision/reaction cell technology effectively reduces polyatomic interferences by introducing collision gases (helium) or reaction gases (hydrogen, oxygen) that selectively interact with interfering species. High-resolution ICP-MS instruments can separate analytes from interferences based on slight mass differences, though at the cost of reduced sensitivity.
Sample preparation strategies constitute another critical dimension of matrix effect management. Dilution, while straightforward, can effectively reduce matrix effects when analyte concentrations remain sufficiently high. More sophisticated approaches include matrix separation techniques such as solid-phase extraction, co-precipitation, or chromatographic methods that physically separate analytes from interfering matrix components.
Mathematical correction models have gained prominence with advances in computational capabilities. These include multivariate calibration methods like partial least squares (PLS) and artificial neural networks (ANN) that can model complex matrix-analyte interactions. Such approaches are particularly valuable when dealing with highly variable or unpredictable sample matrices where physical mitigation strategies alone prove insufficient.
For optimal precision in ICP-MS calibration curves, laboratories typically implement a combination of these strategies tailored to specific analytical requirements. The selection depends on factors including sample complexity, required detection limits, available instrumentation, and throughput considerations. Regular validation using certified reference materials remains essential to verify the effectiveness of the chosen matrix effect mitigation approach.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!