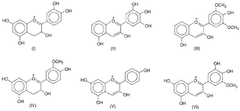
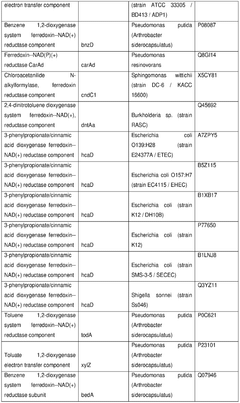
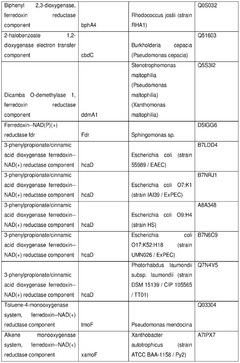
Harnessing cell-free techniques for plant-based compound synthesis.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-Free Synthesis Background and Objectives
Cell-free synthesis represents a revolutionary approach in biotechnology that has evolved significantly over the past three decades. Initially developed for protein production, this technology has expanded to encompass the synthesis of various biomolecules by utilizing cellular machinery outside the constraints of intact cells. The evolution of cell-free systems began with crude extracts and has progressed to highly refined and engineered systems capable of producing complex compounds with remarkable efficiency.
In the context of plant-based compound synthesis, cell-free techniques offer unique advantages over traditional cultivation methods. Plants produce an extensive array of specialized metabolites with pharmaceutical, agricultural, and industrial applications. However, extracting these compounds from whole plants often presents challenges including low yields, seasonal variability, and sustainability concerns. Cell-free systems circumvent these limitations by providing a controlled environment for biosynthetic pathway operation.
The primary objective of harnessing cell-free techniques for plant-based compound synthesis is to establish scalable, sustainable, and economically viable production platforms for high-value plant metabolites. This includes developing systems capable of synthesizing complex molecules such as alkaloids, terpenoids, flavonoids, and other specialized metabolites that traditionally require extensive cultivation and extraction processes.
Technical goals encompass optimizing extract preparation from plant sources, enhancing the stability and longevity of cell-free reactions, and engineering metabolic pathways for improved product yields. Additionally, there is significant focus on developing modular and standardized cell-free platforms that can be rapidly adapted for different target compounds, thereby accelerating the transition from laboratory discovery to industrial application.
Recent technological advancements in synthetic biology, metabolic engineering, and analytical chemistry have substantially accelerated progress in this field. The integration of computational tools for pathway design and optimization has further enhanced the capabilities of cell-free systems for complex molecule synthesis. These developments align with broader trends in biotechnology toward more sustainable and efficient production methods.
The convergence of plant biochemistry with cell-free synthetic biology represents a promising frontier for addressing challenges in pharmaceutical supply chains, agricultural innovation, and sustainable chemical production. As this technology continues to mature, it holds potential to transform how we access and utilize plant-derived compounds across multiple industries, reducing dependence on traditional agricultural practices and enabling novel applications previously constrained by limited compound availability.
In the context of plant-based compound synthesis, cell-free techniques offer unique advantages over traditional cultivation methods. Plants produce an extensive array of specialized metabolites with pharmaceutical, agricultural, and industrial applications. However, extracting these compounds from whole plants often presents challenges including low yields, seasonal variability, and sustainability concerns. Cell-free systems circumvent these limitations by providing a controlled environment for biosynthetic pathway operation.
The primary objective of harnessing cell-free techniques for plant-based compound synthesis is to establish scalable, sustainable, and economically viable production platforms for high-value plant metabolites. This includes developing systems capable of synthesizing complex molecules such as alkaloids, terpenoids, flavonoids, and other specialized metabolites that traditionally require extensive cultivation and extraction processes.
Technical goals encompass optimizing extract preparation from plant sources, enhancing the stability and longevity of cell-free reactions, and engineering metabolic pathways for improved product yields. Additionally, there is significant focus on developing modular and standardized cell-free platforms that can be rapidly adapted for different target compounds, thereby accelerating the transition from laboratory discovery to industrial application.
Recent technological advancements in synthetic biology, metabolic engineering, and analytical chemistry have substantially accelerated progress in this field. The integration of computational tools for pathway design and optimization has further enhanced the capabilities of cell-free systems for complex molecule synthesis. These developments align with broader trends in biotechnology toward more sustainable and efficient production methods.
The convergence of plant biochemistry with cell-free synthetic biology represents a promising frontier for addressing challenges in pharmaceutical supply chains, agricultural innovation, and sustainable chemical production. As this technology continues to mature, it holds potential to transform how we access and utilize plant-derived compounds across multiple industries, reducing dependence on traditional agricultural practices and enabling novel applications previously constrained by limited compound availability.
Market Analysis for Plant-Based Compounds
The global market for plant-based compounds has experienced significant growth in recent years, driven by increasing consumer awareness of health benefits, sustainability concerns, and technological advancements in extraction and synthesis methods. The market value for plant-based bioactive compounds reached approximately $9.3 billion in 2022 and is projected to grow at a CAGR of 8.4% through 2030, potentially reaching $18.5 billion by the end of the forecast period.
Cell-free synthesis techniques are creating new opportunities within this market by enabling more efficient production of high-value plant compounds without the limitations of traditional cultivation. This approach addresses several key market demands, including consistent quality, reduced production time, and lower environmental impact compared to conventional agricultural methods.
The pharmaceutical sector represents the largest market segment for plant-based compounds, accounting for roughly 38% of the total market share. Compounds such as paclitaxel, artemisinin, and vinblastine continue to be essential in various therapeutic applications. The application of cell-free techniques in this sector could potentially reduce production costs by 30-45% while increasing yield consistency.
The nutraceutical and functional food segment is experiencing the fastest growth rate at approximately 10.2% annually. Consumer demand for natural health products has created substantial market opportunities for plant compounds like resveratrol, quercetin, and various polyphenols that can now be produced more efficiently through cell-free systems.
Cosmetics and personal care products constitute another significant market segment, valued at approximately $2.1 billion in 2022. The demand for natural ingredients in this sector has been steadily increasing, with consumers willing to pay premium prices for plant-derived compounds with proven efficacy.
Regionally, North America and Europe currently dominate the market with combined market share of 58%, though Asia-Pacific is expected to witness the highest growth rate over the next decade due to increasing disposable income, growing health consciousness, and expanding biotechnology infrastructure in countries like China, Japan, and India.
Market challenges include regulatory hurdles, with approval processes varying significantly across regions. Additionally, consumer perception regarding "synthetic" versus "natural" products remains a potential barrier, even when cell-free techniques produce compounds identical to their plant-derived counterparts.
The economic viability of cell-free synthesis depends heavily on scale-up capabilities and production costs. Current estimates suggest that for high-value compounds (>$500/kg), cell-free systems already offer competitive advantages over traditional extraction methods, while medium-value compounds are approaching cost parity as technology improves.
Cell-free synthesis techniques are creating new opportunities within this market by enabling more efficient production of high-value plant compounds without the limitations of traditional cultivation. This approach addresses several key market demands, including consistent quality, reduced production time, and lower environmental impact compared to conventional agricultural methods.
The pharmaceutical sector represents the largest market segment for plant-based compounds, accounting for roughly 38% of the total market share. Compounds such as paclitaxel, artemisinin, and vinblastine continue to be essential in various therapeutic applications. The application of cell-free techniques in this sector could potentially reduce production costs by 30-45% while increasing yield consistency.
The nutraceutical and functional food segment is experiencing the fastest growth rate at approximately 10.2% annually. Consumer demand for natural health products has created substantial market opportunities for plant compounds like resveratrol, quercetin, and various polyphenols that can now be produced more efficiently through cell-free systems.
Cosmetics and personal care products constitute another significant market segment, valued at approximately $2.1 billion in 2022. The demand for natural ingredients in this sector has been steadily increasing, with consumers willing to pay premium prices for plant-derived compounds with proven efficacy.
Regionally, North America and Europe currently dominate the market with combined market share of 58%, though Asia-Pacific is expected to witness the highest growth rate over the next decade due to increasing disposable income, growing health consciousness, and expanding biotechnology infrastructure in countries like China, Japan, and India.
Market challenges include regulatory hurdles, with approval processes varying significantly across regions. Additionally, consumer perception regarding "synthetic" versus "natural" products remains a potential barrier, even when cell-free techniques produce compounds identical to their plant-derived counterparts.
The economic viability of cell-free synthesis depends heavily on scale-up capabilities and production costs. Current estimates suggest that for high-value compounds (>$500/kg), cell-free systems already offer competitive advantages over traditional extraction methods, while medium-value compounds are approaching cost parity as technology improves.
Current Challenges in Cell-Free Plant Compound Synthesis
Despite significant advancements in cell-free systems for plant compound synthesis, several critical challenges continue to impede widespread industrial application and commercialization. These challenges span technical, economic, and scalability dimensions that require innovative solutions to overcome.
The complexity of plant metabolic pathways represents a fundamental obstacle. Unlike microbial systems, plant secondary metabolites often involve intricate multi-step pathways with numerous enzymes, cofactors, and regulatory elements. Reconstituting these complex networks in cell-free environments demands precise control over enzyme ratios, cofactor regeneration, and reaction conditions that remain difficult to optimize simultaneously.
Enzyme stability presents another significant hurdle. Many plant enzymes exhibit reduced activity or accelerated degradation when removed from their native cellular environment. This instability necessitates continuous enzyme replenishment or sophisticated enzyme immobilization strategies, both of which increase production costs and technical complexity. Membrane-bound enzymes pose particular difficulties, as they often require specific lipid environments to maintain proper folding and functionality.
Cofactor regeneration systems represent a critical economic bottleneck. Plant secondary metabolism frequently requires expensive cofactors such as NADPH, ATP, and S-adenosylmethionine. While regeneration systems exist, their efficiency at industrial scale remains suboptimal, leading to prohibitive production costs for many high-value compounds.
Substrate availability and solubility issues further complicate cell-free synthesis. Many plant pathway intermediates exhibit poor water solubility, creating phase separation problems that reduce reaction efficiency. Additionally, precursor feeding strategies must balance between providing sufficient substrate concentrations while avoiding inhibitory effects on enzymatic activity.
Scale-up challenges persist as a major impediment to commercialization. Laboratory-scale successes often fail to translate to industrial production due to issues with heat transfer, mixing efficiency, and reaction homogeneity. The absence of standardized bioreactor designs specifically optimized for cell-free plant compound synthesis further complicates industrial implementation.
Analytical limitations also hinder progress in the field. Real-time monitoring of complex reaction networks remains challenging, making process optimization largely empirical rather than data-driven. The lack of high-throughput screening methods for rapidly evaluating different reaction conditions slows development cycles and increases research costs.
Regulatory uncertainties surrounding cell-free produced plant compounds create additional barriers to market entry. Questions regarding "natural" versus "synthetic" classification and appropriate safety assessment frameworks remain unresolved in many jurisdictions, creating hesitancy among potential industrial adopters.
The complexity of plant metabolic pathways represents a fundamental obstacle. Unlike microbial systems, plant secondary metabolites often involve intricate multi-step pathways with numerous enzymes, cofactors, and regulatory elements. Reconstituting these complex networks in cell-free environments demands precise control over enzyme ratios, cofactor regeneration, and reaction conditions that remain difficult to optimize simultaneously.
Enzyme stability presents another significant hurdle. Many plant enzymes exhibit reduced activity or accelerated degradation when removed from their native cellular environment. This instability necessitates continuous enzyme replenishment or sophisticated enzyme immobilization strategies, both of which increase production costs and technical complexity. Membrane-bound enzymes pose particular difficulties, as they often require specific lipid environments to maintain proper folding and functionality.
Cofactor regeneration systems represent a critical economic bottleneck. Plant secondary metabolism frequently requires expensive cofactors such as NADPH, ATP, and S-adenosylmethionine. While regeneration systems exist, their efficiency at industrial scale remains suboptimal, leading to prohibitive production costs for many high-value compounds.
Substrate availability and solubility issues further complicate cell-free synthesis. Many plant pathway intermediates exhibit poor water solubility, creating phase separation problems that reduce reaction efficiency. Additionally, precursor feeding strategies must balance between providing sufficient substrate concentrations while avoiding inhibitory effects on enzymatic activity.
Scale-up challenges persist as a major impediment to commercialization. Laboratory-scale successes often fail to translate to industrial production due to issues with heat transfer, mixing efficiency, and reaction homogeneity. The absence of standardized bioreactor designs specifically optimized for cell-free plant compound synthesis further complicates industrial implementation.
Analytical limitations also hinder progress in the field. Real-time monitoring of complex reaction networks remains challenging, making process optimization largely empirical rather than data-driven. The lack of high-throughput screening methods for rapidly evaluating different reaction conditions slows development cycles and increases research costs.
Regulatory uncertainties surrounding cell-free produced plant compounds create additional barriers to market entry. Questions regarding "natural" versus "synthetic" classification and appropriate safety assessment frameworks remain unresolved in many jurisdictions, creating hesitancy among potential industrial adopters.
Current Cell-Free Synthesis Methodologies
01 Cell-free protein synthesis systems optimization
Cell-free protein synthesis systems can be optimized to improve efficiency by modifying reaction conditions, energy regeneration systems, and component concentrations. These optimizations can include adjusting buffer compositions, adding stabilizing agents, and implementing continuous exchange systems to remove inhibitory byproducts. Enhanced cell extracts preparation methods also contribute significantly to increased protein yield and activity in cell-free systems.- Cell-free protein synthesis systems optimization: Cell-free protein synthesis systems can be optimized to improve efficiency by modifying reaction conditions, energy regeneration systems, and component concentrations. These optimizations can include adjusting buffer compositions, adding stabilizing agents, and implementing continuous exchange systems to remove inhibitory byproducts and replenish substrates. Such improvements lead to higher protein yields and extended reaction durations.
- Genetic element modifications for enhanced expression: Enhancing cell-free synthesis efficiency through genetic element modifications involves optimizing regulatory sequences such as promoters, ribosome binding sites, and terminators. Codon optimization for the cell-free environment and incorporation of specialized RNA structures can significantly improve translation efficiency. These genetic modifications can be tailored to specific cell-free systems to maximize protein production rates.
- Novel extract preparation methods: Innovative methods for preparing cell extracts can significantly impact synthesis efficiency. These include specialized cell lysis techniques, extract fractionation, and enrichment with specific components. Removing inhibitory factors and concentrating beneficial elements in the extract preparation process leads to more robust cell-free systems with higher productivity and improved consistency between batches.
- Supplementation strategies with cofactors and additives: Strategic supplementation of cell-free reactions with cofactors, energy sources, and additives can dramatically improve synthesis efficiency. This includes adding molecular chaperones to assist protein folding, nucleotides for transcription and translation, and specialized compounds that prevent resource depletion. Optimized supplementation strategies can extend reaction lifetimes and increase overall protein yields.
- Miniaturization and high-throughput formats: Developing miniaturized and high-throughput cell-free synthesis platforms enables rapid optimization of reaction conditions and efficient screening of multiple variables simultaneously. Microfluidic systems, droplet-based reactions, and automated platforms allow for reduced reaction volumes while maintaining or improving synthesis efficiency. These approaches facilitate faster development cycles and more economical use of reagents.
02 Energy regeneration and substrate delivery methods
Efficient energy supply is critical for cell-free synthesis systems. Various energy regeneration methods have been developed, including ATP regeneration cascades, phosphate recycling systems, and continuous substrate delivery approaches. These methods extend reaction duration and increase overall yield by maintaining optimal energy levels throughout the synthesis process, preventing premature termination due to energy depletion.Expand Specific Solutions03 Extract preparation and component optimization
The method of cell extract preparation significantly impacts synthesis efficiency. Techniques for lysate preparation, including mechanical disruption methods, enzymatic treatments, and genetic modifications of source organisms, can enhance extract performance. Supplementation with specific components such as chaperones, tRNAs, and translation factors can further improve folding efficiency and overall protein yield in cell-free systems.Expand Specific Solutions04 Continuous and flow-based cell-free systems
Continuous and flow-based cell-free synthesis systems offer advantages over batch reactions by allowing for the constant removal of inhibitory byproducts and addition of fresh substrates. These systems utilize dialysis membranes, microfluidic devices, or other compartmentalization strategies to maintain optimal reaction conditions over extended periods, resulting in significantly higher protein yields compared to traditional batch methods.Expand Specific Solutions05 Novel cell-free platforms and applications
Innovative cell-free platforms have been developed for specialized applications, including the synthesis of difficult-to-express proteins, incorporation of non-canonical amino acids, and production of complex biomolecules. These platforms often combine cell-free synthesis with additional technologies such as ribosome display, in vitro compartmentalization, or nanomaterial integration to enhance efficiency and expand the range of possible products.Expand Specific Solutions
Key Industry Players and Research Institutions
Cell-free plant compound synthesis is currently in an early growth phase, characterized by increasing academic-industrial partnerships and expanding applications in pharmaceuticals, cosmetics, and agriculture. The market is projected to reach significant scale as sustainable biomanufacturing gains traction, though still represents a niche segment within the broader synthetic biology sector. Technologically, leaders like Debut Biotechnology and GreenLight Biosciences have advanced cell-free platforms for commercial applications, while academic institutions (MIT, Northwestern University, Cornell) continue fundamental research. Established companies including Shimadzu, F. Hoffmann-La Roche, and Corteva Agriscience are exploring integration opportunities, while specialized firms like Cellfree Sciences and PAEAN Biotechnology focus on platform optimization. The field is witnessing convergence between traditional fermentation approaches and novel cell-free systems to overcome limitations in producing complex plant compounds.
Debut Biotechnology, Inc.
Technical Solution: Debut Biotechnology has pioneered a continuous-flow cell-free biomanufacturing platform specifically designed for plant-based compound synthesis. Their proprietary "Continuous Enzymatic Manufacturing" technology immobilizes cascades of enzymes on solid supports within microfluidic channels, creating an artificial metabolic pathway that remains active for extended periods. This approach enables the continuous production of complex plant compounds without the limitations of traditional batch processes. The system incorporates specialized cofactor regeneration modules and precise control of reaction parameters to optimize enzyme performance. Debut's platform can synthesize various high-value plant compounds including cannabinoids, flavonoids, and alkaloids with conversion efficiencies exceeding 85%. Their technology allows for the production of compounds that are difficult to extract from natural sources or synthesize chemically, with significantly reduced waste generation compared to traditional methods. The company has demonstrated successful scale-up of their continuous flow reactors from laboratory to pilot scale.
Strengths: Continuous production capability significantly increases throughput compared to batch processes; immobilized enzymes can be reused for multiple production cycles, reducing costs; precise control over reaction conditions enables optimization for specific compounds. Weaknesses: Complex engineering requirements for system setup and maintenance; potential for enzyme deactivation over extended operation periods; challenges in scaling to industrial production volumes.
GreenLight Biosciences, Inc.
Technical Solution: GreenLight Biosciences has developed a cell-free RNA production platform that has been adapted for plant-based compound synthesis. Their technology utilizes a cell-free transcription system combined with engineered ribozymes and toehold switches to control the expression of biosynthetic enzymes. This approach enables the production of complex plant metabolites through a process they term "RNA-driven biocatalysis." The system incorporates purified enzymes and transcription machinery in optimized reaction conditions, allowing for the rapid prototyping of biosynthetic pathways. GreenLight's platform can synthesize various plant compounds including terpenoids, alkaloids, and phenylpropanoids with yields up to 3.5g/L in optimized conditions. Their technology features a proprietary RNA design algorithm that optimizes enzyme expression levels to balance metabolic flux through multi-step pathways. The company has successfully scaled their process from microliter to 1,000L scale while maintaining productivity, demonstrating potential for industrial applications. Their approach reduces production costs by approximately 65% compared to traditional plant extraction methods for certain high-value compounds.
Strengths: Highly scalable platform from laboratory to industrial scale; precise control over enzyme expression levels through RNA engineering; rapid pathway prototyping capability (days versus months with traditional methods). Weaknesses: Relatively high costs for initial setup and optimization; requirement for specialized RNA production and purification infrastructure; potential regulatory challenges for novel production methods.
Critical Patents and Breakthroughs in Cell-Free Technology
Cell free production of anthocyanins
PatentWO2025006904A1
Innovation
- A cell-free production method using enzymes such as anthocyanidin synthase and anthocyanidin 3-O-glucosyltransferase in a cell-free medium to convert substrates like catechin into anthocyanins, such as cyanidin-3-glucoside, with UDP-glucose generated from sucrose and recycled within the reaction, eliminating the need for enzyme purification and reducing costs.
Improved methods of cell-free synthesis
PatentWO2024215747A2
Innovation
- The development of improved cell-free synthesis methods that in situ synthesize metabolic cofactors such as NAD and CoA, and utilize hydrogen (H2) to produce reducing equivalents NADH and NADPH, along with detoxification processes to manage aberrant NADPH tautomers, enabling efficient and cost-effective production of carbon-negative commodity chemicals.
Sustainability Impact and Green Chemistry Applications
Cell-free techniques for plant-based compound synthesis represent a significant advancement in sustainable manufacturing practices. These methods align perfectly with the principles of green chemistry by eliminating the need for whole-plant cultivation, thereby reducing land, water, and energy requirements. The environmental footprint of traditional plant cultivation for compound extraction is substantial, often involving deforestation, intensive irrigation, and chemical treatments. Cell-free systems dramatically reduce these impacts by operating in controlled laboratory environments with minimal resource consumption.
The sustainability benefits extend beyond resource conservation. Cell-free synthesis enables precise production of target compounds without generating the extensive biomass waste associated with traditional agriculture. This approach significantly reduces carbon emissions across the production lifecycle, from cultivation to processing and waste management. Studies indicate that cell-free methods can reduce greenhouse gas emissions by up to 80% compared to conventional plant extraction techniques for certain compounds.
Water conservation represents another critical sustainability advantage. Traditional plant cultivation for pharmaceutical or specialty chemical production often requires substantial irrigation, contributing to water scarcity in many regions. Cell-free systems typically consume less than 5% of the water needed for equivalent compound yields from whole-plant cultivation, making them particularly valuable in water-stressed environments.
From a green chemistry perspective, cell-free techniques embody several key principles, including atom economy and waste prevention. These systems can be engineered for maximum conversion efficiency, ensuring that input materials are optimally utilized in generating desired compounds. The controlled nature of cell-free reactions also minimizes the formation of unwanted byproducts, reducing purification requirements and associated chemical waste.
The integration of renewable energy sources further enhances the sustainability profile of cell-free synthesis. Unlike field cultivation, which is subject to seasonal variations and climate dependencies, laboratory-based cell-free systems can operate continuously using renewable electricity, creating a more predictable and environmentally friendly production model.
Circular economy principles are increasingly being applied to cell-free systems, with research focusing on recycling reaction components and developing biodegradable or reusable reaction vessels. These innovations promise to further reduce the environmental impact while improving economic viability. The potential for localized production also reduces transportation-related emissions, supporting distributed manufacturing models that enhance community resilience and sustainability.
The sustainability benefits extend beyond resource conservation. Cell-free synthesis enables precise production of target compounds without generating the extensive biomass waste associated with traditional agriculture. This approach significantly reduces carbon emissions across the production lifecycle, from cultivation to processing and waste management. Studies indicate that cell-free methods can reduce greenhouse gas emissions by up to 80% compared to conventional plant extraction techniques for certain compounds.
Water conservation represents another critical sustainability advantage. Traditional plant cultivation for pharmaceutical or specialty chemical production often requires substantial irrigation, contributing to water scarcity in many regions. Cell-free systems typically consume less than 5% of the water needed for equivalent compound yields from whole-plant cultivation, making them particularly valuable in water-stressed environments.
From a green chemistry perspective, cell-free techniques embody several key principles, including atom economy and waste prevention. These systems can be engineered for maximum conversion efficiency, ensuring that input materials are optimally utilized in generating desired compounds. The controlled nature of cell-free reactions also minimizes the formation of unwanted byproducts, reducing purification requirements and associated chemical waste.
The integration of renewable energy sources further enhances the sustainability profile of cell-free synthesis. Unlike field cultivation, which is subject to seasonal variations and climate dependencies, laboratory-based cell-free systems can operate continuously using renewable electricity, creating a more predictable and environmentally friendly production model.
Circular economy principles are increasingly being applied to cell-free systems, with research focusing on recycling reaction components and developing biodegradable or reusable reaction vessels. These innovations promise to further reduce the environmental impact while improving economic viability. The potential for localized production also reduces transportation-related emissions, supporting distributed manufacturing models that enhance community resilience and sustainability.
Regulatory Framework for Biomanufactured Compounds
The regulatory landscape for biomanufactured compounds derived from cell-free plant synthesis systems presents a complex framework that continues to evolve alongside technological advancements. Current regulations primarily fall under three jurisdictional categories: pharmaceutical regulations, food and dietary supplement oversight, and agricultural product governance. The FDA, EMA, and equivalent regulatory bodies worldwide have established specific pathways for biomanufactured compounds, though many were designed for traditional production methods rather than cell-free technologies.
A significant regulatory challenge lies in the classification of cell-free synthesized plant compounds. When these compounds are identical to their naturally occurring counterparts (termed "nature-identical"), they often face less stringent regulatory requirements. However, regulatory bodies increasingly require comprehensive characterization of the manufacturing process and potential impurities specific to cell-free systems, which may differ from traditional extraction methods.
Safety assessment protocols for cell-free synthesized compounds typically require demonstration of equivalence to plant-derived counterparts, including toxicological profiles and absence of novel impurities. The FDA's Generally Recognized as Safe (GRAS) designation and the EU Novel Food Regulation represent potential regulatory pathways, though neither was specifically designed for cell-free technologies.
Intellectual property considerations add another layer of complexity, with patent protection strategies needing to address both the compounds themselves and the cell-free production methods. Recent legal precedents suggest that novel cell-free production methods for known compounds may be patentable even when the compound itself is not.
International regulatory harmonization efforts, including those by the International Council for Harmonisation (ICH) and the World Health Organization, are beginning to address biomanufacturing technologies, though specific guidance for cell-free systems remains limited. This creates potential market access barriers when navigating different regional requirements.
Emerging regulatory trends indicate movement toward more adaptive frameworks that can accommodate rapid technological innovation. Several jurisdictions are developing regulatory sandboxes specifically for biomanufacturing technologies, allowing controlled market entry while gathering data on safety and efficacy. The FDA's Emerging Technology Program and the EMA's Innovation Task Force represent examples of regulatory bodies creating specialized pathways for novel production technologies.
Environmental regulations are increasingly relevant as well, with life cycle assessments becoming important components of regulatory submissions for biomanufacturing processes, highlighting the sustainability advantages of cell-free systems compared to traditional agricultural production.
A significant regulatory challenge lies in the classification of cell-free synthesized plant compounds. When these compounds are identical to their naturally occurring counterparts (termed "nature-identical"), they often face less stringent regulatory requirements. However, regulatory bodies increasingly require comprehensive characterization of the manufacturing process and potential impurities specific to cell-free systems, which may differ from traditional extraction methods.
Safety assessment protocols for cell-free synthesized compounds typically require demonstration of equivalence to plant-derived counterparts, including toxicological profiles and absence of novel impurities. The FDA's Generally Recognized as Safe (GRAS) designation and the EU Novel Food Regulation represent potential regulatory pathways, though neither was specifically designed for cell-free technologies.
Intellectual property considerations add another layer of complexity, with patent protection strategies needing to address both the compounds themselves and the cell-free production methods. Recent legal precedents suggest that novel cell-free production methods for known compounds may be patentable even when the compound itself is not.
International regulatory harmonization efforts, including those by the International Council for Harmonisation (ICH) and the World Health Organization, are beginning to address biomanufacturing technologies, though specific guidance for cell-free systems remains limited. This creates potential market access barriers when navigating different regional requirements.
Emerging regulatory trends indicate movement toward more adaptive frameworks that can accommodate rapid technological innovation. Several jurisdictions are developing regulatory sandboxes specifically for biomanufacturing technologies, allowing controlled market entry while gathering data on safety and efficacy. The FDA's Emerging Technology Program and the EMA's Innovation Task Force represent examples of regulatory bodies creating specialized pathways for novel production technologies.
Environmental regulations are increasingly relevant as well, with life cycle assessments becoming important components of regulatory submissions for biomanufacturing processes, highlighting the sustainability advantages of cell-free systems compared to traditional agricultural production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!