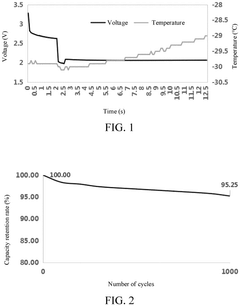
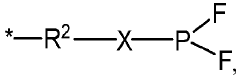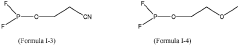High-Temperature Performance of Lithium Iron Phosphate Batteries
AUG 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LFP Battery Evolution
Lithium Iron Phosphate (LFP) batteries have undergone significant evolution since their inception in the late 1990s. Initially developed as a safer alternative to traditional lithium-ion batteries, LFP technology has seen remarkable advancements in performance, particularly in high-temperature environments.
The early 2000s marked the beginning of commercial LFP battery production, with a focus on improving energy density and cycle life. During this period, researchers concentrated on optimizing the cathode material's structure and composition to enhance its electrochemical properties. The introduction of carbon coating techniques for LFP particles emerged as a crucial breakthrough, significantly improving the material's conductivity and rate capability.
By the mid-2000s, LFP batteries had gained traction in various applications, including electric vehicles and stationary energy storage systems. This period saw the development of advanced synthesis methods, such as solid-state and hydrothermal reactions, which led to more uniform and smaller LFP particles, further enhancing battery performance.
The late 2000s and early 2010s witnessed a surge in research aimed at addressing the challenges of high-temperature performance. Scientists explored doping strategies, introducing elements like manganese, cobalt, and nickel into the LFP structure to improve its thermal stability and capacity retention at elevated temperatures. Concurrently, advancements in electrolyte formulations played a crucial role in enhancing the overall high-temperature performance of LFP batteries.
From 2010 to 2015, the focus shifted towards nano-engineering of LFP materials. The development of nano-sized LFP particles and innovative carbon-based composites resulted in batteries with superior rate capability and improved performance under high-temperature conditions. This period also saw the emergence of novel electrode designs, such as 3D-structured electrodes, which facilitated better ion transport and heat dissipation.
Recent years have seen a renewed interest in LFP technology, driven by its inherent safety advantages and improving performance characteristics. Researchers have made significant strides in enhancing the high-temperature stability of LFP batteries through advanced surface modification techniques and the development of heat-resistant binders and separators. These innovations have extended the operational temperature range of LFP batteries, making them increasingly suitable for demanding applications in extreme environments.
The ongoing evolution of LFP batteries continues to focus on pushing the boundaries of high-temperature performance. Current research trends include the exploration of solid-state electrolytes, advanced thermal management systems, and the integration of artificial intelligence for optimized battery management under varying temperature conditions. These developments promise to further enhance the high-temperature capabilities of LFP batteries, solidifying their position as a key technology in the transition towards sustainable energy solutions.
The early 2000s marked the beginning of commercial LFP battery production, with a focus on improving energy density and cycle life. During this period, researchers concentrated on optimizing the cathode material's structure and composition to enhance its electrochemical properties. The introduction of carbon coating techniques for LFP particles emerged as a crucial breakthrough, significantly improving the material's conductivity and rate capability.
By the mid-2000s, LFP batteries had gained traction in various applications, including electric vehicles and stationary energy storage systems. This period saw the development of advanced synthesis methods, such as solid-state and hydrothermal reactions, which led to more uniform and smaller LFP particles, further enhancing battery performance.
The late 2000s and early 2010s witnessed a surge in research aimed at addressing the challenges of high-temperature performance. Scientists explored doping strategies, introducing elements like manganese, cobalt, and nickel into the LFP structure to improve its thermal stability and capacity retention at elevated temperatures. Concurrently, advancements in electrolyte formulations played a crucial role in enhancing the overall high-temperature performance of LFP batteries.
From 2010 to 2015, the focus shifted towards nano-engineering of LFP materials. The development of nano-sized LFP particles and innovative carbon-based composites resulted in batteries with superior rate capability and improved performance under high-temperature conditions. This period also saw the emergence of novel electrode designs, such as 3D-structured electrodes, which facilitated better ion transport and heat dissipation.
Recent years have seen a renewed interest in LFP technology, driven by its inherent safety advantages and improving performance characteristics. Researchers have made significant strides in enhancing the high-temperature stability of LFP batteries through advanced surface modification techniques and the development of heat-resistant binders and separators. These innovations have extended the operational temperature range of LFP batteries, making them increasingly suitable for demanding applications in extreme environments.
The ongoing evolution of LFP batteries continues to focus on pushing the boundaries of high-temperature performance. Current research trends include the exploration of solid-state electrolytes, advanced thermal management systems, and the integration of artificial intelligence for optimized battery management under varying temperature conditions. These developments promise to further enhance the high-temperature capabilities of LFP batteries, solidifying their position as a key technology in the transition towards sustainable energy solutions.
Market Demand Analysis
The market demand for high-temperature performance lithium iron phosphate (LFP) batteries has been steadily increasing, driven by the growing need for energy storage solutions in extreme environments. Industries such as electric vehicles, renewable energy systems, and aerospace applications are particularly interested in batteries that can maintain stability and efficiency at elevated temperatures.
In the electric vehicle sector, there is a significant push for batteries that can withstand high temperatures without compromising safety or performance. This demand is especially pronounced in regions with hot climates, where conventional lithium-ion batteries may experience accelerated degradation. The ability of LFP batteries to operate efficiently at higher temperatures makes them an attractive option for automakers looking to expand their market reach in these areas.
The renewable energy sector is another key driver of demand for high-temperature LFP batteries. As solar and wind power installations continue to grow globally, there is an increasing need for energy storage systems that can function reliably in diverse environmental conditions. LFP batteries with enhanced thermal stability are well-suited for grid-scale storage applications in hot climates, where they can help manage peak loads and integrate intermittent renewable sources more effectively.
Industrial applications represent another significant market segment for high-temperature LFP batteries. Manufacturing facilities, data centers, and telecommunications infrastructure in warm regions require robust energy storage solutions that can operate consistently in challenging thermal environments. The improved safety profile of LFP chemistry at high temperatures makes these batteries particularly appealing for use in sensitive industrial settings.
The aerospace and defense sectors are also showing interest in high-temperature LFP batteries. These industries require power sources that can function reliably under extreme conditions, including high-altitude and desert environments. The potential for LFP batteries to meet these demanding requirements is driving research and development efforts in this area.
Market analysts project substantial growth in the high-temperature battery market over the coming years. This growth is expected to be fueled by advancements in LFP technology that further enhance thermal stability and performance. As manufacturers continue to improve the energy density and cycle life of LFP batteries at elevated temperatures, their adoption across various industries is likely to accelerate.
The increasing focus on sustainability and environmental regulations is also contributing to the demand for high-temperature LFP batteries. Their longer lifespan and reduced environmental impact compared to some other battery chemistries make them an attractive option for companies looking to improve their ecological footprint while meeting their energy storage needs in challenging thermal conditions.
In the electric vehicle sector, there is a significant push for batteries that can withstand high temperatures without compromising safety or performance. This demand is especially pronounced in regions with hot climates, where conventional lithium-ion batteries may experience accelerated degradation. The ability of LFP batteries to operate efficiently at higher temperatures makes them an attractive option for automakers looking to expand their market reach in these areas.
The renewable energy sector is another key driver of demand for high-temperature LFP batteries. As solar and wind power installations continue to grow globally, there is an increasing need for energy storage systems that can function reliably in diverse environmental conditions. LFP batteries with enhanced thermal stability are well-suited for grid-scale storage applications in hot climates, where they can help manage peak loads and integrate intermittent renewable sources more effectively.
Industrial applications represent another significant market segment for high-temperature LFP batteries. Manufacturing facilities, data centers, and telecommunications infrastructure in warm regions require robust energy storage solutions that can operate consistently in challenging thermal environments. The improved safety profile of LFP chemistry at high temperatures makes these batteries particularly appealing for use in sensitive industrial settings.
The aerospace and defense sectors are also showing interest in high-temperature LFP batteries. These industries require power sources that can function reliably under extreme conditions, including high-altitude and desert environments. The potential for LFP batteries to meet these demanding requirements is driving research and development efforts in this area.
Market analysts project substantial growth in the high-temperature battery market over the coming years. This growth is expected to be fueled by advancements in LFP technology that further enhance thermal stability and performance. As manufacturers continue to improve the energy density and cycle life of LFP batteries at elevated temperatures, their adoption across various industries is likely to accelerate.
The increasing focus on sustainability and environmental regulations is also contributing to the demand for high-temperature LFP batteries. Their longer lifespan and reduced environmental impact compared to some other battery chemistries make them an attractive option for companies looking to improve their ecological footprint while meeting their energy storage needs in challenging thermal conditions.
High-Temp Challenges
Lithium iron phosphate (LiFePO4) batteries have gained significant attention in the energy storage industry due to their excellent safety profile and long cycle life. However, their performance at high temperatures remains a critical challenge that needs to be addressed for widespread adoption in various applications.
One of the primary challenges faced by LiFePO4 batteries at elevated temperatures is the accelerated degradation of the cathode material. As temperatures rise, the crystal structure of LiFePO4 becomes less stable, leading to increased iron dissolution and subsequent capacity loss. This phenomenon is particularly pronounced at temperatures above 55°C, where the rate of capacity fade can increase dramatically.
The electrolyte stability is another crucial factor affecting high-temperature performance. Conventional carbonate-based electrolytes tend to decompose at elevated temperatures, forming a thick solid electrolyte interphase (SEI) layer on the electrode surface. This excessive SEI growth not only consumes active lithium but also increases internal resistance, resulting in reduced power capability and overall battery performance.
Thermal runaway is a severe safety concern for LiFePO4 batteries operating at high temperatures. Although LiFePO4 is inherently more stable than other cathode materials, prolonged exposure to extreme temperatures can still trigger exothermic reactions, potentially leading to battery failure or, in rare cases, fire hazards.
The lithium plating issue becomes more pronounced at high temperatures, especially during fast charging. The increased kinetics of lithium-ion transport at elevated temperatures can lead to uneven deposition of lithium on the anode surface, causing capacity loss and potential safety risks.
Furthermore, the separator's integrity is compromised at high temperatures. Most commercial separators are made of polyolefin materials, which can shrink or melt at temperatures above 130°C. This can lead to internal short circuits and catastrophic failure of the battery.
The battery management system (BMS) faces additional challenges in high-temperature environments. Accurate state-of-charge (SOC) and state-of-health (SOH) estimation becomes more difficult due to the non-linear behavior of the battery at elevated temperatures. This can result in suboptimal charging and discharging strategies, further exacerbating the degradation issues.
Addressing these high-temperature challenges requires a multifaceted approach, including the development of more thermally stable cathode materials, advanced electrolyte formulations, and improved thermal management systems. Research efforts are ongoing to enhance the high-temperature performance of LiFePO4 batteries, with promising advancements in areas such as doped cathode materials, solid-state electrolytes, and advanced cooling technologies.
One of the primary challenges faced by LiFePO4 batteries at elevated temperatures is the accelerated degradation of the cathode material. As temperatures rise, the crystal structure of LiFePO4 becomes less stable, leading to increased iron dissolution and subsequent capacity loss. This phenomenon is particularly pronounced at temperatures above 55°C, where the rate of capacity fade can increase dramatically.
The electrolyte stability is another crucial factor affecting high-temperature performance. Conventional carbonate-based electrolytes tend to decompose at elevated temperatures, forming a thick solid electrolyte interphase (SEI) layer on the electrode surface. This excessive SEI growth not only consumes active lithium but also increases internal resistance, resulting in reduced power capability and overall battery performance.
Thermal runaway is a severe safety concern for LiFePO4 batteries operating at high temperatures. Although LiFePO4 is inherently more stable than other cathode materials, prolonged exposure to extreme temperatures can still trigger exothermic reactions, potentially leading to battery failure or, in rare cases, fire hazards.
The lithium plating issue becomes more pronounced at high temperatures, especially during fast charging. The increased kinetics of lithium-ion transport at elevated temperatures can lead to uneven deposition of lithium on the anode surface, causing capacity loss and potential safety risks.
Furthermore, the separator's integrity is compromised at high temperatures. Most commercial separators are made of polyolefin materials, which can shrink or melt at temperatures above 130°C. This can lead to internal short circuits and catastrophic failure of the battery.
The battery management system (BMS) faces additional challenges in high-temperature environments. Accurate state-of-charge (SOC) and state-of-health (SOH) estimation becomes more difficult due to the non-linear behavior of the battery at elevated temperatures. This can result in suboptimal charging and discharging strategies, further exacerbating the degradation issues.
Addressing these high-temperature challenges requires a multifaceted approach, including the development of more thermally stable cathode materials, advanced electrolyte formulations, and improved thermal management systems. Research efforts are ongoing to enhance the high-temperature performance of LiFePO4 batteries, with promising advancements in areas such as doped cathode materials, solid-state electrolytes, and advanced cooling technologies.
Current Solutions
01 Electrolyte additives for high-temperature stability
Incorporating specific additives into the electrolyte can enhance the high-temperature performance of lithium iron phosphate batteries. These additives can include flame retardants, stabilizers, or compounds that form protective films on electrodes, improving the battery's thermal stability and safety at elevated temperatures.- Electrolyte additives for high-temperature stability: Incorporating specific additives into the electrolyte can enhance the high-temperature performance of lithium iron phosphate batteries. These additives may include flame retardants, stabilizers, or compounds that form protective films on electrodes, improving the battery's thermal stability and safety at elevated temperatures.
- Electrode material modifications: Modifying the lithium iron phosphate cathode material or the anode material can improve high-temperature performance. This may involve doping with other elements, surface coating, or altering the particle size and morphology to enhance stability and conductivity at higher temperatures.
- Thermal management systems: Implementing advanced thermal management systems can help maintain optimal operating temperatures for lithium iron phosphate batteries in high-temperature environments. This may include innovative cooling mechanisms, heat-dissipating materials, or intelligent temperature control systems.
- Separator enhancements: Developing high-performance separators that maintain their integrity and functionality at elevated temperatures can significantly improve the high-temperature performance of lithium iron phosphate batteries. This may involve using heat-resistant materials or novel separator designs.
- Battery pack design optimization: Optimizing the overall battery pack design can contribute to better high-temperature performance. This may include improved cell arrangement, enhanced insulation, strategic placement of cooling elements, and the use of heat-resistant packaging materials to mitigate the effects of high ambient temperatures on battery performance.
02 Electrode material modifications
Modifying the lithium iron phosphate cathode material or the anode material can improve high-temperature performance. This may involve doping with other elements, surface coating, or altering the particle size and morphology to enhance stability and conductivity at higher temperatures.Expand Specific Solutions03 Thermal management systems
Implementing advanced thermal management systems can help maintain optimal operating temperatures for lithium iron phosphate batteries in high-temperature environments. This may include innovative cooling mechanisms, heat dissipation materials, or intelligent temperature control systems.Expand Specific Solutions04 Separator enhancements
Developing high-performance separators that maintain their integrity and ion permeability at elevated temperatures can significantly improve the high-temperature performance of lithium iron phosphate batteries. This may involve using heat-resistant materials or novel separator designs.Expand Specific Solutions05 Battery pack design optimization
Optimizing the overall battery pack design can enhance high-temperature performance. This includes improving cell arrangement, using advanced insulation materials, and implementing smart battery management systems that can adapt to temperature fluctuations and maintain battery efficiency in hot conditions.Expand Specific Solutions
Key Industry Players
The high-temperature performance of Lithium Iron Phosphate (LFP) batteries is a critical area of research in the energy storage industry, currently in a growth phase. The market for LFP batteries is expanding rapidly, driven by their thermal stability and safety advantages. Key players like Contemporary Amperex Technology Co., Ltd. (CATL), BYD Co., Ltd., and Panasonic Energy Co. Ltd. are at the forefront of technological advancements. These companies are investing heavily in R&D to improve the high-temperature performance of LFP batteries, focusing on enhancing energy density and cycle life at elevated temperatures. The technology is maturing, with significant progress in thermal management systems and electrode materials, but there's still room for innovation to fully address high-temperature challenges.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed advanced lithium iron phosphate (LFP) batteries with improved high-temperature performance. Their CTP (cell-to-pack) technology integrates cells directly into the battery pack, increasing energy density by 10-15% and improving thermal management[1]. CATL's LFP batteries utilize a novel electrolyte formulation and surface coating on cathode particles to enhance stability at elevated temperatures. The company has also implemented a smart thermal management system that actively regulates battery temperature, maintaining optimal performance even in high-temperature environments[2]. CATL's LFP batteries have demonstrated stable capacity retention of over 90% after 1000 cycles at 45°C, significantly outperforming conventional LFP batteries[3].
Strengths: High energy density, excellent thermal stability, and long cycle life at elevated temperatures. Weaknesses: Higher production costs compared to standard LFP batteries, and potential intellectual property constraints.
BYD Co., Ltd.
Technical Solution: BYD has pioneered the Blade Battery, an innovative LFP battery design optimized for high-temperature performance. The Blade Battery utilizes a unique cell arrangement that maximizes space utilization and improves heat dissipation. BYD's proprietary electrolyte additives and cathode coatings enhance the battery's thermal stability[4]. The company has implemented an advanced battery management system (BMS) that employs predictive algorithms to optimize charging and discharging patterns based on temperature conditions. BYD's Blade Battery has demonstrated remarkable safety performance, passing nail penetration tests without ignition even at high temperatures[5]. The battery maintains over 95% capacity retention after 3000 cycles at 40°C, showcasing its exceptional high-temperature durability[6].
Strengths: Excellent safety performance, high energy density, and superior cycle life at elevated temperatures. Weaknesses: Limited production capacity compared to some competitors, and potential challenges in scaling up manufacturing.
Innovative Materials
Battery core, battery core preparation method, starting battery for fuel engine, and vehicle
PatentPendingEP4567913A2
Innovation
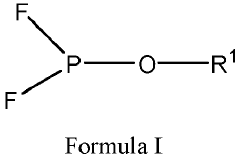
- A battery core is developed using composite lithium iron phosphate particles with different D50 particle diameters for the positive electrode and composite graphite particles with different D50 particle diameters for the negative electrode, along with an optimized electrolyte solution, to enhance lithium ion transmission and reduce impedance.
Electrolyte, electrochemical device and electronic device
PatentPendingEP3876329A1
Innovation
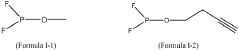
- An electrolyte composition including lithium difluorophosphate (LiPO2F2) and a compound containing (F)2PO-, along with additional components like multiple-cyano compounds, sultone compounds, and fluoroether compounds, which form protective films to reduce resistance and enhance capacitance retention at varying temperatures.
Safety Regulations
Safety regulations play a crucial role in the development and deployment of lithium iron phosphate (LFP) batteries, especially when considering their high-temperature performance. As these batteries gain popularity in various applications, including electric vehicles and energy storage systems, regulatory bodies have established stringent guidelines to ensure their safe operation under elevated temperature conditions.
The primary focus of safety regulations for LFP batteries at high temperatures is to prevent thermal runaway, which can lead to fires or explosions. Regulatory agencies, such as the International Electrotechnical Commission (IEC) and the Society of Automotive Engineers (SAE), have developed specific standards addressing thermal management and safety requirements for lithium-ion batteries, including LFP chemistry.
One key aspect of these regulations is the mandatory implementation of battery management systems (BMS) that can monitor and control battery temperature. These systems are required to have built-in safeguards that can detect abnormal temperature increases and initiate protective measures, such as reducing charging current or completely shutting down the battery if necessary.
Additionally, safety regulations mandate extensive testing procedures to evaluate the high-temperature performance of LFP batteries. These tests typically include thermal abuse tests, where batteries are subjected to extreme temperature conditions to assess their stability and safety features. Manufacturers must demonstrate that their batteries can maintain safe operation within specified temperature ranges and withstand short-term exposure to higher temperatures without compromising safety.
Regulatory bodies also require the implementation of passive and active cooling systems in battery packs to manage heat generation during operation. This includes the use of thermal insulation materials, heat sinks, and forced air or liquid cooling systems to maintain optimal operating temperatures and prevent overheating.
Furthermore, safety regulations address the importance of proper battery packaging and cell design to mitigate the risks associated with high-temperature operation. This includes requirements for robust cell casings, thermal barriers between cells, and the use of fire-resistant materials in battery pack construction.
In recent years, there has been an increased focus on developing regulations specific to the unique characteristics of LFP batteries. These regulations take into account the inherent thermal stability of LFP chemistry compared to other lithium-ion chemistries, allowing for potentially less stringent cooling requirements while maintaining high safety standards.
As the technology continues to evolve, regulatory bodies are working closely with industry stakeholders to update and refine safety regulations. This ongoing collaboration aims to strike a balance between ensuring the highest levels of safety and enabling the continued advancement of LFP battery technology for high-temperature applications.
The primary focus of safety regulations for LFP batteries at high temperatures is to prevent thermal runaway, which can lead to fires or explosions. Regulatory agencies, such as the International Electrotechnical Commission (IEC) and the Society of Automotive Engineers (SAE), have developed specific standards addressing thermal management and safety requirements for lithium-ion batteries, including LFP chemistry.
One key aspect of these regulations is the mandatory implementation of battery management systems (BMS) that can monitor and control battery temperature. These systems are required to have built-in safeguards that can detect abnormal temperature increases and initiate protective measures, such as reducing charging current or completely shutting down the battery if necessary.
Additionally, safety regulations mandate extensive testing procedures to evaluate the high-temperature performance of LFP batteries. These tests typically include thermal abuse tests, where batteries are subjected to extreme temperature conditions to assess their stability and safety features. Manufacturers must demonstrate that their batteries can maintain safe operation within specified temperature ranges and withstand short-term exposure to higher temperatures without compromising safety.
Regulatory bodies also require the implementation of passive and active cooling systems in battery packs to manage heat generation during operation. This includes the use of thermal insulation materials, heat sinks, and forced air or liquid cooling systems to maintain optimal operating temperatures and prevent overheating.
Furthermore, safety regulations address the importance of proper battery packaging and cell design to mitigate the risks associated with high-temperature operation. This includes requirements for robust cell casings, thermal barriers between cells, and the use of fire-resistant materials in battery pack construction.
In recent years, there has been an increased focus on developing regulations specific to the unique characteristics of LFP batteries. These regulations take into account the inherent thermal stability of LFP chemistry compared to other lithium-ion chemistries, allowing for potentially less stringent cooling requirements while maintaining high safety standards.
As the technology continues to evolve, regulatory bodies are working closely with industry stakeholders to update and refine safety regulations. This ongoing collaboration aims to strike a balance between ensuring the highest levels of safety and enabling the continued advancement of LFP battery technology for high-temperature applications.
Environmental Impact
The environmental impact of lithium iron phosphate (LiFePO4) batteries, particularly in high-temperature conditions, is a critical consideration in their widespread adoption. These batteries have gained popularity due to their improved safety and longer lifespan compared to other lithium-ion chemistries. However, their performance and environmental implications at elevated temperatures require careful examination.
High-temperature operation of LiFePO4 batteries can lead to accelerated degradation of the electrolyte and electrode materials. This degradation not only affects the battery's performance but also has environmental consequences. The breakdown of electrolytes can result in the release of volatile organic compounds (VOCs) and other potentially harmful substances. While these emissions are generally lower than those from other battery types, they still contribute to air pollution and pose potential health risks in enclosed spaces.
The thermal management systems required to maintain optimal operating temperatures for LiFePO4 batteries in hot environments also have environmental implications. These systems often consume additional energy, potentially offsetting some of the efficiency gains provided by the batteries themselves. The production and disposal of thermal management components add to the overall environmental footprint of the battery system.
In terms of resource consumption, the high-temperature performance of LiFePO4 batteries influences their lifespan and, consequently, the frequency of replacement. Improved high-temperature resilience can lead to longer battery life, reducing the demand for raw materials and the environmental impact associated with battery production and disposal. However, the manufacturing process of heat-resistant components may require more energy-intensive techniques or specialized materials, potentially increasing the initial environmental cost.
The end-of-life management of LiFePO4 batteries exposed to high temperatures presents unique challenges. Thermal stress can alter the chemical composition and physical structure of battery components, potentially complicating recycling processes. This may necessitate the development of new recycling technologies or modifications to existing ones, ensuring efficient recovery of valuable materials while minimizing environmental hazards.
Water usage is another environmental concern related to high-temperature operation of LiFePO4 batteries. Cooling systems, often essential in hot climates, may require significant water resources. In water-scarce regions, this could strain local ecosystems and compete with other essential water needs. The development of more efficient cooling technologies or alternative heat management strategies is crucial to mitigate this impact.
Overall, while LiFePO4 batteries offer environmental advantages over some alternative energy storage technologies, their high-temperature performance characteristics present a complex set of environmental considerations. Balancing the benefits of extended battery life and improved safety with the potential environmental costs of thermal management and accelerated degradation is essential for sustainable implementation of this technology.
High-temperature operation of LiFePO4 batteries can lead to accelerated degradation of the electrolyte and electrode materials. This degradation not only affects the battery's performance but also has environmental consequences. The breakdown of electrolytes can result in the release of volatile organic compounds (VOCs) and other potentially harmful substances. While these emissions are generally lower than those from other battery types, they still contribute to air pollution and pose potential health risks in enclosed spaces.
The thermal management systems required to maintain optimal operating temperatures for LiFePO4 batteries in hot environments also have environmental implications. These systems often consume additional energy, potentially offsetting some of the efficiency gains provided by the batteries themselves. The production and disposal of thermal management components add to the overall environmental footprint of the battery system.
In terms of resource consumption, the high-temperature performance of LiFePO4 batteries influences their lifespan and, consequently, the frequency of replacement. Improved high-temperature resilience can lead to longer battery life, reducing the demand for raw materials and the environmental impact associated with battery production and disposal. However, the manufacturing process of heat-resistant components may require more energy-intensive techniques or specialized materials, potentially increasing the initial environmental cost.
The end-of-life management of LiFePO4 batteries exposed to high temperatures presents unique challenges. Thermal stress can alter the chemical composition and physical structure of battery components, potentially complicating recycling processes. This may necessitate the development of new recycling technologies or modifications to existing ones, ensuring efficient recovery of valuable materials while minimizing environmental hazards.
Water usage is another environmental concern related to high-temperature operation of LiFePO4 batteries. Cooling systems, often essential in hot climates, may require significant water resources. In water-scarce regions, this could strain local ecosystems and compete with other essential water needs. The development of more efficient cooling technologies or alternative heat management strategies is crucial to mitigate this impact.
Overall, while LiFePO4 batteries offer environmental advantages over some alternative energy storage technologies, their high-temperature performance characteristics present a complex set of environmental considerations. Balancing the benefits of extended battery life and improved safety with the potential environmental costs of thermal management and accelerated degradation is essential for sustainable implementation of this technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!