High-throughput screening using cell-free biomanufacturing technologies.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Biomanufacturing Background and Objectives
Cell-free biomanufacturing represents a paradigm shift in biotechnology, emerging from decades of research into biological systems outside living cells. This approach harnesses cellular machinery without the constraints of cell walls and metabolic requirements, offering unprecedented flexibility for protein production and metabolic engineering. The evolution of cell-free systems traces back to the 1950s with pioneering work on in vitro protein synthesis, progressing through significant milestones including the development of coupled transcription-translation systems in the 1980s and the optimization of extract preparation methods in the early 2000s.
Recent technological advancements have dramatically improved the efficiency and scalability of cell-free systems, transforming them from laboratory curiosities into viable manufacturing platforms. The integration of high-throughput screening (HTS) methodologies with cell-free systems represents the latest frontier in this evolution, enabling rapid prototyping and optimization of biological pathways and products at unprecedented scales.
The primary objective of high-throughput screening in cell-free biomanufacturing is to accelerate the design-build-test-learn cycle in synthetic biology and bioengineering. By eliminating the constraints of cellular growth and viability, these systems enable the parallel testing of thousands to millions of genetic variants or reaction conditions in timeframes previously unimaginable with traditional cell-based methods.
Technical goals include developing robust platforms that maintain consistency across microscale reactions, creating compatible detection systems for diverse biomolecular outputs, and establishing computational frameworks to manage and interpret the massive datasets generated. Additionally, there is a push toward miniaturization and automation to further increase throughput while reducing reagent costs.
The field aims to bridge fundamental research with practical applications, particularly in areas such as therapeutic protein production, enzyme engineering, biosensor development, and sustainable chemical synthesis. Long-term objectives include the creation of standardized, modular cell-free platforms that can be rapidly deployed for diverse applications, from personalized medicine to distributed biomanufacturing.
As the technology matures, researchers are increasingly focused on addressing challenges related to scalability, reproducibility, and cost-effectiveness. The convergence of cell-free systems with other cutting-edge technologies—including microfluidics, artificial intelligence, and advanced biomolecular engineering—promises to further expand the capabilities and applications of high-throughput cell-free screening methodologies.
Recent technological advancements have dramatically improved the efficiency and scalability of cell-free systems, transforming them from laboratory curiosities into viable manufacturing platforms. The integration of high-throughput screening (HTS) methodologies with cell-free systems represents the latest frontier in this evolution, enabling rapid prototyping and optimization of biological pathways and products at unprecedented scales.
The primary objective of high-throughput screening in cell-free biomanufacturing is to accelerate the design-build-test-learn cycle in synthetic biology and bioengineering. By eliminating the constraints of cellular growth and viability, these systems enable the parallel testing of thousands to millions of genetic variants or reaction conditions in timeframes previously unimaginable with traditional cell-based methods.
Technical goals include developing robust platforms that maintain consistency across microscale reactions, creating compatible detection systems for diverse biomolecular outputs, and establishing computational frameworks to manage and interpret the massive datasets generated. Additionally, there is a push toward miniaturization and automation to further increase throughput while reducing reagent costs.
The field aims to bridge fundamental research with practical applications, particularly in areas such as therapeutic protein production, enzyme engineering, biosensor development, and sustainable chemical synthesis. Long-term objectives include the creation of standardized, modular cell-free platforms that can be rapidly deployed for diverse applications, from personalized medicine to distributed biomanufacturing.
As the technology matures, researchers are increasingly focused on addressing challenges related to scalability, reproducibility, and cost-effectiveness. The convergence of cell-free systems with other cutting-edge technologies—including microfluidics, artificial intelligence, and advanced biomolecular engineering—promises to further expand the capabilities and applications of high-throughput cell-free screening methodologies.
Market Analysis for High-throughput Screening Applications
The high-throughput screening (HTS) market utilizing cell-free biomanufacturing technologies has experienced substantial growth in recent years, driven by increasing demand for efficient drug discovery processes and advancements in synthetic biology. The global HTS market was valued at approximately $16 billion in 2020 and is projected to reach $28 billion by 2027, representing a compound annual growth rate of 8.2%.
Pharmaceutical and biotechnology companies constitute the largest segment of end-users, accounting for nearly 65% of the market share. These organizations leverage HTS technologies to accelerate drug discovery processes and reduce development costs. Academic research institutions represent the second-largest market segment at 20%, followed by contract research organizations at 15%.
Geographically, North America dominates the market with approximately 45% share, attributed to the presence of major pharmaceutical companies, substantial R&D investments, and favorable regulatory frameworks. Europe follows with 30% market share, while the Asia-Pacific region is experiencing the fastest growth rate of 10.5% annually, primarily driven by expanding biotechnology sectors in China, Japan, and India.
The cell-free biomanufacturing segment within the HTS market is witnessing particularly rapid expansion, with a projected growth rate of 12% annually through 2027. This acceleration stems from the technology's advantages in rapid prototyping, reduced biosafety concerns, and ability to express proteins that would be toxic in living cells.
Key market drivers include increasing drug discovery costs, growing prevalence of chronic diseases, rising demand for personalized medicine, and technological advancements in automation and miniaturization. The COVID-19 pandemic has further accelerated market growth by highlighting the need for rapid screening technologies in vaccine and therapeutic development.
Challenges facing market expansion include high initial investment costs, technical complexities in assay development, and standardization issues. The average cost of implementing a comprehensive HTS platform utilizing cell-free systems ranges from $500,000 to $2 million, creating barriers for smaller organizations and academic institutions.
Emerging trends shaping the market include integration of artificial intelligence for data analysis, development of microfluidic-based HTS platforms, increasing adoption of label-free detection technologies, and growing focus on phenotypic screening approaches. The combination of cell-free systems with microfluidic technologies is particularly promising, potentially reducing reagent consumption by up to 90% while increasing throughput by 50-100 times compared to conventional methods.
Pharmaceutical and biotechnology companies constitute the largest segment of end-users, accounting for nearly 65% of the market share. These organizations leverage HTS technologies to accelerate drug discovery processes and reduce development costs. Academic research institutions represent the second-largest market segment at 20%, followed by contract research organizations at 15%.
Geographically, North America dominates the market with approximately 45% share, attributed to the presence of major pharmaceutical companies, substantial R&D investments, and favorable regulatory frameworks. Europe follows with 30% market share, while the Asia-Pacific region is experiencing the fastest growth rate of 10.5% annually, primarily driven by expanding biotechnology sectors in China, Japan, and India.
The cell-free biomanufacturing segment within the HTS market is witnessing particularly rapid expansion, with a projected growth rate of 12% annually through 2027. This acceleration stems from the technology's advantages in rapid prototyping, reduced biosafety concerns, and ability to express proteins that would be toxic in living cells.
Key market drivers include increasing drug discovery costs, growing prevalence of chronic diseases, rising demand for personalized medicine, and technological advancements in automation and miniaturization. The COVID-19 pandemic has further accelerated market growth by highlighting the need for rapid screening technologies in vaccine and therapeutic development.
Challenges facing market expansion include high initial investment costs, technical complexities in assay development, and standardization issues. The average cost of implementing a comprehensive HTS platform utilizing cell-free systems ranges from $500,000 to $2 million, creating barriers for smaller organizations and academic institutions.
Emerging trends shaping the market include integration of artificial intelligence for data analysis, development of microfluidic-based HTS platforms, increasing adoption of label-free detection technologies, and growing focus on phenotypic screening approaches. The combination of cell-free systems with microfluidic technologies is particularly promising, potentially reducing reagent consumption by up to 90% while increasing throughput by 50-100 times compared to conventional methods.
Technical Challenges in Cell-free HTS Systems
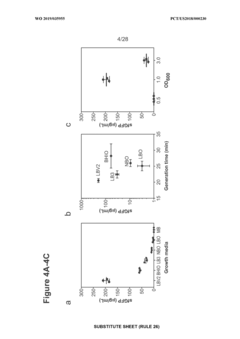
Despite the promising potential of cell-free high-throughput screening (HTS) systems, several significant technical challenges impede their widespread adoption and optimal performance. One primary obstacle is the limited stability of cell-free extracts, which typically remain active for only 4-8 hours under standard conditions. This short operational window restricts screening duration and throughput capacity, particularly for reactions requiring extended incubation periods.
Extract preparation consistency presents another major challenge. Batch-to-batch variations in extract quality can introduce significant data variability, compromising screening reliability and reproducibility. These inconsistencies stem from subtle differences in cell growth conditions, lysis efficiency, and extract processing methods, necessitating rigorous standardization protocols.
Energy system limitations further constrain cell-free HTS applications. The rapid depletion of ATP and other energy-rich compounds in cell-free systems leads to declining metabolic activity over time. Current continuous energy regeneration systems remain suboptimal for extended screening campaigns, requiring sophisticated engineering solutions to maintain consistent metabolic performance.
Scale-down challenges also persist in cell-free HTS implementation. Miniaturizing reaction volumes to nanoliter or picoliter scales—essential for ultra-high-throughput applications—introduces surface tension effects, evaporation issues, and mixing inefficiencies that can compromise assay performance and data quality.
Detection sensitivity represents a critical technical barrier, particularly when screening for subtle phenotypic changes or low-abundance products. The absence of cellular amplification mechanisms in cell-free systems often necessitates more sensitive analytical methods than those used in cell-based screening approaches.
Computational infrastructure limitations further complicate cell-free HTS deployment. The massive datasets generated during high-throughput campaigns require sophisticated data processing pipelines, machine learning algorithms, and predictive models that remain underdeveloped for cell-free specific applications.
Reagent cost considerations pose significant economic challenges. High-quality cell-free extracts and supplementary components remain expensive to produce at scale, limiting accessibility for many research groups and increasing per-sample screening costs compared to cell-based alternatives.
Finally, the integration of cell-free HTS with downstream validation workflows presents technical hurdles. Translating hits from cell-free screens to cellular or in vivo contexts often reveals discrepancies due to fundamental differences in biochemical environments, requiring careful validation strategies and correlation studies to ensure biological relevance.
Extract preparation consistency presents another major challenge. Batch-to-batch variations in extract quality can introduce significant data variability, compromising screening reliability and reproducibility. These inconsistencies stem from subtle differences in cell growth conditions, lysis efficiency, and extract processing methods, necessitating rigorous standardization protocols.
Energy system limitations further constrain cell-free HTS applications. The rapid depletion of ATP and other energy-rich compounds in cell-free systems leads to declining metabolic activity over time. Current continuous energy regeneration systems remain suboptimal for extended screening campaigns, requiring sophisticated engineering solutions to maintain consistent metabolic performance.
Scale-down challenges also persist in cell-free HTS implementation. Miniaturizing reaction volumes to nanoliter or picoliter scales—essential for ultra-high-throughput applications—introduces surface tension effects, evaporation issues, and mixing inefficiencies that can compromise assay performance and data quality.
Detection sensitivity represents a critical technical barrier, particularly when screening for subtle phenotypic changes or low-abundance products. The absence of cellular amplification mechanisms in cell-free systems often necessitates more sensitive analytical methods than those used in cell-based screening approaches.
Computational infrastructure limitations further complicate cell-free HTS deployment. The massive datasets generated during high-throughput campaigns require sophisticated data processing pipelines, machine learning algorithms, and predictive models that remain underdeveloped for cell-free specific applications.
Reagent cost considerations pose significant economic challenges. High-quality cell-free extracts and supplementary components remain expensive to produce at scale, limiting accessibility for many research groups and increasing per-sample screening costs compared to cell-based alternatives.
Finally, the integration of cell-free HTS with downstream validation workflows presents technical hurdles. Translating hits from cell-free screens to cellular or in vivo contexts often reveals discrepancies due to fundamental differences in biochemical environments, requiring careful validation strategies and correlation studies to ensure biological relevance.
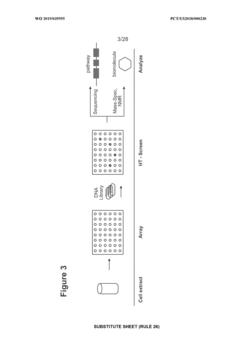
Current High-throughput Screening Methodologies
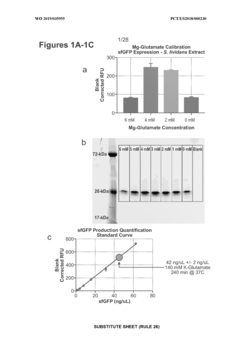
01 Cell-free protein synthesis systems for high-throughput screening
Cell-free protein synthesis systems enable rapid production of proteins without the constraints of living cells, making them ideal for high-throughput screening applications. These systems utilize transcription and translation machinery extracted from cells to produce proteins directly from DNA templates. By eliminating cell growth requirements, these platforms allow for faster screening cycles and direct access to the reaction environment for manipulation and analysis, significantly accelerating the discovery and optimization of bioproducts.- Cell-free protein synthesis systems for high-throughput screening: Cell-free protein synthesis systems enable rapid production of proteins without the constraints of living cells, making them ideal for high-throughput screening applications. These systems utilize cellular extracts containing the necessary machinery for transcription and translation, allowing for direct manipulation of reaction conditions and rapid protein production. This approach facilitates screening of large libraries of proteins or enzymes for desired properties or activities in a controlled environment.
- Microfluidic platforms for cell-free biomanufacturing screening: Microfluidic technologies provide miniaturized platforms for cell-free biomanufacturing processes, enabling high-throughput screening with minimal reagent consumption. These systems incorporate microchannels, chambers, and sensors to perform multiple parallel reactions while monitoring various parameters in real-time. The integration of microfluidics with cell-free systems allows for automation, precise control of reaction conditions, and rapid analysis of results, significantly accelerating the screening and optimization of biomanufacturing processes.
- Biosensor integration with cell-free systems for screening applications: The integration of biosensors with cell-free systems enables real-time monitoring and high-throughput screening of biological processes. These biosensors can detect specific molecules, metabolites, or reaction products, providing immediate feedback on the performance of the cell-free system. This approach allows for rapid assessment of multiple conditions or variants simultaneously, facilitating the optimization of biomanufacturing processes and the discovery of novel biocatalysts or pathways.
- Computational tools and AI for cell-free biomanufacturing optimization: Computational tools and artificial intelligence are increasingly being applied to optimize cell-free biomanufacturing processes through high-throughput screening. These technologies enable the analysis of large datasets generated from screening experiments, identifying patterns and relationships that can inform process improvements. Machine learning algorithms can predict optimal conditions, component concentrations, or genetic modifications, reducing the experimental burden and accelerating the development of efficient cell-free biomanufacturing systems.
- Cell-free metabolic engineering for pathway optimization: Cell-free metabolic engineering enables the rapid assembly and testing of metabolic pathways outside of living cells, facilitating high-throughput screening of pathway variants. This approach allows for direct manipulation of enzyme concentrations, cofactor levels, and reaction conditions to optimize product yield and specificity. By eliminating cellular constraints such as growth requirements or toxicity issues, cell-free systems provide a versatile platform for screening and optimizing complex biosynthetic pathways for various applications.
02 Microfluidic platforms for cell-free biomanufacturing screening
Microfluidic technologies provide miniaturized platforms for cell-free biomanufacturing screening with significantly reduced reagent consumption and increased throughput. These systems integrate multiple functions including sample preparation, reaction, separation, and detection into a single chip. The small reaction volumes and parallel processing capabilities enable thousands of reactions to be performed simultaneously, making them particularly valuable for screening enzyme variants, reaction conditions, and production parameters in cell-free systems.Expand Specific Solutions03 Biosensor integration with cell-free systems for screening applications
Biosensors integrated with cell-free systems provide real-time monitoring capabilities for high-throughput screening applications. These biosensors can detect specific metabolites, proteins, or reaction conditions without the interference of cellular components. The combination enables rapid feedback on production efficiency, product quality, and reaction kinetics. Advanced biosensor designs incorporate fluorescent proteins, electrochemical sensors, or aptamer-based detection methods to provide quantitative measurements suitable for automated screening platforms.Expand Specific Solutions04 Computational tools and AI for cell-free biomanufacturing optimization
Computational tools and artificial intelligence approaches enhance high-throughput screening in cell-free biomanufacturing by predicting optimal reaction conditions and identifying promising candidates. These tools analyze large datasets generated from screening experiments to establish correlations between reaction parameters and production outcomes. Machine learning algorithms can identify patterns not readily apparent to human researchers, accelerating the optimization process and reducing the number of physical experiments required to achieve desired production characteristics.Expand Specific Solutions05 Enzyme and pathway engineering for enhanced cell-free production
Engineering enzymes and metabolic pathways specifically for cell-free environments enables enhanced production capabilities and screening efficiency. Without cellular constraints, enzymes can be optimized for stability, activity, and specificity under broader reaction conditions. Pathway engineering in cell-free systems allows for the rapid testing of novel biosynthetic routes and enzyme combinations. High-throughput screening methods can rapidly evaluate these engineered components to identify optimal configurations for producing desired compounds with increased yields and purity.Expand Specific Solutions
Leading Companies in Cell-free Biomanufacturing
High-throughput screening using cell-free biomanufacturing technologies is currently in a growth phase, with the market expanding rapidly due to increasing demand for efficient drug discovery and development processes. The global market size is estimated to reach several billion dollars by 2025, driven by pharmaceutical R&D investments and biotechnology advancements. Technologically, the field shows varying maturity levels across applications. Leading players like Corning, Inc. and BASF Corp. have established robust platforms for industrial applications, while companies such as Life Technologies Corp. and Boehringer Ingelheim are advancing pharmaceutical applications. Academic institutions including Harvard College, The Scripps Research Institute, and École Polytechnique Fédérale de Lausanne are driving fundamental innovations. Emerging players like Bifrost Biosystems and EdiGene Biotechnology are introducing disruptive approaches, particularly in synthetic biology applications.
Life Technologies Corp.
Technical Solution: Life Technologies (now part of Thermo Fisher Scientific) has developed a comprehensive cell-free high-throughput screening platform marketed under their PURExpress® and ExpresswayTM product lines. Their technology utilizes reconstituted cell-free protein synthesis systems with defined components, eliminating variability associated with crude cell extracts. The platform incorporates automated liquid handling systems compatible with standard laboratory robotics, enabling seamless integration into existing high-throughput screening workflows. Life Technologies' system features specialized reaction formulations optimized for different protein classes, including membrane proteins, antibodies, and enzymes. Their technology includes proprietary translation enhancers that increase protein yield and reduce reaction times. The platform has been widely adopted in pharmaceutical research for target validation, hit identification, and lead optimization applications, demonstrating consistent performance across diverse screening campaigns.
Strengths: Commercial maturity and widespread adoption; excellent technical support infrastructure; compatibility with standard laboratory automation. Weaknesses: Less customizable than some academic platforms; potentially higher cost per reaction compared to in-house developed systems.
President & Fellows of Harvard College
Technical Solution: Harvard University has developed sophisticated cell-free biomanufacturing platforms for high-throughput screening applications. Their technology centers on a highly optimized cell-free protein synthesis system derived from multiple organisms, including E. coli, yeast, and mammalian cells, each tailored for specific protein classes. Harvard's platform incorporates a proprietary ribosome display system that allows for the direct evolution and screening of proteins without cellular transformation steps. The technology features miniaturized reaction formats compatible with 1536-well plates and beyond, enabling massive parallelization of screening efforts. Harvard researchers have pioneered the integration of synthetic genetic circuits into cell-free systems, creating programmable screening platforms that can detect and respond to specific molecular interactions. Their system has been successfully applied to antibody engineering, enzyme optimization, and the discovery of novel antimicrobial peptides.
Strengths: Exceptional scientific foundation; highly versatile platform applicable to diverse protein classes; advanced genetic circuit integration. Weaknesses: Complex technology requiring specialized expertise; potentially higher implementation costs compared to more standardized platforms.
Key Innovations in Cell-free Protein Synthesis
High-throughput system using a cell-free expression system and in SITU sequencing
PatentWO2019035955A1
Innovation
- A high-throughput system utilizing a cell-free expression system combined with in situ sequencing for rapid screening of nucleic acid templates, allowing for the detection and characterization of small molecules produced in multiple reaction volumes, enabling the identification of biosynthetic pathways and optimization of conditions for biomolecule production.
High-throughput Screening System Based on Multi-manipulators
PatentActiveUS20190004073A1
Innovation
- A high-throughput screening system utilizing multi-manipulators that automates sample handling, transfer, and detection processes, integrating components like samplers, pipettes, plate washers, microplate readers, and centrifuges to streamline the screening of microorganisms, enabling rapid and precise analysis of multiple samples simultaneously.
Scalability and Industrial Implementation Considerations
Scaling cell-free biomanufacturing technologies from laboratory to industrial scale presents significant challenges that must be addressed for successful implementation. The transition requires careful consideration of several factors, including production volume capabilities, equipment requirements, and process optimization. Current cell-free systems typically operate at milliliter scales in research settings, but industrial applications demand liter to cubic meter volumes, necessitating substantial engineering solutions to maintain reaction efficiency and consistency.
Material costs represent a critical barrier to industrial implementation. Cell-free systems require expensive components such as enzymes, energy sources, and nucleotides. Developing cost-effective production methods for these components and establishing recycling systems for reusable elements could significantly improve economic viability. Companies pioneering industrial cell-free processes have reported up to 60% cost reduction through optimization of extract preparation and component recycling.
Process standardization and quality control systems must be established to ensure reproducibility across batches and production sites. This includes developing robust protocols for extract preparation, reaction conditions, and analytical methods. The inherent variability in biological components necessitates comprehensive quality management systems that can detect and correct deviations in real-time, potentially through integration of advanced monitoring technologies and machine learning algorithms.
Regulatory considerations present another dimension of complexity for industrial implementation. Cell-free systems occupy a unique position between chemical and biological manufacturing processes, creating uncertainty in regulatory frameworks. Early engagement with regulatory bodies is essential to establish appropriate guidelines for safety assessment, product characterization, and quality control. Several pioneering companies have successfully navigated these challenges by developing comprehensive regulatory strategies specific to cell-free products.
Infrastructure requirements for large-scale cell-free manufacturing differ significantly from traditional biomanufacturing. The absence of living cells eliminates the need for sterile cultivation environments but introduces requirements for specialized mixing systems, temperature control, and component addition mechanisms. Modular and continuous processing approaches show particular promise for cell-free systems, potentially offering advantages in scalability and process control compared to batch operations.
Workforce development represents an often-overlooked aspect of industrial implementation. Cell-free biomanufacturing requires specialized knowledge spanning biochemistry, process engineering, and analytical sciences. Organizations implementing these technologies must invest in training programs and knowledge management systems to build and maintain the necessary expertise for successful operation and troubleshooting of these complex systems.
Material costs represent a critical barrier to industrial implementation. Cell-free systems require expensive components such as enzymes, energy sources, and nucleotides. Developing cost-effective production methods for these components and establishing recycling systems for reusable elements could significantly improve economic viability. Companies pioneering industrial cell-free processes have reported up to 60% cost reduction through optimization of extract preparation and component recycling.
Process standardization and quality control systems must be established to ensure reproducibility across batches and production sites. This includes developing robust protocols for extract preparation, reaction conditions, and analytical methods. The inherent variability in biological components necessitates comprehensive quality management systems that can detect and correct deviations in real-time, potentially through integration of advanced monitoring technologies and machine learning algorithms.
Regulatory considerations present another dimension of complexity for industrial implementation. Cell-free systems occupy a unique position between chemical and biological manufacturing processes, creating uncertainty in regulatory frameworks. Early engagement with regulatory bodies is essential to establish appropriate guidelines for safety assessment, product characterization, and quality control. Several pioneering companies have successfully navigated these challenges by developing comprehensive regulatory strategies specific to cell-free products.
Infrastructure requirements for large-scale cell-free manufacturing differ significantly from traditional biomanufacturing. The absence of living cells eliminates the need for sterile cultivation environments but introduces requirements for specialized mixing systems, temperature control, and component addition mechanisms. Modular and continuous processing approaches show particular promise for cell-free systems, potentially offering advantages in scalability and process control compared to batch operations.
Workforce development represents an often-overlooked aspect of industrial implementation. Cell-free biomanufacturing requires specialized knowledge spanning biochemistry, process engineering, and analytical sciences. Organizations implementing these technologies must invest in training programs and knowledge management systems to build and maintain the necessary expertise for successful operation and troubleshooting of these complex systems.
Regulatory Pathway for Cell-free Bioproduction Systems
Cell-free bioproduction systems represent a paradigm shift in biomanufacturing, offering significant advantages in speed, flexibility, and scalability. However, their regulatory pathway remains complex and evolving, presenting unique challenges for developers seeking market approval.
The regulatory framework for cell-free bioproduction systems primarily falls under the jurisdiction of the FDA in the United States, the EMA in Europe, and similar regulatory bodies worldwide. These systems are typically evaluated based on their end products rather than the production platform itself, with classification depending on the intended use—pharmaceuticals, food ingredients, or industrial enzymes.
For pharmaceutical applications, cell-free produced biologics must navigate the same rigorous approval process as traditional biologics, requiring comprehensive characterization of the product, demonstration of batch-to-batch consistency, and extensive safety and efficacy data. The FDA's Center for Biologics Evaluation and Research (CBER) or Center for Drug Evaluation and Research (CDER) oversees these approvals, depending on the specific product classification.
A critical regulatory consideration for cell-free systems is the demonstration of product equivalence when replicating existing approved biologics. Developers must provide substantial evidence that the cell-free produced molecule is structurally and functionally identical to its cell-based counterpart, with particular attention to post-translational modifications and impurity profiles.
Quality control represents another significant regulatory hurdle. Manufacturers must implement robust analytical methods to ensure consistent product quality, with particular emphasis on detecting and removing potential contaminants from the cell-free reaction components, including nucleic acids, endotoxins, and host cell proteins.
Environmental regulations also apply to cell-free bioproduction, particularly regarding waste management and potential environmental impact of reaction components. While generally considered more environmentally friendly than traditional cell-based methods, developers must still address these concerns in their regulatory submissions.
Recent regulatory developments show increasing recognition of cell-free technologies. The FDA's Emerging Technology Program and similar initiatives in other regions provide pathways for early engagement with regulators, helping developers address potential concerns before formal submission. Several precedents have been established with the approval of cell-free produced enzymes for food applications and diagnostic reagents, gradually paving the way for more complex therapeutics.
The regulatory framework for cell-free bioproduction systems primarily falls under the jurisdiction of the FDA in the United States, the EMA in Europe, and similar regulatory bodies worldwide. These systems are typically evaluated based on their end products rather than the production platform itself, with classification depending on the intended use—pharmaceuticals, food ingredients, or industrial enzymes.
For pharmaceutical applications, cell-free produced biologics must navigate the same rigorous approval process as traditional biologics, requiring comprehensive characterization of the product, demonstration of batch-to-batch consistency, and extensive safety and efficacy data. The FDA's Center for Biologics Evaluation and Research (CBER) or Center for Drug Evaluation and Research (CDER) oversees these approvals, depending on the specific product classification.
A critical regulatory consideration for cell-free systems is the demonstration of product equivalence when replicating existing approved biologics. Developers must provide substantial evidence that the cell-free produced molecule is structurally and functionally identical to its cell-based counterpart, with particular attention to post-translational modifications and impurity profiles.
Quality control represents another significant regulatory hurdle. Manufacturers must implement robust analytical methods to ensure consistent product quality, with particular emphasis on detecting and removing potential contaminants from the cell-free reaction components, including nucleic acids, endotoxins, and host cell proteins.
Environmental regulations also apply to cell-free bioproduction, particularly regarding waste management and potential environmental impact of reaction components. While generally considered more environmentally friendly than traditional cell-based methods, developers must still address these concerns in their regulatory submissions.
Recent regulatory developments show increasing recognition of cell-free technologies. The FDA's Emerging Technology Program and similar initiatives in other regions provide pathways for early engagement with regulators, helping developers address potential concerns before formal submission. Several precedents have been established with the approval of cell-free produced enzymes for food applications and diagnostic reagents, gradually paving the way for more complex therapeutics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!