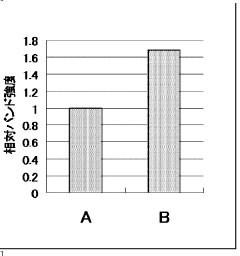
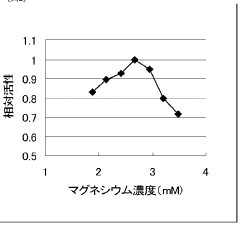
How cell-free systems improve protein synthesis rates?
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Protein Synthesis Background and Objectives
Cell-free protein synthesis (CFPS) has evolved significantly since its inception in the 1960s when Nirenberg and Matthaei first demonstrated protein synthesis outside living cells. This groundbreaking technology has transformed from a fundamental research tool into a versatile platform with applications spanning from pharmaceutical production to synthetic biology. The evolution of CFPS systems reflects broader trends in biotechnology, moving toward more efficient, scalable, and customizable protein production methods that overcome limitations inherent to traditional cell-based expression systems.
The primary objective of modern CFPS technology is to achieve protein synthesis rates comparable to or exceeding those of living cells while offering greater flexibility and control. This goal addresses critical challenges in biopharmaceutical manufacturing, where rapid production of complex proteins remains a significant bottleneck. By eliminating cellular barriers and constraints, CFPS systems aim to revolutionize protein production across multiple industries, potentially reducing development timelines from months to days.
Recent technological advancements have dramatically improved CFPS efficiency, with systems derived from various organisms including Escherichia coli, wheat germ, rabbit reticulocytes, and insect cells. Each system offers unique advantages for specific applications, with E. coli-based systems generally providing the highest protein yields while eukaryotic systems better accommodate complex post-translational modifications. The continuous refinement of these systems represents a critical frontier in biotechnology research.
The trajectory of CFPS development has been characterized by several key innovations, including improved energy regeneration systems, enhanced translation machinery, and optimized reaction conditions. These developments have collectively increased protein yields from micrograms to milligrams per milliliter of reaction volume, representing orders of magnitude improvement over early systems. This progress has expanded the potential applications of CFPS from basic research to industrial-scale production.
Looking forward, CFPS technology aims to address several ambitious objectives: achieving consistent gram-scale protein production, incorporating complex post-translational modifications, enabling continuous-flow synthesis systems, and reducing production costs to economically viable levels for commercial applications. These objectives align with broader industry needs for flexible, rapid-response manufacturing platforms capable of producing complex biological molecules on demand.
The convergence of CFPS with other emerging technologies, including artificial intelligence for reaction optimization, microfluidics for reaction compartmentalization, and synthetic biology for novel pathway engineering, promises to further accelerate progress in this field. These interdisciplinary approaches may unlock unprecedented capabilities in protein synthesis, potentially transforming biomanufacturing paradigms across multiple sectors.
The primary objective of modern CFPS technology is to achieve protein synthesis rates comparable to or exceeding those of living cells while offering greater flexibility and control. This goal addresses critical challenges in biopharmaceutical manufacturing, where rapid production of complex proteins remains a significant bottleneck. By eliminating cellular barriers and constraints, CFPS systems aim to revolutionize protein production across multiple industries, potentially reducing development timelines from months to days.
Recent technological advancements have dramatically improved CFPS efficiency, with systems derived from various organisms including Escherichia coli, wheat germ, rabbit reticulocytes, and insect cells. Each system offers unique advantages for specific applications, with E. coli-based systems generally providing the highest protein yields while eukaryotic systems better accommodate complex post-translational modifications. The continuous refinement of these systems represents a critical frontier in biotechnology research.
The trajectory of CFPS development has been characterized by several key innovations, including improved energy regeneration systems, enhanced translation machinery, and optimized reaction conditions. These developments have collectively increased protein yields from micrograms to milligrams per milliliter of reaction volume, representing orders of magnitude improvement over early systems. This progress has expanded the potential applications of CFPS from basic research to industrial-scale production.
Looking forward, CFPS technology aims to address several ambitious objectives: achieving consistent gram-scale protein production, incorporating complex post-translational modifications, enabling continuous-flow synthesis systems, and reducing production costs to economically viable levels for commercial applications. These objectives align with broader industry needs for flexible, rapid-response manufacturing platforms capable of producing complex biological molecules on demand.
The convergence of CFPS with other emerging technologies, including artificial intelligence for reaction optimization, microfluidics for reaction compartmentalization, and synthetic biology for novel pathway engineering, promises to further accelerate progress in this field. These interdisciplinary approaches may unlock unprecedented capabilities in protein synthesis, potentially transforming biomanufacturing paradigms across multiple sectors.
Market Analysis for Cell-free Expression Systems
The cell-free expression systems market has experienced significant growth in recent years, driven by increasing demand for rapid protein production methods across various industries. The global market value reached approximately $208 million in 2020 and is projected to grow at a compound annual growth rate (CAGR) of 6.5% through 2027, potentially reaching $325 million by that time. This growth trajectory reflects the expanding applications of cell-free systems in pharmaceutical development, diagnostics, and synthetic biology.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 45% of the total market share. These companies leverage cell-free systems for drug discovery, protein engineering, and therapeutic protein production. The academic and research institutions segment follows closely, comprising approximately 30% of the market, where cell-free systems are extensively used for fundamental research and educational purposes.
Geographically, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and Japan, is expected to witness the fastest growth rate due to increasing investments in biotechnology research and development infrastructure.
The market is characterized by a mix of established players and innovative startups. Key market drivers include the growing demand for personalized medicine, increasing research activities in synthetic biology, and the need for rapid vaccine development platforms as highlighted during the COVID-19 pandemic. Cell-free systems offer significant advantages in these applications due to their ability to accelerate protein synthesis rates compared to traditional cell-based methods.
Customer demand is increasingly focused on systems that can deliver higher protein yields, greater reproducibility, and simplified workflows. End-users are particularly interested in cell-free platforms that can be easily integrated with automation technologies to enable high-throughput applications. This trend is driving manufacturers to develop more sophisticated and user-friendly cell-free expression kits.
Pricing strategies in the market vary significantly, with basic research-grade kits starting at $200-300 per reaction set, while more specialized systems for industrial applications can command premium prices of $1,000-2,000 per kit. The consumables and reagents segment represents a substantial recurring revenue stream for suppliers, accounting for approximately 65% of the total market value.
Market challenges include the relatively high cost of cell-free systems compared to conventional expression methods, technical limitations in producing complex proteins, and regulatory uncertainties surrounding products developed using these technologies. Despite these challenges, the market outlook remains positive as technological advancements continue to enhance protein synthesis rates and expand the application scope of cell-free systems.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 45% of the total market share. These companies leverage cell-free systems for drug discovery, protein engineering, and therapeutic protein production. The academic and research institutions segment follows closely, comprising approximately 30% of the market, where cell-free systems are extensively used for fundamental research and educational purposes.
Geographically, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and Japan, is expected to witness the fastest growth rate due to increasing investments in biotechnology research and development infrastructure.
The market is characterized by a mix of established players and innovative startups. Key market drivers include the growing demand for personalized medicine, increasing research activities in synthetic biology, and the need for rapid vaccine development platforms as highlighted during the COVID-19 pandemic. Cell-free systems offer significant advantages in these applications due to their ability to accelerate protein synthesis rates compared to traditional cell-based methods.
Customer demand is increasingly focused on systems that can deliver higher protein yields, greater reproducibility, and simplified workflows. End-users are particularly interested in cell-free platforms that can be easily integrated with automation technologies to enable high-throughput applications. This trend is driving manufacturers to develop more sophisticated and user-friendly cell-free expression kits.
Pricing strategies in the market vary significantly, with basic research-grade kits starting at $200-300 per reaction set, while more specialized systems for industrial applications can command premium prices of $1,000-2,000 per kit. The consumables and reagents segment represents a substantial recurring revenue stream for suppliers, accounting for approximately 65% of the total market value.
Market challenges include the relatively high cost of cell-free systems compared to conventional expression methods, technical limitations in producing complex proteins, and regulatory uncertainties surrounding products developed using these technologies. Despite these challenges, the market outlook remains positive as technological advancements continue to enhance protein synthesis rates and expand the application scope of cell-free systems.
Technical Challenges in Cell-free Protein Synthesis
Despite significant advancements in cell-free protein synthesis (CFPS) systems, several technical challenges continue to limit their efficiency and widespread application. One of the primary obstacles is the rapid depletion of energy resources during the synthesis process. ATP and GTP, essential for translation, are quickly consumed, leading to premature termination of protein production. Current energy regeneration systems often struggle to maintain consistent energy levels throughout the reaction duration.
Extract preparation represents another significant hurdle. The quality and composition of cell extracts vary considerably between batches, affecting reproducibility and scalability. The presence of proteases and nucleases in these extracts can degrade essential components, while the concentration of ribosomes and translation factors directly impacts synthesis rates. Standardization of extract preparation protocols remains elusive across different laboratories and applications.
Post-translational modifications present unique challenges in CFPS systems. Many proteins require specific modifications such as glycosylation, phosphorylation, or disulfide bond formation to achieve proper folding and functionality. Conventional CFPS systems often lack the necessary cellular machinery to perform these modifications efficiently, resulting in inactive or improperly folded proteins.
Scale-up difficulties constitute a major barrier to industrial implementation. While CFPS works effectively at small laboratory scales, transitioning to industrial production volumes introduces complications in maintaining reaction homogeneity, temperature control, and oxygen transfer. The cost of reagents at larger scales also presents economic challenges that limit commercial viability.
Reaction environment optimization remains complex due to the multifactorial nature of CFPS systems. Parameters including pH, ion concentrations, temperature, and molecular crowding agents must be precisely controlled to maximize protein yield. These parameters often interact in unpredictable ways, making systematic optimization difficult.
The limited reaction lifetime of CFPS systems typically restricts production to several hours before yields plateau. This constraint stems from multiple factors including resource depletion, byproduct accumulation, and component degradation. Extending this productive window represents a significant technical challenge that researchers continue to address through various approaches.
Lastly, the formation of inclusion bodies and protein aggregation frequently occurs when synthesizing complex proteins at high rates. These aggregates result from improper folding kinetics when translation rates exceed the capacity of chaperone systems to assist proper folding. Developing strategies to maintain solubility while preserving high synthesis rates remains an active area of research in the field.
Extract preparation represents another significant hurdle. The quality and composition of cell extracts vary considerably between batches, affecting reproducibility and scalability. The presence of proteases and nucleases in these extracts can degrade essential components, while the concentration of ribosomes and translation factors directly impacts synthesis rates. Standardization of extract preparation protocols remains elusive across different laboratories and applications.
Post-translational modifications present unique challenges in CFPS systems. Many proteins require specific modifications such as glycosylation, phosphorylation, or disulfide bond formation to achieve proper folding and functionality. Conventional CFPS systems often lack the necessary cellular machinery to perform these modifications efficiently, resulting in inactive or improperly folded proteins.
Scale-up difficulties constitute a major barrier to industrial implementation. While CFPS works effectively at small laboratory scales, transitioning to industrial production volumes introduces complications in maintaining reaction homogeneity, temperature control, and oxygen transfer. The cost of reagents at larger scales also presents economic challenges that limit commercial viability.
Reaction environment optimization remains complex due to the multifactorial nature of CFPS systems. Parameters including pH, ion concentrations, temperature, and molecular crowding agents must be precisely controlled to maximize protein yield. These parameters often interact in unpredictable ways, making systematic optimization difficult.
The limited reaction lifetime of CFPS systems typically restricts production to several hours before yields plateau. This constraint stems from multiple factors including resource depletion, byproduct accumulation, and component degradation. Extending this productive window represents a significant technical challenge that researchers continue to address through various approaches.
Lastly, the formation of inclusion bodies and protein aggregation frequently occurs when synthesizing complex proteins at high rates. These aggregates result from improper folding kinetics when translation rates exceed the capacity of chaperone systems to assist proper folding. Developing strategies to maintain solubility while preserving high synthesis rates remains an active area of research in the field.
Current Methodologies for Enhancing Synthesis Rates
01 Cell-free protein synthesis systems optimization
Cell-free protein synthesis systems can be optimized to increase protein production rates through various methods. These include modifying reaction conditions such as temperature, pH, and ionic strength, as well as optimizing the concentration of key components like ribosomes, tRNAs, and translation factors. Enhanced systems allow for rapid protein synthesis without the constraints of cellular metabolism, enabling higher yields and faster production rates.- Cell-free protein synthesis systems optimization: Cell-free protein synthesis systems can be optimized to increase protein production rates through various approaches. These include modifying reaction conditions, enhancing energy regeneration systems, and optimizing the composition of reaction mixtures. Such optimizations can significantly improve the efficiency and yield of protein synthesis in vitro, making these systems more suitable for industrial and research applications.
- Continuous-flow cell-free protein synthesis: Continuous-flow systems for cell-free protein synthesis allow for sustained production by continuously supplying substrates and removing inhibitory byproducts. These systems can maintain protein synthesis rates for extended periods compared to batch reactions. The implementation of microfluidic devices and specialized bioreactors enables precise control over reaction parameters, resulting in higher overall protein yields and synthesis rates.
- Enhanced energy regeneration for sustained synthesis: Energy supply is a critical factor affecting protein synthesis rates in cell-free systems. Advanced energy regeneration methods, including ATP regeneration systems and alternative energy sources, can significantly extend reaction lifetimes and increase protein yields. These approaches address one of the main limitations of cell-free systems by preventing energy depletion that typically leads to decreased synthesis rates over time.
- Engineered components for improved translation efficiency: The use of engineered ribosomes, tRNAs, and translation factors can significantly enhance protein synthesis rates in cell-free systems. These modified components are designed to overcome rate-limiting steps in translation, resulting in faster and more efficient protein production. Additionally, optimized genetic elements such as enhanced promoters and ribosome binding sites contribute to higher expression levels.
- High-throughput screening and monitoring methods: Advanced techniques for real-time monitoring and high-throughput screening of cell-free protein synthesis enable rapid optimization of reaction conditions. These methods allow researchers to quickly identify factors affecting synthesis rates and make adjustments to maximize productivity. Fluorescent reporters, mass spectrometry, and automated platforms facilitate the development of more efficient cell-free systems with enhanced protein production rates.
02 Energy regeneration systems for sustained protein synthesis
Energy regeneration systems are crucial for maintaining high protein synthesis rates in cell-free systems. These systems provide a continuous supply of ATP and GTP, which are essential energy sources for translation. Various approaches include enzyme-based regeneration systems, phosphate donor compounds, and metabolic engineering strategies that extend the duration of active protein synthesis, resulting in higher overall yields.Expand Specific Solutions03 Extract preparation methods affecting synthesis rates
The method of preparing cell extracts significantly impacts protein synthesis rates in cell-free systems. Techniques for cell disruption, extract clarification, and component preservation all affect the quality and activity of the resulting system. Optimized extract preparation protocols can preserve essential translation machinery, remove inhibitory components, and enhance the overall efficiency of protein production.Expand Specific Solutions04 Supplementation strategies for enhanced synthesis
Supplementation of cell-free systems with specific components can dramatically increase protein synthesis rates. These supplements include amino acids, nucleotides, chaperones, and other factors that may become limiting during the reaction. Strategic addition of these components at optimized concentrations and timing can overcome bottlenecks in the translation process, leading to improved protein yields and synthesis rates.Expand Specific Solutions05 Continuous-flow and microfluidic cell-free systems
Continuous-flow and microfluidic platforms represent advanced approaches to cell-free protein synthesis that can achieve higher production rates. These systems allow for the continuous supply of substrates and removal of inhibitory byproducts, extending reaction lifetimes. Miniaturized formats enable precise control over reaction conditions, reduced reagent consumption, and parallelization for high-throughput applications, resulting in improved synthesis rates and yields.Expand Specific Solutions
Leading Companies and Research Institutions
Cell-free protein synthesis technology is currently in a growth phase, with increasing market adoption driven by its ability to overcome traditional cellular limitations. The market is expanding rapidly, projected to reach significant scale due to applications in pharmaceuticals, diagnostics, and research. Technologically, the field shows varying maturity levels among key players. Companies like Sutro Biopharma and Cellfree Sciences have developed proprietary platforms demonstrating commercial viability, while academic institutions including Northwestern University, Tsinghua University, and Cornell University contribute fundamental research advancements. Research organizations such as Fraunhofer-Gesellschaft and Riken Corp are bridging the gap between academic discoveries and industrial applications. Japanese firms including Toyobo and Shimadzu are advancing instrumentation and reagent technologies, positioning themselves as important suppliers in this emerging ecosystem.
Cellfree Sciences Co., Ltd.
Technical Solution: Cellfree Sciences has developed the WEPRO® cell-free protein synthesis system based on wheat germ extract technology. Their approach utilizes the eukaryotic translation machinery from wheat germ to achieve high-yield protein production without cellular constraints. The company's bilayer reaction format physically separates the translation reaction from the energy supply and amino acids, allowing for continuous protein synthesis over extended periods[1]. Their system incorporates optimized mRNA templates with enhanced stability features and specialized cap structures to improve translation efficiency. Additionally, Cellfree Sciences has engineered their cell-free system to incorporate non-natural amino acids and perform post-translational modifications that are critical for producing functional proteins[2]. The company has also developed high-throughput screening platforms using their cell-free technology for rapid protein expression and analysis.
Strengths: Superior expression of complex eukaryotic proteins with proper folding and functionality; scalable production from micrograms to milligrams; rapid protein synthesis (typically 24-48 hours from gene to protein). Weaknesses: Higher cost compared to bacterial cell-free systems; limited post-translational modifications compared to mammalian cell-based systems; requires specialized equipment and expertise for optimal results.
Sutro Biopharma, Inc.
Technical Solution: Sutro Biopharma has pioneered the XpressCF® platform, an E. coli-based cell-free protein synthesis system specifically optimized for the production of complex therapeutic proteins and antibody-drug conjugates (ADCs). Their technology enables site-specific incorporation of non-natural amino acids through their proprietary tRNA synthetase/tRNA pairs, allowing precise conjugation of cytotoxic payloads to antibodies[3]. The XpressCF+ system includes chaperones and disulfide isomerases to facilitate proper protein folding and disulfide bond formation, significantly improving the production of complex proteins like antibodies. Sutro has engineered their cell-free system to operate at scales suitable for commercial manufacturing, with optimized energy regeneration systems that extend reaction durations and increase protein yields[4]. Their approach eliminates the need for cell culture, cell lysis, and extensive purification steps, reducing production time from weeks to days while maintaining consistent quality and homogeneity in the final product.
Strengths: Precise control over protein modifications enabling novel conjugated therapeutics; rapid iteration cycles for drug development (days vs weeks/months); elimination of cell viability concerns allowing production of proteins toxic to host cells. Weaknesses: Higher production costs compared to traditional cell-based methods; challenges in scaling to very large commercial quantities; limited to certain types of post-translational modifications.
Key Innovations in Extract Preparation and Reaction Conditions
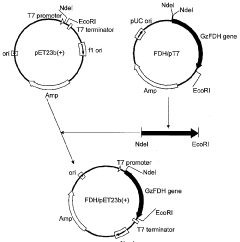
Cell-free protein synthesis solution, cell-free protein synthesis kit, and protein synthesis method
PatentWO2010147111A1
Innovation
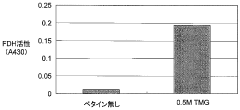
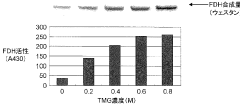
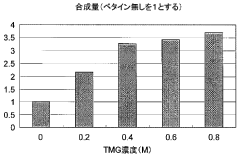
- Incorporating specific compounds such as trimethylglycine, L-carnitine, sarcosine, and their derivatives into the cell-free protein synthesis solution to enhance protein synthesis efficiency, allowing for the production of proteins that are challenging to synthesize in conventional systems.
Improved composition for cell-free protein synthesis
PatentWO2005075660A1
Innovation
- Optimizing the concentration of ATP, magnesium, and creatine phosphate in cell-free protein synthesis compositions to enhance protein synthesis efficiency, using a multi-layer method that continuously supplies necessary substrates and removes by-products, thereby increasing yield without adding new components.
Scalability and Manufacturing Considerations
Scaling up cell-free protein synthesis (CFPS) systems from laboratory scale to industrial production presents significant challenges that must be addressed to realize their full commercial potential. Current laboratory-scale CFPS reactions typically range from microliters to milliliters, while industrial applications require volumes in liters or even cubic meters. This substantial increase in scale introduces issues related to mixing efficiency, heat transfer, and maintaining homogeneity throughout the reaction vessel.
The economics of large-scale CFPS production remains a critical consideration. The cost of extract preparation, particularly for E. coli-based systems, decreases significantly with scale, but still represents a substantial portion of overall production expenses. Energy regeneration components and nucleotides contribute approximately 30% to the total cost, while amino acids account for roughly 15%. Recent advancements have reduced costs from dollars per milligram to cents per milligram of protein, making industrial applications increasingly viable.
Continuous-flow CFPS systems offer promising solutions for scaling challenges. These systems allow for the continuous addition of substrates and removal of products, maintaining optimal reaction conditions over extended periods. This approach has demonstrated up to 10-fold improvements in volumetric productivity compared to batch processes. Additionally, microfluidic technologies enable precise control over reaction parameters in continuous systems, further enhancing productivity and consistency.
Lyophilization (freeze-drying) of cell extracts represents another significant advancement for manufacturing scalability. Freeze-dried extracts maintain activity for months at room temperature, dramatically reducing cold chain requirements and storage costs. This technology enables the development of "just-add-water" CFPS kits that can be deployed in resource-limited settings or for on-demand production scenarios.
Standardization of manufacturing protocols presents another challenge for industrial adoption. Current research laboratories often employ proprietary methods for extract preparation and reaction conditions, leading to variability in performance across different facilities. Establishing industry standards for extract quality, component specifications, and process parameters will be essential for consistent large-scale production.
Regulatory considerations also impact manufacturing scale-up. As CFPS moves toward pharmaceutical applications, compliance with Good Manufacturing Practice (GMP) standards becomes necessary. The cell-free nature of these systems potentially simplifies regulatory approval compared to whole-cell systems, as concerns about living organisms are eliminated, but validation of consistent product quality remains paramount.
The economics of large-scale CFPS production remains a critical consideration. The cost of extract preparation, particularly for E. coli-based systems, decreases significantly with scale, but still represents a substantial portion of overall production expenses. Energy regeneration components and nucleotides contribute approximately 30% to the total cost, while amino acids account for roughly 15%. Recent advancements have reduced costs from dollars per milligram to cents per milligram of protein, making industrial applications increasingly viable.
Continuous-flow CFPS systems offer promising solutions for scaling challenges. These systems allow for the continuous addition of substrates and removal of products, maintaining optimal reaction conditions over extended periods. This approach has demonstrated up to 10-fold improvements in volumetric productivity compared to batch processes. Additionally, microfluidic technologies enable precise control over reaction parameters in continuous systems, further enhancing productivity and consistency.
Lyophilization (freeze-drying) of cell extracts represents another significant advancement for manufacturing scalability. Freeze-dried extracts maintain activity for months at room temperature, dramatically reducing cold chain requirements and storage costs. This technology enables the development of "just-add-water" CFPS kits that can be deployed in resource-limited settings or for on-demand production scenarios.
Standardization of manufacturing protocols presents another challenge for industrial adoption. Current research laboratories often employ proprietary methods for extract preparation and reaction conditions, leading to variability in performance across different facilities. Establishing industry standards for extract quality, component specifications, and process parameters will be essential for consistent large-scale production.
Regulatory considerations also impact manufacturing scale-up. As CFPS moves toward pharmaceutical applications, compliance with Good Manufacturing Practice (GMP) standards becomes necessary. The cell-free nature of these systems potentially simplifies regulatory approval compared to whole-cell systems, as concerns about living organisms are eliminated, but validation of consistent product quality remains paramount.
Regulatory Framework for Cell-free Produced Biologics
The regulatory landscape for cell-free produced biologics represents a complex and evolving framework that significantly impacts the commercial viability and implementation of these innovative protein synthesis technologies. Currently, regulatory bodies including the FDA, EMA, and other international agencies are working to establish appropriate guidelines that address the unique characteristics of cell-free systems while ensuring product safety and efficacy.
Unlike traditional cell-based production methods, cell-free systems present novel regulatory considerations due to their acellular nature and distinct production processes. Regulatory frameworks must address concerns regarding raw material sourcing, extract preparation standardization, and potential contaminants specific to cell-free systems. The absence of cellular components eliminates certain biosafety concerns but introduces new questions about process validation and product characterization.
Quality control parameters for cell-free produced biologics require specialized approaches that differ from traditional biomanufacturing. Regulatory bodies are developing specific guidelines for analytical methods to assess product quality, purity, and consistency. These include requirements for demonstrating the absence of host cell proteins, nucleic acids, and other potential contaminants that might be present in cell-free reaction components.
Intellectual property considerations also play a crucial role in the regulatory framework. Patents covering cell-free expression systems, genetic elements, and production methodologies may impact regulatory approval pathways and commercial development strategies. Companies must navigate this complex IP landscape while ensuring compliance with evolving regulatory requirements.
Harmonization efforts between different regulatory jurisdictions are underway to facilitate global development and commercialization of cell-free produced biologics. These initiatives aim to establish consistent standards for safety assessment, manufacturing controls, and product characterization across international markets, reducing regulatory barriers to market entry.
For companies developing cell-free protein synthesis technologies, early engagement with regulatory authorities through formal consultation processes is increasingly important. These interactions help address potential regulatory concerns proactively and establish appropriate development pathways for novel cell-free produced biologics. Regulatory agencies have shown willingness to adapt existing frameworks to accommodate these innovative production platforms while maintaining their mandate to ensure public safety.
As the field advances, regulatory frameworks will likely continue to evolve, potentially creating accelerated approval pathways for certain cell-free produced biologics that demonstrate significant advantages in production efficiency, consistency, or safety profiles compared to traditional methods.
Unlike traditional cell-based production methods, cell-free systems present novel regulatory considerations due to their acellular nature and distinct production processes. Regulatory frameworks must address concerns regarding raw material sourcing, extract preparation standardization, and potential contaminants specific to cell-free systems. The absence of cellular components eliminates certain biosafety concerns but introduces new questions about process validation and product characterization.
Quality control parameters for cell-free produced biologics require specialized approaches that differ from traditional biomanufacturing. Regulatory bodies are developing specific guidelines for analytical methods to assess product quality, purity, and consistency. These include requirements for demonstrating the absence of host cell proteins, nucleic acids, and other potential contaminants that might be present in cell-free reaction components.
Intellectual property considerations also play a crucial role in the regulatory framework. Patents covering cell-free expression systems, genetic elements, and production methodologies may impact regulatory approval pathways and commercial development strategies. Companies must navigate this complex IP landscape while ensuring compliance with evolving regulatory requirements.
Harmonization efforts between different regulatory jurisdictions are underway to facilitate global development and commercialization of cell-free produced biologics. These initiatives aim to establish consistent standards for safety assessment, manufacturing controls, and product characterization across international markets, reducing regulatory barriers to market entry.
For companies developing cell-free protein synthesis technologies, early engagement with regulatory authorities through formal consultation processes is increasingly important. These interactions help address potential regulatory concerns proactively and establish appropriate development pathways for novel cell-free produced biologics. Regulatory agencies have shown willingness to adapt existing frameworks to accommodate these innovative production platforms while maintaining their mandate to ensure public safety.
As the field advances, regulatory frameworks will likely continue to evolve, potentially creating accelerated approval pathways for certain cell-free produced biologics that demonstrate significant advantages in production efficiency, consistency, or safety profiles compared to traditional methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!