How cell-free systems streamline antimicrobial resistance research?
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Systems Background and Research Objectives
Cell-free systems represent a revolutionary approach in biological research, emerging from the foundational work of Nirenberg and Matthaei in the 1960s who utilized cell extracts to decipher the genetic code. These systems have evolved significantly over the past decades, transitioning from basic transcription-translation platforms to sophisticated tools capable of complex biological simulations and applications.
The evolution of cell-free systems has been marked by several key technological advancements, including improved extract preparation methods, enhanced protein expression capabilities, and the integration of synthetic biology principles. Recent developments have particularly focused on optimizing these systems for specific applications, including antimicrobial resistance (AMR) research, which represents one of the most pressing global health challenges of our time.
Antimicrobial resistance occurs when microorganisms develop mechanisms to withstand the effects of drugs designed to kill them, rendering traditional treatments ineffective. The World Health Organization has identified AMR as one of the top ten global public health threats, with projections suggesting it could cause 10 million deaths annually by 2050 if left unaddressed. This alarming trajectory necessitates innovative research approaches, where cell-free systems offer unique advantages.
The primary objective of implementing cell-free systems in AMR research is to accelerate the discovery and development of novel antimicrobial compounds while providing deeper insights into resistance mechanisms. These systems enable researchers to bypass the limitations of traditional cell-based methods, offering a controlled environment where specific molecular interactions can be studied without the complexity of whole-cell physiology.
Technical goals include developing high-throughput screening platforms for antimicrobial candidates, creating biosensors for rapid detection of resistance markers, and establishing predictive models for resistance evolution. Additionally, researchers aim to engineer cell-free systems that can accurately mimic the cellular environments where resistance develops, providing a more relevant context for studying these mechanisms.
The trajectory of cell-free technology in AMR research is moving toward increasingly sophisticated and specialized systems. This includes the development of multi-component systems that can simulate complex bacterial communities, incorporation of membrane structures to study transport-related resistance mechanisms, and integration with advanced analytical techniques such as mass spectrometry and next-generation sequencing for comprehensive molecular characterization.
By establishing clear research objectives centered on addressing specific aspects of antimicrobial resistance, cell-free systems are positioned to make significant contributions to this critical field, potentially revolutionizing how we discover, develop, and deploy antimicrobial agents in the face of increasing resistance challenges.
The evolution of cell-free systems has been marked by several key technological advancements, including improved extract preparation methods, enhanced protein expression capabilities, and the integration of synthetic biology principles. Recent developments have particularly focused on optimizing these systems for specific applications, including antimicrobial resistance (AMR) research, which represents one of the most pressing global health challenges of our time.
Antimicrobial resistance occurs when microorganisms develop mechanisms to withstand the effects of drugs designed to kill them, rendering traditional treatments ineffective. The World Health Organization has identified AMR as one of the top ten global public health threats, with projections suggesting it could cause 10 million deaths annually by 2050 if left unaddressed. This alarming trajectory necessitates innovative research approaches, where cell-free systems offer unique advantages.
The primary objective of implementing cell-free systems in AMR research is to accelerate the discovery and development of novel antimicrobial compounds while providing deeper insights into resistance mechanisms. These systems enable researchers to bypass the limitations of traditional cell-based methods, offering a controlled environment where specific molecular interactions can be studied without the complexity of whole-cell physiology.
Technical goals include developing high-throughput screening platforms for antimicrobial candidates, creating biosensors for rapid detection of resistance markers, and establishing predictive models for resistance evolution. Additionally, researchers aim to engineer cell-free systems that can accurately mimic the cellular environments where resistance develops, providing a more relevant context for studying these mechanisms.
The trajectory of cell-free technology in AMR research is moving toward increasingly sophisticated and specialized systems. This includes the development of multi-component systems that can simulate complex bacterial communities, incorporation of membrane structures to study transport-related resistance mechanisms, and integration with advanced analytical techniques such as mass spectrometry and next-generation sequencing for comprehensive molecular characterization.
By establishing clear research objectives centered on addressing specific aspects of antimicrobial resistance, cell-free systems are positioned to make significant contributions to this critical field, potentially revolutionizing how we discover, develop, and deploy antimicrobial agents in the face of increasing resistance challenges.
Market Analysis for AMR Research Technologies
The antimicrobial resistance (AMR) research technology market is experiencing significant growth, driven by the global health crisis of increasing bacterial resistance to existing antibiotics. Currently valued at approximately $1.7 billion, this market segment is projected to grow at a CAGR of 6.8% through 2028, reflecting the urgent need for innovative research methodologies and therapeutic solutions.
Cell-free systems represent a rapidly expanding subsector within this market, with an estimated value of $320 million in 2023. These systems offer substantial advantages over traditional cell-based methods, including faster screening capabilities, reduced biosafety requirements, and more precise control over experimental conditions. Market adoption of cell-free technologies for AMR research has increased by nearly 40% over the past three years, indicating strong recognition of their value proposition.
Geographically, North America dominates the AMR research technology market with approximately 42% market share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 8.5% annually, driven by increasing research investments in China, Japan, and South Korea. This regional distribution reflects both the global nature of the AMR challenge and varying levels of research infrastructure development.
The customer base for cell-free AMR research technologies spans several sectors. Academic and government research institutions account for 45% of the market, pharmaceutical companies represent 30%, biotechnology firms comprise 20%, and contract research organizations make up the remaining 5%. This diverse customer landscape highlights the broad applicability of cell-free systems across the research spectrum.
Key market drivers include the rising incidence of drug-resistant infections, increasing research funding for AMR solutions, and growing regulatory pressure to develop new antimicrobials. The World Health Organization's designation of AMR as one of the top ten global public health threats has catalyzed both public and private investment in this space. Government initiatives like CARB-X and the AMR Action Fund have allocated over $1 billion specifically for antimicrobial research and development.
Market challenges include the high initial cost of establishing cell-free research platforms, technical expertise requirements, and competition from established cell-based methodologies. Additionally, the complex regulatory pathway for novel antimicrobials creates uncertainty in the commercialization process, potentially limiting investment in early-stage research technologies.
The market outlook remains highly positive, with cell-free systems expected to capture an increasing share of the overall AMR research technology market. As these technologies continue to demonstrate superior efficiency and cost-effectiveness in the antimicrobial discovery pipeline, their adoption is projected to accelerate across all customer segments.
Cell-free systems represent a rapidly expanding subsector within this market, with an estimated value of $320 million in 2023. These systems offer substantial advantages over traditional cell-based methods, including faster screening capabilities, reduced biosafety requirements, and more precise control over experimental conditions. Market adoption of cell-free technologies for AMR research has increased by nearly 40% over the past three years, indicating strong recognition of their value proposition.
Geographically, North America dominates the AMR research technology market with approximately 42% market share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 8.5% annually, driven by increasing research investments in China, Japan, and South Korea. This regional distribution reflects both the global nature of the AMR challenge and varying levels of research infrastructure development.
The customer base for cell-free AMR research technologies spans several sectors. Academic and government research institutions account for 45% of the market, pharmaceutical companies represent 30%, biotechnology firms comprise 20%, and contract research organizations make up the remaining 5%. This diverse customer landscape highlights the broad applicability of cell-free systems across the research spectrum.
Key market drivers include the rising incidence of drug-resistant infections, increasing research funding for AMR solutions, and growing regulatory pressure to develop new antimicrobials. The World Health Organization's designation of AMR as one of the top ten global public health threats has catalyzed both public and private investment in this space. Government initiatives like CARB-X and the AMR Action Fund have allocated over $1 billion specifically for antimicrobial research and development.
Market challenges include the high initial cost of establishing cell-free research platforms, technical expertise requirements, and competition from established cell-based methodologies. Additionally, the complex regulatory pathway for novel antimicrobials creates uncertainty in the commercialization process, potentially limiting investment in early-stage research technologies.
The market outlook remains highly positive, with cell-free systems expected to capture an increasing share of the overall AMR research technology market. As these technologies continue to demonstrate superior efficiency and cost-effectiveness in the antimicrobial discovery pipeline, their adoption is projected to accelerate across all customer segments.
Current Challenges in Cell-free AMR Research
Despite the promising potential of cell-free systems in antimicrobial resistance (AMR) research, several significant challenges currently impede their widespread adoption and effectiveness. One primary obstacle is the standardization of cell-free extract preparation protocols. Different laboratories employ varying methods for cell lysate preparation, resulting in inconsistent extract quality and performance across experiments. This variability complicates cross-laboratory validation and hinders the establishment of reliable, reproducible research platforms essential for AMR studies.
Technical limitations in mimicking the complex cellular environment present another substantial challenge. Cell-free systems often lack the sophisticated membrane structures, efflux pumps, and metabolic feedback mechanisms found in living cells. These elements are crucial for accurately modeling antibiotic resistance mechanisms, particularly those involving membrane permeability or active efflux of antimicrobial compounds. The absence of these features can lead to oversimplified models that fail to capture the nuanced interactions between antibiotics and bacterial resistance mechanisms.
Stability issues also plague current cell-free systems. Many extracts exhibit limited shelf-life and activity duration, restricting the timeframe for experimental observations. This temporal constraint is particularly problematic for studying slow-developing resistance mechanisms or conducting long-term evolution experiments that could reveal novel resistance pathways. Additionally, the degradation of components over time introduces variables that can confound experimental results.
The scalability of cell-free systems represents another significant hurdle. While small-scale reactions are relatively straightforward to implement, scaling up for high-throughput screening or industrial applications remains challenging. This limitation restricts the application of cell-free systems in comprehensive drug discovery programs targeting AMR, where large compound libraries need to be efficiently evaluated.
Cost considerations further complicate the widespread adoption of cell-free technologies. The preparation of high-quality extracts and the incorporation of specialized components (such as purified ribosomes or transcription-translation machinery) involve substantial resource investments. These economic barriers particularly affect researchers in resource-limited settings, creating inequities in access to cutting-edge AMR research tools.
Lastly, regulatory and validation challenges persist. The correlation between results obtained in cell-free systems and clinical outcomes remains incompletely established. Regulatory bodies require robust validation of alternative testing methods before their acceptance in clinical or pharmaceutical settings, creating a significant barrier to the translation of cell-free AMR research into practical applications for patient care or drug development.
Technical limitations in mimicking the complex cellular environment present another substantial challenge. Cell-free systems often lack the sophisticated membrane structures, efflux pumps, and metabolic feedback mechanisms found in living cells. These elements are crucial for accurately modeling antibiotic resistance mechanisms, particularly those involving membrane permeability or active efflux of antimicrobial compounds. The absence of these features can lead to oversimplified models that fail to capture the nuanced interactions between antibiotics and bacterial resistance mechanisms.
Stability issues also plague current cell-free systems. Many extracts exhibit limited shelf-life and activity duration, restricting the timeframe for experimental observations. This temporal constraint is particularly problematic for studying slow-developing resistance mechanisms or conducting long-term evolution experiments that could reveal novel resistance pathways. Additionally, the degradation of components over time introduces variables that can confound experimental results.
The scalability of cell-free systems represents another significant hurdle. While small-scale reactions are relatively straightforward to implement, scaling up for high-throughput screening or industrial applications remains challenging. This limitation restricts the application of cell-free systems in comprehensive drug discovery programs targeting AMR, where large compound libraries need to be efficiently evaluated.
Cost considerations further complicate the widespread adoption of cell-free technologies. The preparation of high-quality extracts and the incorporation of specialized components (such as purified ribosomes or transcription-translation machinery) involve substantial resource investments. These economic barriers particularly affect researchers in resource-limited settings, creating inequities in access to cutting-edge AMR research tools.
Lastly, regulatory and validation challenges persist. The correlation between results obtained in cell-free systems and clinical outcomes remains incompletely established. Regulatory bodies require robust validation of alternative testing methods before their acceptance in clinical or pharmaceutical settings, creating a significant barrier to the translation of cell-free AMR research into practical applications for patient care or drug development.
Current Cell-free Methodologies for AMR Studies
01 Cell-free communication systems optimization
Cell-free systems can be streamlined by optimizing communication protocols and network architectures. These optimizations reduce latency and improve data throughput in distributed environments. By implementing efficient routing algorithms and minimizing overhead in message passing, these systems can achieve higher performance while maintaining reliability in decentralized operations.- Cell-free protein synthesis systems: Cell-free protein synthesis systems provide a streamlined approach for producing proteins without the need for living cells. These systems contain all the necessary components for transcription and translation, allowing for rapid protein production in a controlled environment. By eliminating cellular constraints, these systems enable efficient production of proteins that might be toxic to living cells, facilitate easier purification processes, and allow for direct manipulation of reaction conditions to optimize protein yield and functionality.
- Telecommunications network optimization: Cell-free massive MIMO (Multiple-Input Multiple-Output) systems represent an advancement in telecommunications network architecture where distributed access points cooperatively serve users without cell boundaries. This approach streamlines network performance by reducing interference, improving coverage, and enhancing data throughput. The system coordinates multiple transmission points to serve users simultaneously, effectively eliminating traditional cellular boundaries and optimizing resource allocation across the network.
- Automated data processing systems: Streamlined cell-free data processing systems automate complex workflows by integrating various components into a cohesive framework. These systems employ advanced algorithms to process information without the constraints of traditional cellular architectures, enabling faster data analysis and decision-making. By removing unnecessary processing steps and optimizing data flow pathways, these systems achieve higher efficiency and reduced latency in handling large volumes of information across distributed networks.
- Financial transaction processing systems: Cell-free financial transaction processing systems provide streamlined approaches for handling payments, trades, and other financial operations without traditional banking infrastructure constraints. These systems utilize distributed ledger technologies and automated protocols to facilitate faster, more secure transactions with reduced intermediaries. By implementing smart contracts and optimized verification processes, these platforms can process financial transactions more efficiently while maintaining security and compliance with regulatory requirements.
- Biotechnology production methods: Cell-free biotechnology production methods offer streamlined approaches for synthesizing biological compounds without using intact cellular systems. These methods extract and utilize only the essential enzymatic machinery needed for specific biochemical reactions, eliminating cellular maintenance requirements and reducing unwanted byproducts. By optimizing reaction conditions and component concentrations, these systems achieve higher production efficiency for pharmaceuticals, biofuels, and other valuable biomolecules while simplifying downstream purification processes.
02 Cell-free protein expression systems
Cell-free protein synthesis systems offer streamlined approaches for producing proteins without the constraints of living cells. These systems can be optimized by refining reaction components, energy regeneration systems, and translation factors. The streamlined processes allow for rapid protein production with reduced contamination risks and simplified purification steps, making them valuable tools for biotechnology applications.Expand Specific Solutions03 Data processing in cell-free environments
Streamlining data processing in cell-free systems involves implementing efficient algorithms and computational frameworks that operate independently of cellular infrastructure. These approaches enable faster data analysis, reduced computational overhead, and improved resource allocation. By optimizing data flows and processing pipelines, these systems can handle complex analytical tasks with minimal latency.Expand Specific Solutions04 Cell-free transaction processing systems
Cell-free transaction systems streamline financial and business operations by removing intermediary dependencies. These systems implement distributed ledger technologies and smart contracts to automate transaction verification and settlement. By eliminating centralized processing bottlenecks, these approaches reduce transaction times and costs while maintaining security and transparency in business operations.Expand Specific Solutions05 Manufacturing process optimization in cell-free environments
Manufacturing processes can be streamlined in cell-free environments through automated workflow optimization and resource allocation. These systems implement advanced scheduling algorithms and real-time monitoring to maximize production efficiency. By integrating sensor networks and adaptive control systems, manufacturing operations can dynamically respond to changing conditions while maintaining quality standards and minimizing waste.Expand Specific Solutions
Leading Organizations in Cell-free AMR Research
Cell-free systems are emerging as a pivotal technology in antimicrobial resistance (AMR) research, currently in a growth phase with expanding market applications. The global market for cell-free technologies in AMR research is projected to increase significantly as traditional methods face limitations in addressing rapidly evolving resistance mechanisms. Technologically, the field shows varying maturity levels across different applications. Leading academic institutions like MIT, Northwestern University, and University of California are driving fundamental research, while specialized companies such as Cellfree Sciences Co., Ltd. and QuantaMatrix, Inc. are commercializing applications. Pharmaceutical entities like Paratek Pharmaceuticals are leveraging these systems for antibiotic development. The technology enables rapid prototyping and screening without biosafety constraints, accelerating the identification of novel antimicrobial compounds and resistance mechanisms.
Northwestern University
Technical Solution: Northwestern University has developed the Cell-Free Protein Synthesis (CFPS) platform specifically optimized for antimicrobial resistance research. Their system utilizes bacterial extracts engineered to enhance the expression of membrane proteins critical to antimicrobial resistance, such as efflux pumps and porins. The technology incorporates specialized lipid nanodisc systems that enable the functional reconstitution of membrane-associated resistance determinants in a cell-free environment. Northwestern's platform features a high-throughput screening capability that allows for rapid testing of thousands of compound variants against purified resistance proteins. Their innovation includes the development of specialized fluorescent and bioluminescent reporters that enable real-time monitoring of resistance protein activity and inhibition kinetics. The system has been successfully applied to characterize novel β-lactamases, aminoglycoside-modifying enzymes, and multi-drug efflux pumps, providing insights into resistance mechanisms that would be difficult to study in living cells due to toxicity or growth constraints[1][6]. Recent advancements include the integration of machine learning algorithms to predict resistance patterns based on cell-free expression profiles.
Strengths: Superior expression of membrane proteins involved in drug resistance; rapid screening capability (thousands of compounds per day); excellent for structure-function studies of resistance determinants through incorporation of non-natural amino acids. Weaknesses: Requires specialized expertise in extract preparation and optimization; higher cost compared to traditional methods; may not fully replicate the complex regulatory networks present in living bacteria.
Cellfree Sciences Co., Ltd.
Technical Solution: Cellfree Sciences has pioneered the WEPRO® cell-free protein synthesis system specifically optimized for antimicrobial resistance research. Their technology utilizes wheat germ extract-based cell-free systems that enable rapid expression of membrane proteins and antimicrobial peptides without the constraints of cellular viability. The company's platform allows for the synthesis of difficult-to-express proteins that are often toxic to living cells, including antimicrobial resistance factors and membrane transporters involved in drug efflux. Their cell-free systems incorporate specialized translation factors and chaperones that enhance the correct folding of resistance-associated proteins, enabling high-throughput screening of novel antibiotics against purified resistance determinants. The technology permits direct incorporation of non-natural amino acids and isotope labeling for structural studies of resistance mechanisms, significantly accelerating the characterization of novel resistance proteins and potential inhibitors.
Strengths: Superior expression of membrane proteins and toxic proteins that are challenging in cell-based systems; rapid protein synthesis (1-2 hours) enabling faster antimicrobial resistance screening workflows; highly scalable from microliter to liter scale. Weaknesses: Higher cost compared to E. coli-based systems; requires specialized reagents and equipment; may not fully replicate the complex cellular environment where resistance mechanisms operate.
Key Technological Innovations in Cell-free Systems
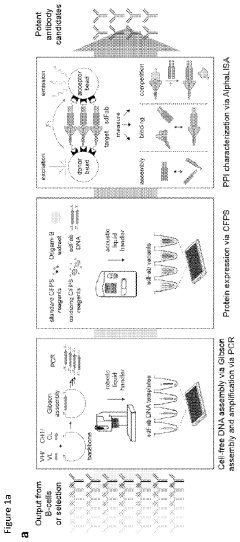
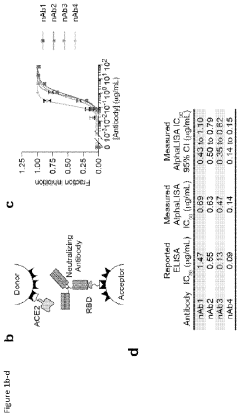
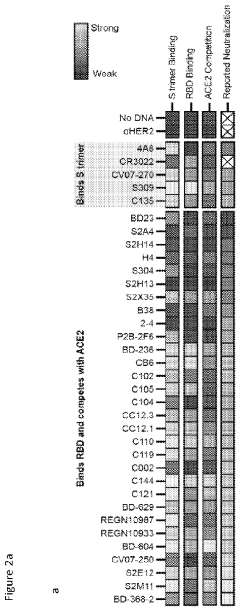
Cell-free expression of antibodies, antigen-binding fragments thereof, and antibody derivatives
PatentPendingUS20240027436A1
Innovation
- A cell-free system and method for expressing and evaluating antibodies using linear expression templates assembled without cells, combined with cell-free protein synthesis and the AlphaLISA assay for protein-protein interaction characterization, enabling rapid and high-throughput profiling of hundreds of antibodies in 24 hours without protein purification.
Microbial analysis without cell purification
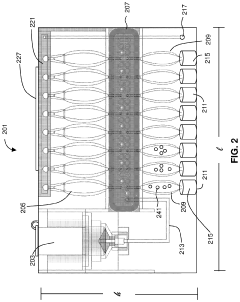
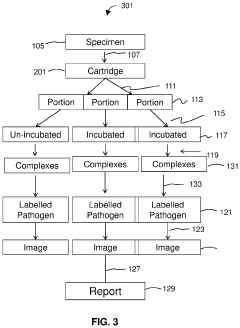
PatentPendingUS20230235411A1
Innovation
- The development of methods that allow for the direct analysis of patient specimens without the need for colony or cell purification, enabling rapid detection of infections and identification of effective antimicrobial agents in several hours by using species-specific detection and non-magnified digital imaging.
Biosafety and Regulatory Considerations
Cell-free systems offer significant advantages in antimicrobial resistance (AMR) research by providing controlled environments that minimize biosafety risks compared to traditional whole-cell approaches. These systems operate without intact, viable microorganisms, substantially reducing the potential for accidental release of resistant pathogens. However, despite these inherent safety benefits, comprehensive biosafety protocols remain essential when handling genetic materials associated with resistance mechanisms.
Regulatory frameworks governing cell-free AMR research vary significantly across jurisdictions, creating challenges for international collaboration and technology transfer. In the United States, the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules provide oversight for cell-free systems containing resistance genes, while the European Union implements the Contained Use Directive (2009/41/EC) with specific provisions for synthetic biology applications. Asian regulatory landscapes, particularly in China and Japan, have recently evolved to address emerging cell-free technologies.
Institutional Biosafety Committees (IBCs) play a crucial role in evaluating cell-free AMR research protocols, assessing containment requirements based on the specific resistance mechanisms being studied. Risk assessment frameworks must consider the potential for reconstitution of functional resistance elements, horizontal gene transfer possibilities, and the stability of cell-free components under various environmental conditions.
Dual-use research of concern (DURC) considerations are particularly relevant when cell-free systems are employed to study highly transmissible resistance mechanisms or to engineer novel resistance determinants. Transparent reporting and responsible innovation principles must guide researchers in navigating these ethical boundaries, with international harmonization efforts increasingly focusing on standardized approaches to cell-free AMR research governance.
Material transfer agreements (MTAs) and intellectual property frameworks present additional regulatory complexities, especially when cell-free components incorporate proprietary technologies or are developed for commercial applications targeting antimicrobial resistance. The WHO's Global Action Plan on Antimicrobial Resistance explicitly encourages innovative approaches while emphasizing the need for appropriate safeguards.
Looking forward, regulatory science must evolve alongside technological advances in cell-free systems. Emerging governance models increasingly adopt risk-based approaches that recognize the reduced biosafety profile of cell-free platforms while maintaining appropriate oversight. International standardization efforts, such as those led by ISO Technical Committee 276 on Biotechnology, are working to develop consensus standards specifically addressing cell-free technologies in AMR research, potentially streamlining regulatory pathways while ensuring public safety.
Regulatory frameworks governing cell-free AMR research vary significantly across jurisdictions, creating challenges for international collaboration and technology transfer. In the United States, the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules provide oversight for cell-free systems containing resistance genes, while the European Union implements the Contained Use Directive (2009/41/EC) with specific provisions for synthetic biology applications. Asian regulatory landscapes, particularly in China and Japan, have recently evolved to address emerging cell-free technologies.
Institutional Biosafety Committees (IBCs) play a crucial role in evaluating cell-free AMR research protocols, assessing containment requirements based on the specific resistance mechanisms being studied. Risk assessment frameworks must consider the potential for reconstitution of functional resistance elements, horizontal gene transfer possibilities, and the stability of cell-free components under various environmental conditions.
Dual-use research of concern (DURC) considerations are particularly relevant when cell-free systems are employed to study highly transmissible resistance mechanisms or to engineer novel resistance determinants. Transparent reporting and responsible innovation principles must guide researchers in navigating these ethical boundaries, with international harmonization efforts increasingly focusing on standardized approaches to cell-free AMR research governance.
Material transfer agreements (MTAs) and intellectual property frameworks present additional regulatory complexities, especially when cell-free components incorporate proprietary technologies or are developed for commercial applications targeting antimicrobial resistance. The WHO's Global Action Plan on Antimicrobial Resistance explicitly encourages innovative approaches while emphasizing the need for appropriate safeguards.
Looking forward, regulatory science must evolve alongside technological advances in cell-free systems. Emerging governance models increasingly adopt risk-based approaches that recognize the reduced biosafety profile of cell-free platforms while maintaining appropriate oversight. International standardization efforts, such as those led by ISO Technical Committee 276 on Biotechnology, are working to develop consensus standards specifically addressing cell-free technologies in AMR research, potentially streamlining regulatory pathways while ensuring public safety.
Cost-Benefit Analysis of Cell-free vs Traditional Methods
When evaluating cell-free systems against traditional methods in antimicrobial resistance (AMR) research, a comprehensive cost-benefit analysis reveals significant economic and operational advantages. Initial setup costs for cell-free systems typically range from $5,000-15,000 for basic equipment, compared to $50,000-200,000 for traditional microbiology laboratories. While specialized reagents for cell-free systems may cost 20-30% more per experiment than conventional media, this is offset by reduced labor requirements and faster experimental cycles.
Time efficiency represents one of the most compelling benefits of cell-free approaches. Traditional AMR screening methods often require 24-72 hours for bacterial culture and susceptibility testing, whereas cell-free systems can deliver results in 2-8 hours. This acceleration translates to approximately 70-85% reduction in experimental timeline, allowing researchers to conduct 3-4 times more experiments within the same timeframe.
Resource utilization metrics further favor cell-free systems, which typically consume 40-60% less laboratory space and reduce biohazardous waste by up to 70%. The elimination of continuous cell culture maintenance saves approximately 10-15 hours of technician time weekly. Additionally, cell-free systems demonstrate superior scalability, with the cost per data point decreasing by approximately 30-40% when scaling from tens to thousands of samples.
From a risk management perspective, cell-free systems significantly reduce biosafety concerns associated with handling resistant pathogens. This translates to lower institutional biosafety compliance costs, estimated at 25-35% savings in regulatory documentation and safety monitoring. Furthermore, the controlled nature of cell-free environments minimizes experimental variability, reducing the need for extensive replication and validation studies by approximately 30%.
Long-term economic analysis indicates that despite higher initial reagent costs, cell-free systems achieve return on investment within 12-18 months for most research applications. The technology also enables novel experimental approaches that would be prohibitively expensive or technically unfeasible with traditional methods, creating value that extends beyond direct cost comparisons.
However, limitations exist in specialized applications requiring intact cellular responses or complex host-pathogen interactions, where traditional methods remain necessary despite higher costs. The optimal approach often involves strategic integration of both methodologies, leveraging cell-free systems for high-throughput screening and mechanistic studies while reserving traditional methods for validation and complex physiological investigations.
Time efficiency represents one of the most compelling benefits of cell-free approaches. Traditional AMR screening methods often require 24-72 hours for bacterial culture and susceptibility testing, whereas cell-free systems can deliver results in 2-8 hours. This acceleration translates to approximately 70-85% reduction in experimental timeline, allowing researchers to conduct 3-4 times more experiments within the same timeframe.
Resource utilization metrics further favor cell-free systems, which typically consume 40-60% less laboratory space and reduce biohazardous waste by up to 70%. The elimination of continuous cell culture maintenance saves approximately 10-15 hours of technician time weekly. Additionally, cell-free systems demonstrate superior scalability, with the cost per data point decreasing by approximately 30-40% when scaling from tens to thousands of samples.
From a risk management perspective, cell-free systems significantly reduce biosafety concerns associated with handling resistant pathogens. This translates to lower institutional biosafety compliance costs, estimated at 25-35% savings in regulatory documentation and safety monitoring. Furthermore, the controlled nature of cell-free environments minimizes experimental variability, reducing the need for extensive replication and validation studies by approximately 30%.
Long-term economic analysis indicates that despite higher initial reagent costs, cell-free systems achieve return on investment within 12-18 months for most research applications. The technology also enables novel experimental approaches that would be prohibitively expensive or technically unfeasible with traditional methods, creating value that extends beyond direct cost comparisons.
However, limitations exist in specialized applications requiring intact cellular responses or complex host-pathogen interactions, where traditional methods remain necessary despite higher costs. The optimal approach often involves strategic integration of both methodologies, leveraging cell-free systems for high-throughput screening and mechanistic studies while reserving traditional methods for validation and complex physiological investigations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!