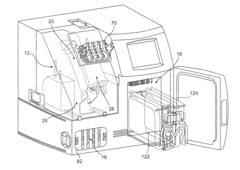
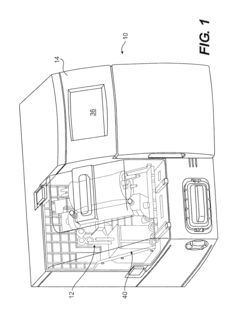
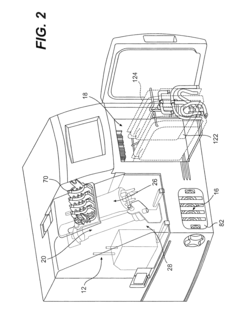
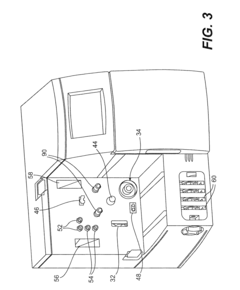
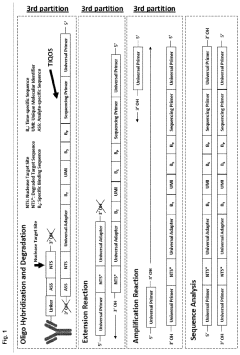
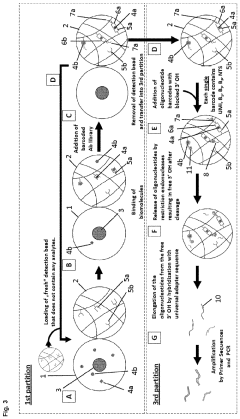
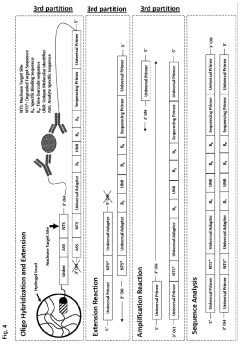
How do gate valves enhance cell-free biomanufacturing?
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-Free Biomanufacturing Background and Objectives
Cell-free biomanufacturing represents a paradigm shift in biotechnology, emerging from traditional cell-based production methods that have dominated the industry for decades. This approach harnesses the cellular machinery necessary for protein synthesis while eliminating the constraints associated with maintaining living cells. The evolution of this technology can be traced back to the 1960s with the development of cell-free protein synthesis (CFPS) systems, which have since undergone significant refinements in efficiency, scalability, and cost-effectiveness.
The trajectory of cell-free systems has accelerated dramatically in the past decade, driven by advances in synthetic biology, metabolic engineering, and process intensification. These developments have transformed cell-free biomanufacturing from a laboratory research tool into a viable industrial production platform for pharmaceuticals, enzymes, and biomaterials. The integration of gate valve technologies represents the latest advancement in this evolutionary process.
Gate valves—mechanical devices that regulate flow through precise opening and closing mechanisms—have emerged as critical components in enhancing the control, efficiency, and scalability of cell-free biomanufacturing processes. Their implementation addresses several fundamental challenges that have historically limited the industrial application of cell-free systems, including reaction longevity, substrate replenishment, and product inhibition.
The primary objective of incorporating gate valve technologies into cell-free biomanufacturing is to establish dynamic control over reaction conditions, enabling continuous or semi-continuous operation modes that significantly extend production capabilities. This approach aims to overcome the batch-mode limitations that have constrained productivity and economic viability of cell-free systems at industrial scales.
Additional technical goals include the development of automated, responsive systems that can maintain optimal reaction environments through real-time monitoring and adjustment of substrate concentrations, cofactor levels, and inhibitory byproduct removal. These capabilities are essential for achieving the consistency and reliability required for commercial biomanufacturing operations.
From a broader perspective, the integration of gate valve technologies with cell-free systems seeks to create a more sustainable and flexible biomanufacturing paradigm. This combination promises reduced resource consumption, minimized waste generation, and enhanced adaptability to changing production requirements—all critical factors for addressing global challenges in healthcare, materials science, and environmental sustainability.
The convergence of these technologies represents a strategic opportunity to revolutionize biomanufacturing by combining the precision and flexibility of cell-free systems with the control and scalability afforded by advanced valve technologies. Success in this domain could fundamentally alter production economics for critical biological products and enable new applications previously constrained by the limitations of traditional manufacturing approaches.
The trajectory of cell-free systems has accelerated dramatically in the past decade, driven by advances in synthetic biology, metabolic engineering, and process intensification. These developments have transformed cell-free biomanufacturing from a laboratory research tool into a viable industrial production platform for pharmaceuticals, enzymes, and biomaterials. The integration of gate valve technologies represents the latest advancement in this evolutionary process.
Gate valves—mechanical devices that regulate flow through precise opening and closing mechanisms—have emerged as critical components in enhancing the control, efficiency, and scalability of cell-free biomanufacturing processes. Their implementation addresses several fundamental challenges that have historically limited the industrial application of cell-free systems, including reaction longevity, substrate replenishment, and product inhibition.
The primary objective of incorporating gate valve technologies into cell-free biomanufacturing is to establish dynamic control over reaction conditions, enabling continuous or semi-continuous operation modes that significantly extend production capabilities. This approach aims to overcome the batch-mode limitations that have constrained productivity and economic viability of cell-free systems at industrial scales.
Additional technical goals include the development of automated, responsive systems that can maintain optimal reaction environments through real-time monitoring and adjustment of substrate concentrations, cofactor levels, and inhibitory byproduct removal. These capabilities are essential for achieving the consistency and reliability required for commercial biomanufacturing operations.
From a broader perspective, the integration of gate valve technologies with cell-free systems seeks to create a more sustainable and flexible biomanufacturing paradigm. This combination promises reduced resource consumption, minimized waste generation, and enhanced adaptability to changing production requirements—all critical factors for addressing global challenges in healthcare, materials science, and environmental sustainability.
The convergence of these technologies represents a strategic opportunity to revolutionize biomanufacturing by combining the precision and flexibility of cell-free systems with the control and scalability afforded by advanced valve technologies. Success in this domain could fundamentally alter production economics for critical biological products and enable new applications previously constrained by the limitations of traditional manufacturing approaches.
Market Analysis for Gate Valve Applications in Bioprocessing
The global market for gate valves in bioprocessing applications is experiencing significant growth, driven by the expanding adoption of cell-free biomanufacturing technologies. Current market valuations indicate that the specialized valve segment for bioprocessing reached approximately 1.2 billion USD in 2022, with gate valves representing a growing share of this market. Industry analysts project a compound annual growth rate of 9.7% for this segment through 2028, outpacing the broader industrial valve market which grows at 5-6% annually.
The demand for gate valves in cell-free biomanufacturing is particularly strong in North America and Europe, which together account for over 65% of the global market. Asia-Pacific, especially China and Singapore, represents the fastest-growing regional market with annual growth exceeding 12%, reflecting the rapid expansion of biomanufacturing capabilities in these regions.
Key market drivers include the increasing shift toward continuous bioprocessing methods, which require precise flow control mechanisms that gate valves effectively provide. The reduction in contamination risk offered by specialized gate valves has become a critical selling point, as contamination events in biomanufacturing can result in batch losses valued at hundreds of thousands to millions of dollars.
End-user segmentation reveals that pharmaceutical companies constitute the largest market share at 48%, followed by contract manufacturing organizations (CMOs) at 32%, and academic and research institutions at 15%. The remaining market share is distributed among various smaller end-users including food and beverage biotechnology applications.
Market penetration analysis indicates that while traditional stainless steel gate valves dominate with 72% market share, there is growing demand for advanced materials such as specialized polymers and single-use valve systems, which currently represent 18% and 10% of the market respectively. This trend reflects the industry's movement toward more flexible manufacturing systems.
Price sensitivity varies significantly by application, with high-containment processes willing to pay premium prices for valves with enhanced sterility assurance. The average selling price for specialized bioprocessing gate valves ranges from 2,000 to 15,000 USD depending on specifications, material construction, and certification requirements.
Customer feedback analysis reveals that reliability and ease of validation are the primary purchase drivers, followed by compatibility with existing systems and total cost of ownership. Interestingly, initial purchase price ranks fifth in importance among decision factors, highlighting the value-driven nature of this market segment.
The demand for gate valves in cell-free biomanufacturing is particularly strong in North America and Europe, which together account for over 65% of the global market. Asia-Pacific, especially China and Singapore, represents the fastest-growing regional market with annual growth exceeding 12%, reflecting the rapid expansion of biomanufacturing capabilities in these regions.
Key market drivers include the increasing shift toward continuous bioprocessing methods, which require precise flow control mechanisms that gate valves effectively provide. The reduction in contamination risk offered by specialized gate valves has become a critical selling point, as contamination events in biomanufacturing can result in batch losses valued at hundreds of thousands to millions of dollars.
End-user segmentation reveals that pharmaceutical companies constitute the largest market share at 48%, followed by contract manufacturing organizations (CMOs) at 32%, and academic and research institutions at 15%. The remaining market share is distributed among various smaller end-users including food and beverage biotechnology applications.
Market penetration analysis indicates that while traditional stainless steel gate valves dominate with 72% market share, there is growing demand for advanced materials such as specialized polymers and single-use valve systems, which currently represent 18% and 10% of the market respectively. This trend reflects the industry's movement toward more flexible manufacturing systems.
Price sensitivity varies significantly by application, with high-containment processes willing to pay premium prices for valves with enhanced sterility assurance. The average selling price for specialized bioprocessing gate valves ranges from 2,000 to 15,000 USD depending on specifications, material construction, and certification requirements.
Customer feedback analysis reveals that reliability and ease of validation are the primary purchase drivers, followed by compatibility with existing systems and total cost of ownership. Interestingly, initial purchase price ranks fifth in importance among decision factors, highlighting the value-driven nature of this market segment.
Current Gate Valve Technologies and Implementation Challenges
Gate valve technologies in cell-free biomanufacturing have evolved significantly in recent years, with several mainstream solutions now available in the market. Microfluidic gate valves represent the most widely adopted technology, utilizing elastomeric membranes that can be pneumatically or hydraulically actuated to control fluid flow with precision at microscale. These valves typically operate through the application of pressure to a control channel that deforms a membrane into the flow channel, effectively stopping flow when closed.
Electromagnetic gate valves offer another solution, particularly valuable in automated biomanufacturing systems. These valves utilize solenoids or other electromagnetic actuators to physically move blocking elements into and out of flow paths. Their advantage lies in rapid actuation speeds and compatibility with electronic control systems, though they may introduce concerns regarding heat generation in temperature-sensitive biological processes.
Piezoelectric gate valves have gained traction for applications requiring extremely precise flow control. By applying voltage to piezoelectric materials that change dimensions in response, these valves can achieve nanoliter-level flow control. This precision makes them ideal for cell-free protein synthesis systems where reagent quantities must be precisely metered.
Despite technological advances, significant implementation challenges persist. Biocompatibility remains a primary concern, as valve materials must not leach compounds that could interfere with sensitive biological reactions or introduce contamination. PDMS (polydimethylsiloxane), while popular for its optical clarity and fabrication ease, can absorb small hydrophobic molecules from reaction mixtures, potentially altering reaction kinetics.
Scalability presents another major challenge. While microfluidic gate valves perform excellently in laboratory settings, scaling to industrial production volumes introduces complications in maintaining uniform flow characteristics and valve performance across larger systems. The transition from micro to macro scales often requires fundamental redesigns rather than simple dimensional scaling.
System integration challenges also exist, particularly in creating interfaces between valve control systems and broader biomanufacturing process control architectures. Standardization remains limited, with many systems requiring custom integration solutions that increase implementation costs and complexity.
Reliability in continuous operation scenarios presents additional concerns. Valve failure in extended biomanufacturing runs can result in significant product loss. Current elastomeric valve technologies may experience mechanical fatigue after repeated actuation cycles, while electromagnetic valves face challenges with heat dissipation during prolonged operation periods.
Electromagnetic gate valves offer another solution, particularly valuable in automated biomanufacturing systems. These valves utilize solenoids or other electromagnetic actuators to physically move blocking elements into and out of flow paths. Their advantage lies in rapid actuation speeds and compatibility with electronic control systems, though they may introduce concerns regarding heat generation in temperature-sensitive biological processes.
Piezoelectric gate valves have gained traction for applications requiring extremely precise flow control. By applying voltage to piezoelectric materials that change dimensions in response, these valves can achieve nanoliter-level flow control. This precision makes them ideal for cell-free protein synthesis systems where reagent quantities must be precisely metered.
Despite technological advances, significant implementation challenges persist. Biocompatibility remains a primary concern, as valve materials must not leach compounds that could interfere with sensitive biological reactions or introduce contamination. PDMS (polydimethylsiloxane), while popular for its optical clarity and fabrication ease, can absorb small hydrophobic molecules from reaction mixtures, potentially altering reaction kinetics.
Scalability presents another major challenge. While microfluidic gate valves perform excellently in laboratory settings, scaling to industrial production volumes introduces complications in maintaining uniform flow characteristics and valve performance across larger systems. The transition from micro to macro scales often requires fundamental redesigns rather than simple dimensional scaling.
System integration challenges also exist, particularly in creating interfaces between valve control systems and broader biomanufacturing process control architectures. Standardization remains limited, with many systems requiring custom integration solutions that increase implementation costs and complexity.
Reliability in continuous operation scenarios presents additional concerns. Valve failure in extended biomanufacturing runs can result in significant product loss. Current elastomeric valve technologies may experience mechanical fatigue after repeated actuation cycles, while electromagnetic valves face challenges with heat dissipation during prolonged operation periods.
Gate Valve Integration Solutions for Cell-Free Systems
01 Improved gate valve sealing mechanisms
Enhanced sealing mechanisms in gate valves improve their performance and reliability. These innovations include specialized sealing surfaces, composite materials, and advanced sealing configurations that prevent leakage under high pressure conditions. The improved designs ensure better fluid control and extended operational life of the valves in various industrial applications.- Gate valve sealing mechanisms: Advanced sealing mechanisms in gate valves improve their performance and longevity. These innovations include specialized sealing materials, dual sealing systems, and pressure-responsive seals that adapt to varying operational conditions. Enhanced sealing technologies reduce leakage, increase operational safety, and extend maintenance intervals in high-pressure or corrosive environments.
- Electronic control systems for gate valves: Integration of electronic control systems enhances gate valve functionality through automated operation and monitoring capabilities. These systems incorporate sensors for real-time status monitoring, remote operation capabilities, and programmable control logic. Electronic enhancements improve precision in flow regulation, enable predictive maintenance, and allow integration with broader industrial control systems.
- Material innovations for valve components: Advanced materials improve gate valve durability and performance in challenging environments. These innovations include corrosion-resistant alloys, composite materials with enhanced wear resistance, and specialized coatings that reduce friction and prevent degradation. Material enhancements extend valve lifespan, improve operational reliability, and enable use in extreme temperature or highly corrosive conditions.
- Semiconductor gate structures: Semiconductor devices utilize specialized gate structures to enhance electronic performance. These innovations include multi-layer gate configurations, novel gate dielectric materials, and advanced doping techniques that improve electron mobility and reduce power consumption. Enhanced semiconductor gates enable faster switching speeds, lower leakage currents, and improved thermal stability in electronic components.
- Flow optimization in gate valve design: Redesigned gate valve geometries optimize fluid flow characteristics and operational efficiency. These innovations include streamlined flow paths, reduced turbulence designs, and variable aperture mechanisms that provide precise flow control. Flow optimization enhancements reduce pressure drops, minimize energy consumption, and improve overall system efficiency while extending valve service life.
02 Gate valve actuation systems
Advanced actuation systems for gate valves provide more precise control and operational efficiency. These systems incorporate innovative mechanisms for opening and closing the valve, including electromagnetic actuators, hydraulic systems, and motorized controls. The enhanced actuation technologies allow for remote operation, improved response times, and better integration with automated control systems.Expand Specific Solutions03 Semiconductor gate structure enhancements
Innovations in semiconductor gate structures improve the performance of electronic devices. These enhancements include modified gate materials, novel fabrication techniques, and optimized gate geometries that result in better electrical characteristics. The advanced gate designs enable higher switching speeds, reduced power consumption, and improved reliability in semiconductor devices.Expand Specific Solutions04 Gate valve material innovations
Novel materials used in gate valve construction enhance durability and performance under extreme conditions. These materials include corrosion-resistant alloys, composite structures, and specialized coatings that withstand high temperatures and pressures. The material innovations extend valve lifespan, reduce maintenance requirements, and enable operation in harsh chemical environments.Expand Specific Solutions05 Safety and monitoring systems for gate valves
Advanced safety and monitoring systems enhance the operational security of gate valves. These systems incorporate sensors, diagnostic tools, and fail-safe mechanisms that detect potential failures and prevent accidents. The monitoring technologies provide real-time data on valve performance, enabling predictive maintenance and reducing the risk of catastrophic failures in critical applications.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Bioprocess Control
Cell-free biomanufacturing is currently in an early growth phase, characterized by rapid technological advancement and expanding applications. The market is projected to reach significant scale as companies overcome production challenges. Gate valves play a crucial role in enhancing process control and contamination prevention. Companies like Presys Co., Ltd. and Eagle Industry Co. Ltd. are developing specialized valve technologies for bioprocessing applications, while Oribiotech Ltd. and GreenLight Biosciences are integrating these components into their cell-free manufacturing platforms. Research institutions including MIT and Tsinghua University are advancing fundamental technologies. The ecosystem shows increasing collaboration between valve manufacturers and biotech firms to optimize fluid handling systems for improved yield, consistency, and scalability in cell-free production processes.
Oribiotech Ltd.
Technical Solution: Oribiotech has developed a proprietary cell-free biomanufacturing platform featuring advanced gate valve technology that enables precise control over reaction conditions. Their system incorporates microfluidic valves fabricated from biocompatible materials that can withstand the chemical environments present in cell-free protein synthesis reactions. These valves enable compartmentalization of different reaction stages, allowing for sequential addition of components and removal of inhibitory byproducts. Oribiotech's gate valves are designed with minimal dead volume to reduce sample loss and feature rapid actuation times that enable dynamic control over reaction conditions. The company has integrated these valves into a fully automated platform that can continuously monitor and adjust reaction parameters in real-time, significantly improving yield and consistency in cell-free protein production. Their technology has been particularly successful in the production of difficult-to-express proteins and has applications in rapid vaccine development and personalized medicine.
Strengths: Highly responsive control system with minimal lag time; excellent compatibility with a wide range of biological reagents; low contamination risk. Weaknesses: Relatively new technology with limited long-term reliability data; higher initial cost compared to conventional systems; requires specialized training for operation and maintenance.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have developed advanced microfluidic gate valve technologies that significantly enhance cell-free biomanufacturing processes. Their innovations include elastomeric membrane valves that enable precise control over nanoliter-scale reaction volumes, allowing for compartmentalization and sequential processing of biological reactions. These valves operate through pneumatic or hydraulic actuation, creating reconfigurable reaction networks that mimic cellular compartmentalization but with greater experimental control. MIT's valve technology has been demonstrated to enable continuous-exchange cell-free (CECF) protein synthesis, where fresh nutrients are continuously supplied and inhibitory byproducts removed through controlled valve operations, extending reaction lifetimes from hours to days. Their multilayer soft lithography approach allows for the integration of thousands of valves on a single chip, enabling complex reaction sequences and high-throughput screening of reaction conditions. This technology has been crucial for developing on-demand biomanufacturing systems for remote or resource-limited settings.
Strengths: Exceptional precision at microscale; highly customizable for different reaction requirements; enables complex, multi-step reaction sequences. Weaknesses: Challenging to scale up to industrial production volumes; requires specialized fabrication facilities; higher complexity compared to traditional bioreactors.
Key Innovations in Valve Technology for Bioprocess Applications
Method and system for the production of cells and cell products and applications thereof
PatentActiveUS20160362650A1
Innovation
- A modular, automated cell culture system with a reusable control module and disposable cultureware that integrates perfusion technology, pH and lactate control, and barcode tracking to minimize operator intervention and prevent contamination, enabling simultaneous culture of multiple cell lines in a compact facility.
Method for analyzing cell released biomolecules
PatentPendingUS20230357822A1
Innovation
- A method involving a cell-laden matrix with capture molecules that mimics the native cell environment, allowing for the release and binding of biomolecules, followed by the use of detection molecules with barcode labels that undergo endonuclease cleavage and amplification for multiplexed analysis.
Scalability and Process Optimization Considerations
Scaling cell-free biomanufacturing systems from laboratory to industrial scale presents significant challenges that gate valve technology can help address. The implementation of gate valves in these systems enables precise control over fluid dynamics, which becomes increasingly critical as production volumes expand. When scaling up, maintaining consistent reaction conditions across larger volumes requires sophisticated flow control mechanisms that gate valves provide through their ability to completely seal pathways when closed and offer unobstructed flow when open.
Process optimization in cell-free biomanufacturing depends heavily on the ability to precisely regulate reaction parameters. Gate valves contribute to this optimization by allowing for rapid switching between different process streams, facilitating the sequential addition of reagents at precisely timed intervals. This capability is particularly valuable in continuous manufacturing setups where maintaining steady-state conditions is essential for consistent product quality.
The integration of automated gate valve systems further enhances scalability by reducing manual intervention requirements. These systems can be programmed to respond to real-time monitoring data, adjusting flow rates and pathway configurations to maintain optimal reaction conditions despite variations in input materials or environmental factors. This adaptability is crucial for robust large-scale operations where minor fluctuations can significantly impact overall yield and product quality.
Energy efficiency considerations also factor into scalability assessments. Gate valves typically require less energy to maintain their position compared to other valve types, resulting in lower operational costs at industrial scales. Additionally, their design minimizes pressure drops across the system, reducing the energy requirements for pumping and circulation—a significant advantage when scaling to commercial production volumes.
Maintenance requirements represent another critical aspect of scalability. Gate valves designed specifically for biomanufacturing applications feature materials and configurations that minimize biofilm formation and facilitate clean-in-place (CIP) procedures. These characteristics extend operational periods between maintenance shutdowns, improving overall process economics and throughput capacity.
The modular nature of gate valve installations supports incremental scaling approaches, allowing manufacturers to expand production capacity without complete system redesigns. This modularity enables a phased investment approach, reducing financial risks associated with scaling operations while providing flexibility to adapt to evolving market demands or technological improvements in cell-free biomanufacturing methodologies.
Process optimization in cell-free biomanufacturing depends heavily on the ability to precisely regulate reaction parameters. Gate valves contribute to this optimization by allowing for rapid switching between different process streams, facilitating the sequential addition of reagents at precisely timed intervals. This capability is particularly valuable in continuous manufacturing setups where maintaining steady-state conditions is essential for consistent product quality.
The integration of automated gate valve systems further enhances scalability by reducing manual intervention requirements. These systems can be programmed to respond to real-time monitoring data, adjusting flow rates and pathway configurations to maintain optimal reaction conditions despite variations in input materials or environmental factors. This adaptability is crucial for robust large-scale operations where minor fluctuations can significantly impact overall yield and product quality.
Energy efficiency considerations also factor into scalability assessments. Gate valves typically require less energy to maintain their position compared to other valve types, resulting in lower operational costs at industrial scales. Additionally, their design minimizes pressure drops across the system, reducing the energy requirements for pumping and circulation—a significant advantage when scaling to commercial production volumes.
Maintenance requirements represent another critical aspect of scalability. Gate valves designed specifically for biomanufacturing applications feature materials and configurations that minimize biofilm formation and facilitate clean-in-place (CIP) procedures. These characteristics extend operational periods between maintenance shutdowns, improving overall process economics and throughput capacity.
The modular nature of gate valve installations supports incremental scaling approaches, allowing manufacturers to expand production capacity without complete system redesigns. This modularity enables a phased investment approach, reducing financial risks associated with scaling operations while providing flexibility to adapt to evolving market demands or technological improvements in cell-free biomanufacturing methodologies.
Regulatory Compliance and Quality Assurance Standards
Cell-free biomanufacturing systems operate under stringent regulatory frameworks that ensure product safety, efficacy, and quality. Gate valve implementation in these systems must adhere to comprehensive regulatory standards established by agencies such as the FDA, EMA, and ICH. These valves, as critical components in bioprocessing equipment, fall under Good Manufacturing Practice (GMP) regulations that govern production facilities, equipment design, and operational protocols.
The FDA's guidance on Process Analytical Technology (PAT) specifically addresses control systems like gate valves, requiring documented validation of their performance characteristics, including precision, accuracy, and reliability. Gate valves used in cell-free biomanufacturing must meet USP Class VI or ISO 10993 biocompatibility standards, ensuring materials do not leach harmful substances into biological products.
Quality assurance for gate valves encompasses multiple dimensions, including material certification, design qualification (DQ), installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). Manufacturers must maintain detailed documentation of these validation processes to demonstrate regulatory compliance during inspections.
ASME-BPE (American Society of Mechanical Engineers - Bioprocessing Equipment) standards provide specific guidelines for valve design in bioprocessing applications, including surface finish requirements, material traceability, and documentation protocols. These standards ensure gate valves maintain sterility and prevent contamination during operation.
Risk management frameworks such as HACCP (Hazard Analysis Critical Control Points) and FMEA (Failure Mode and Effects Analysis) must be applied to gate valve implementation, identifying potential failure points and establishing appropriate control measures. This systematic approach helps manufacturers demonstrate due diligence in risk mitigation.
Cleaning validation presents another critical regulatory consideration for gate valves in cell-free systems. Protocols must verify that cleaning procedures effectively remove product residues, cleaning agents, and potential microbial contaminants from valve surfaces. The design of gate valves specifically enhances cleanability through features like crevice-free construction and smooth surface finishes.
Automated gate valve systems require additional validation of control software under 21 CFR Part 11 compliance for electronic records and signatures. This includes system security, audit trails, and change management protocols that ensure data integrity throughout the manufacturing process.
As regulatory frameworks evolve to address emerging technologies in biomanufacturing, gate valve manufacturers must maintain vigilance regarding changing compliance requirements and implement continuous improvement processes to ensure ongoing adherence to quality standards.
The FDA's guidance on Process Analytical Technology (PAT) specifically addresses control systems like gate valves, requiring documented validation of their performance characteristics, including precision, accuracy, and reliability. Gate valves used in cell-free biomanufacturing must meet USP Class VI or ISO 10993 biocompatibility standards, ensuring materials do not leach harmful substances into biological products.
Quality assurance for gate valves encompasses multiple dimensions, including material certification, design qualification (DQ), installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). Manufacturers must maintain detailed documentation of these validation processes to demonstrate regulatory compliance during inspections.
ASME-BPE (American Society of Mechanical Engineers - Bioprocessing Equipment) standards provide specific guidelines for valve design in bioprocessing applications, including surface finish requirements, material traceability, and documentation protocols. These standards ensure gate valves maintain sterility and prevent contamination during operation.
Risk management frameworks such as HACCP (Hazard Analysis Critical Control Points) and FMEA (Failure Mode and Effects Analysis) must be applied to gate valve implementation, identifying potential failure points and establishing appropriate control measures. This systematic approach helps manufacturers demonstrate due diligence in risk mitigation.
Cleaning validation presents another critical regulatory consideration for gate valves in cell-free systems. Protocols must verify that cleaning procedures effectively remove product residues, cleaning agents, and potential microbial contaminants from valve surfaces. The design of gate valves specifically enhances cleanability through features like crevice-free construction and smooth surface finishes.
Automated gate valve systems require additional validation of control software under 21 CFR Part 11 compliance for electronic records and signatures. This includes system security, audit trails, and change management protocols that ensure data integrity throughout the manufacturing process.
As regulatory frameworks evolve to address emerging technologies in biomanufacturing, gate valve manufacturers must maintain vigilance regarding changing compliance requirements and implement continuous improvement processes to ensure ongoing adherence to quality standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!