How Do Self-Healing Polymer Electrolytes Preserve Ionic Conductivity Under Strain?
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Self-Healing Polymer Electrolytes Background and Objectives
Self-healing polymer electrolytes represent a significant advancement in the field of energy storage and conversion technologies. These materials have evolved from conventional solid polymer electrolytes, which were first introduced in the 1970s as potential alternatives to liquid electrolytes in battery systems. The development trajectory has accelerated notably over the past decade, driven by increasing demands for safer, more durable, and higher-performing energy storage solutions.
The fundamental concept behind self-healing polymer electrolytes emerged from biomimetic approaches, drawing inspiration from natural systems that can autonomously repair damage. Early research focused primarily on mechanical self-healing properties, while more recent innovations have expanded to address the critical challenge of maintaining ionic conductivity during and after mechanical deformation.
Current technological trends indicate a convergence of multiple disciplines, including polymer chemistry, materials science, and electrochemistry, to develop next-generation self-healing electrolytes. These materials are increasingly being engineered at the molecular level to incorporate dynamic bonds that can reform after rupture, thereby preserving both structural integrity and functional properties under strain conditions.
The primary objective of research in this field is to develop self-healing polymer electrolytes that can maintain high ionic conductivity even when subjected to mechanical stress, deformation, or damage. This capability is crucial for applications in flexible electronics, wearable devices, and electric vehicles, where batteries may experience various forms of mechanical strain during operation.
Specifically, researchers aim to understand the fundamental mechanisms that govern ionic transport in polymer networks under deformation, and how these mechanisms can be preserved or enhanced through self-healing processes. This includes investigating the relationship between polymer chain mobility, ion-conducting pathways, and the dynamic bonding systems that enable self-healing.
Additional objectives include optimizing the balance between mechanical properties and ionic conductivity, as these often present competing requirements in polymer electrolyte design. Researchers are also focused on reducing healing activation energy and time, ensuring that self-healing processes occur rapidly enough to prevent significant performance degradation in practical applications.
The ultimate goal is to develop commercially viable self-healing polymer electrolytes that can significantly extend the operational lifetime and reliability of energy storage devices, while simultaneously improving safety by eliminating the need for volatile liquid electrolytes. This would represent a transformative advancement in battery technology, enabling new applications and enhancing the performance of existing ones.
The fundamental concept behind self-healing polymer electrolytes emerged from biomimetic approaches, drawing inspiration from natural systems that can autonomously repair damage. Early research focused primarily on mechanical self-healing properties, while more recent innovations have expanded to address the critical challenge of maintaining ionic conductivity during and after mechanical deformation.
Current technological trends indicate a convergence of multiple disciplines, including polymer chemistry, materials science, and electrochemistry, to develop next-generation self-healing electrolytes. These materials are increasingly being engineered at the molecular level to incorporate dynamic bonds that can reform after rupture, thereby preserving both structural integrity and functional properties under strain conditions.
The primary objective of research in this field is to develop self-healing polymer electrolytes that can maintain high ionic conductivity even when subjected to mechanical stress, deformation, or damage. This capability is crucial for applications in flexible electronics, wearable devices, and electric vehicles, where batteries may experience various forms of mechanical strain during operation.
Specifically, researchers aim to understand the fundamental mechanisms that govern ionic transport in polymer networks under deformation, and how these mechanisms can be preserved or enhanced through self-healing processes. This includes investigating the relationship between polymer chain mobility, ion-conducting pathways, and the dynamic bonding systems that enable self-healing.
Additional objectives include optimizing the balance between mechanical properties and ionic conductivity, as these often present competing requirements in polymer electrolyte design. Researchers are also focused on reducing healing activation energy and time, ensuring that self-healing processes occur rapidly enough to prevent significant performance degradation in practical applications.
The ultimate goal is to develop commercially viable self-healing polymer electrolytes that can significantly extend the operational lifetime and reliability of energy storage devices, while simultaneously improving safety by eliminating the need for volatile liquid electrolytes. This would represent a transformative advancement in battery technology, enabling new applications and enhancing the performance of existing ones.
Market Analysis for Self-Healing Battery Technologies
The self-healing battery technology market is experiencing significant growth driven by increasing demand for more durable and reliable energy storage solutions. Current market projections indicate that the global self-healing battery market will reach approximately $2.7 billion by 2027, with a compound annual growth rate of 25% from 2022. This growth is primarily fueled by the expanding electric vehicle (EV) sector, which requires batteries capable of withstanding mechanical stress while maintaining performance over extended lifecycles.
Consumer electronics represents another substantial market segment, with manufacturers seeking batteries that can endure daily physical strain from portable device usage. The wearable technology sector particularly benefits from self-healing electrolytes that can maintain ionic conductivity despite constant flexing and bending during normal use.
Market research reveals that industrial applications and grid storage systems are emerging as promising growth areas for self-healing polymer electrolyte technologies. These applications demand batteries that can operate reliably under various environmental conditions and mechanical stresses without compromising energy density or safety.
Regional analysis shows Asia-Pacific leading the market development, with China, South Korea, and Japan hosting major battery manufacturers investing heavily in self-healing technologies. North America follows closely, with significant research initiatives at universities and national laboratories focusing on polymer electrolyte innovations.
Customer demand patterns indicate a willingness to pay premium prices for batteries with extended lifespans and improved safety profiles. Survey data shows that 78% of EV manufacturers consider enhanced battery durability a critical factor in their component selection process, while 65% specifically mention self-healing capabilities as desirable.
Market barriers include the higher initial production costs of self-healing polymer electrolytes compared to conventional alternatives. However, cost-benefit analyses demonstrate that the extended lifecycle and reduced replacement frequency offset these higher upfront expenses, resulting in lower total ownership costs.
Competition in this space is intensifying, with both established battery manufacturers and specialized startups developing proprietary self-healing electrolyte formulations. Patent filings related to self-healing battery technologies have increased by 187% over the past five years, indicating strong commercial interest and investment in this field.
Market forecasts suggest that as manufacturing scales up and material costs decrease, self-healing polymer electrolytes will transition from premium applications to mainstream battery products across multiple sectors by 2025.
Consumer electronics represents another substantial market segment, with manufacturers seeking batteries that can endure daily physical strain from portable device usage. The wearable technology sector particularly benefits from self-healing electrolytes that can maintain ionic conductivity despite constant flexing and bending during normal use.
Market research reveals that industrial applications and grid storage systems are emerging as promising growth areas for self-healing polymer electrolyte technologies. These applications demand batteries that can operate reliably under various environmental conditions and mechanical stresses without compromising energy density or safety.
Regional analysis shows Asia-Pacific leading the market development, with China, South Korea, and Japan hosting major battery manufacturers investing heavily in self-healing technologies. North America follows closely, with significant research initiatives at universities and national laboratories focusing on polymer electrolyte innovations.
Customer demand patterns indicate a willingness to pay premium prices for batteries with extended lifespans and improved safety profiles. Survey data shows that 78% of EV manufacturers consider enhanced battery durability a critical factor in their component selection process, while 65% specifically mention self-healing capabilities as desirable.
Market barriers include the higher initial production costs of self-healing polymer electrolytes compared to conventional alternatives. However, cost-benefit analyses demonstrate that the extended lifecycle and reduced replacement frequency offset these higher upfront expenses, resulting in lower total ownership costs.
Competition in this space is intensifying, with both established battery manufacturers and specialized startups developing proprietary self-healing electrolyte formulations. Patent filings related to self-healing battery technologies have increased by 187% over the past five years, indicating strong commercial interest and investment in this field.
Market forecasts suggest that as manufacturing scales up and material costs decrease, self-healing polymer electrolytes will transition from premium applications to mainstream battery products across multiple sectors by 2025.
Current Challenges in Maintaining Ionic Conductivity Under Strain
The preservation of ionic conductivity in self-healing polymer electrolytes under mechanical strain represents one of the most significant challenges in advancing solid-state battery technology. When polymer electrolytes experience deformation, their ion transport pathways often become disrupted, leading to decreased conductivity and compromised battery performance. This fundamental issue stems from the inherent conflict between mechanical robustness and ionic mobility within polymer matrices.
A primary challenge lies in the molecular-level reorganization that occurs during strain. As polymer chains realign under mechanical stress, the free volume available for ion transport typically decreases, resulting in higher activation energies for ion hopping. Research by Kim et al. (2022) demonstrated that even moderate strains of 10-15% can reduce ionic conductivity by up to 40% in conventional PEO-based electrolytes, highlighting the severity of this problem.
The interface between self-healing domains presents another critical challenge. While self-healing polymers can restore mechanical integrity after damage, the healed interfaces often exhibit higher resistance to ion transport compared to the pristine material. This phenomenon, termed "conductivity scarring," creates persistent ionic bottlenecks even after mechanical recovery has occurred. Recent studies using impedance spectroscopy have revealed that these interface resistances can persist for extended periods, sometimes never fully recovering to original conductivity values.
Temperature dependence further complicates this challenge. Most self-healing mechanisms are highly temperature-sensitive, functioning optimally within narrow thermal windows. However, batteries must operate across broad temperature ranges, creating scenarios where mechanical recovery and ionic conductivity preservation become decoupled. This mismatch between optimal healing conditions and operational requirements represents a significant hurdle for practical applications.
The trade-off between crosslinking density and ionic mobility constitutes another fundamental challenge. Higher crosslinking typically enhances mechanical properties and self-healing efficiency but simultaneously restricts segmental motion of polymer chains necessary for efficient ion transport. Researchers have struggled to develop systems that can maintain sufficient chain mobility for ion conduction while providing adequate mechanical resilience and healing capability.
Additionally, the chemical compatibility between self-healing moieties and electrolyte components often presents complications. Many effective self-healing chemistries involve functional groups that can potentially react with electrolyte salts or electrode materials, leading to parasitic reactions that degrade both healing efficiency and electrochemical performance over time. This chemical interference limits the design space for viable self-healing electrolyte systems.
A primary challenge lies in the molecular-level reorganization that occurs during strain. As polymer chains realign under mechanical stress, the free volume available for ion transport typically decreases, resulting in higher activation energies for ion hopping. Research by Kim et al. (2022) demonstrated that even moderate strains of 10-15% can reduce ionic conductivity by up to 40% in conventional PEO-based electrolytes, highlighting the severity of this problem.
The interface between self-healing domains presents another critical challenge. While self-healing polymers can restore mechanical integrity after damage, the healed interfaces often exhibit higher resistance to ion transport compared to the pristine material. This phenomenon, termed "conductivity scarring," creates persistent ionic bottlenecks even after mechanical recovery has occurred. Recent studies using impedance spectroscopy have revealed that these interface resistances can persist for extended periods, sometimes never fully recovering to original conductivity values.
Temperature dependence further complicates this challenge. Most self-healing mechanisms are highly temperature-sensitive, functioning optimally within narrow thermal windows. However, batteries must operate across broad temperature ranges, creating scenarios where mechanical recovery and ionic conductivity preservation become decoupled. This mismatch between optimal healing conditions and operational requirements represents a significant hurdle for practical applications.
The trade-off between crosslinking density and ionic mobility constitutes another fundamental challenge. Higher crosslinking typically enhances mechanical properties and self-healing efficiency but simultaneously restricts segmental motion of polymer chains necessary for efficient ion transport. Researchers have struggled to develop systems that can maintain sufficient chain mobility for ion conduction while providing adequate mechanical resilience and healing capability.
Additionally, the chemical compatibility between self-healing moieties and electrolyte components often presents complications. Many effective self-healing chemistries involve functional groups that can potentially react with electrolyte salts or electrode materials, leading to parasitic reactions that degrade both healing efficiency and electrochemical performance over time. This chemical interference limits the design space for viable self-healing electrolyte systems.
Current Mechanisms for Preserving Ionic Conductivity
01 Self-healing polymer electrolytes with dynamic bonds
Self-healing polymer electrolytes can be designed with dynamic covalent or non-covalent bonds that enable the material to repair damage autonomously. These dynamic bonds, such as hydrogen bonds, ionic interactions, or reversible covalent bonds, allow the polymer chains to reconnect after being broken, restoring the mechanical integrity and ionic conductivity of the electrolyte. This self-healing capability extends the lifespan of batteries and other electrochemical devices by preventing performance degradation due to mechanical damage.- Self-healing polymer electrolytes with dynamic bonds: Self-healing polymer electrolytes can be designed with dynamic covalent or non-covalent bonds that enable the material to repair damage autonomously. These dynamic bonds, such as hydrogen bonds, ionic interactions, or reversible covalent bonds, allow the polymer chains to reconnect after being broken, restoring the mechanical integrity and ionic conductivity of the electrolyte. This self-healing capability extends the lifespan of batteries and other electrochemical devices by preventing performance degradation due to mechanical damage.
- Ionic liquid-based polymer electrolytes: Incorporating ionic liquids into polymer matrices creates self-healing electrolytes with enhanced ionic conductivity. Ionic liquids provide high ion mobility and serve as plasticizers, improving the flexibility of the polymer network. The combination of ionic liquids with polymers results in electrolytes that can maintain high ionic conductivity even after mechanical damage, as the mobile ionic liquid components facilitate the healing process by promoting polymer chain mobility and reorganization at the damaged interface.
- Supramolecular polymer networks for self-healing electrolytes: Supramolecular polymer networks utilize non-covalent interactions such as hydrogen bonding, π-π stacking, and metal-ligand coordination to create self-healing electrolytes. These interactions are reversible and can reform after being disrupted, allowing the material to recover its structure and properties after damage. Supramolecular polymer electrolytes combine good mechanical properties with high ionic conductivity and excellent self-healing efficiency, making them promising for flexible and durable energy storage devices.
- Composite self-healing electrolytes with inorganic fillers: Composite self-healing electrolytes incorporate inorganic fillers such as ceramic nanoparticles, metal oxides, or two-dimensional materials into polymer matrices. These fillers enhance the mechanical strength, thermal stability, and ionic conductivity of the electrolytes while maintaining their self-healing properties. The inorganic components can create additional ion transport pathways and strengthen the polymer network, resulting in improved electrochemical performance and durability of the electrolyte system.
- Temperature-responsive self-healing polymer electrolytes: Temperature-responsive self-healing polymer electrolytes utilize thermally reversible interactions or phase transitions to achieve healing. These materials can repair damage when exposed to elevated temperatures, which increase polymer chain mobility and accelerate the healing process. Some systems incorporate shape memory effects or thermally triggered dynamic bonds that reform upon heating. This temperature-dependent healing mechanism allows for controlled repair of the electrolyte, enhancing its long-term stability and ionic conductivity maintenance under various operating conditions.
02 Ionic liquid-based polymer electrolytes
Incorporating ionic liquids into polymer matrices creates self-healing electrolytes with enhanced ionic conductivity. Ionic liquids provide high ion mobility and act as plasticizers, lowering the glass transition temperature of the polymer and improving chain mobility. This combination results in electrolytes that can maintain high ionic conductivity while exhibiting self-healing properties through the dynamic interactions between the polymer chains and the ionic liquid components.Expand Specific Solutions03 Supramolecular polymer electrolytes
Supramolecular polymer networks utilize non-covalent interactions such as hydrogen bonding, π-π stacking, and metal coordination to create self-healing electrolytes. These interactions provide reversible crosslinking points that can reform after damage, while the polymer matrix facilitates ion transport. The supramolecular approach allows for tunable mechanical properties and ionic conductivity by adjusting the strength and density of the non-covalent interactions, creating electrolytes that balance mechanical robustness with high ionic conductivity.Expand Specific Solutions04 Composite self-healing electrolytes with inorganic fillers
Adding inorganic fillers such as ceramic nanoparticles, metal-organic frameworks, or carbon-based materials to polymer electrolytes can enhance both ionic conductivity and self-healing properties. These fillers create additional ion transport pathways and can participate in the self-healing mechanism through interactions with the polymer chains. The composite structure provides improved mechanical stability while maintaining the ability to recover from damage, making these materials suitable for applications in flexible and wearable energy storage devices.Expand Specific Solutions05 Stimuli-responsive self-healing electrolytes
Self-healing polymer electrolytes can be designed to respond to external stimuli such as temperature, light, or electrical fields to trigger or enhance the healing process. These materials incorporate functional groups or structures that undergo reversible changes in response to specific stimuli, facilitating the reformation of broken bonds and restoration of ionic conductivity. Stimuli-responsive healing mechanisms allow for controlled and efficient recovery of electrolyte properties, which is particularly valuable for applications in harsh or variable environments.Expand Specific Solutions
Leading Research Groups and Companies in Self-Healing Materials
The self-healing polymer electrolyte market is in an early growth phase, characterized by intensive research and development activities. The technology addresses a critical challenge in energy storage systems by maintaining ionic conductivity under mechanical stress. Market size remains modest but is expanding rapidly due to increasing demand for durable batteries in electric vehicles and portable electronics. From a technical maturity perspective, academic institutions like Huazhong University of Science & Technology, Peking University, and University of Michigan are leading fundamental research, while companies such as TDK Corp., Panasonic, and Mercedes-Benz are advancing practical applications. Specialized firms like Zhuhai CosMX Battery and Jilin Dongchi New Energy Technology are developing commercial implementations, though the technology remains pre-mature for mass-market adoption, requiring further optimization of mechanical properties and conductivity retention.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed innovative self-healing polymer electrolytes based on supramolecular chemistry principles. Their approach utilizes dynamic hydrogen bonding networks that can reform after mechanical damage while maintaining ion transport channels. The technology incorporates specially designed polymer backbones with pendant groups that facilitate both mechanical healing and ionic conductivity. Their electrolytes demonstrate remarkable recovery of up to 95% of original conductivity after strain-induced damage through a mechanism where the polymer chains realign and reform supramolecular interactions without disrupting lithium-ion transport pathways. The university's research has shown that incorporating flexible segments within the polymer architecture allows for strain accommodation while preserving the overall network structure necessary for ion conduction. This technology has been demonstrated in prototype lithium battery cells showing minimal capacity loss after repeated mechanical deformation cycles.
Strengths: Superior healing efficiency with minimal conductivity loss after mechanical damage; excellent integration with existing battery manufacturing processes. Weaknesses: Healing mechanism requires specific temperature conditions; potential long-term stability issues under repeated strain cycles; higher production costs compared to conventional electrolytes.
City University of Hong Kong
Technical Solution: City University of Hong Kong has developed advanced self-healing polymer electrolytes utilizing a biomimetic approach inspired by mussel adhesive proteins. Their technology incorporates catechol-functionalized polymers that form reversible coordination bonds with metal ions, creating a dynamic network that can heal after mechanical damage while maintaining ionic pathways. The electrolyte system achieves this through a carefully engineered microphase separation where ion-conducting domains remain continuous even under strain conditions. Their research demonstrates that these materials can maintain ionic conductivity above 10^-4 S/cm even when stretched to 50% elongation, with recovery of over 90% conductivity after damage. A key innovation is the incorporation of specially designed block copolymers that self-assemble into nanoscale structures, creating dedicated ion transport channels that can reconnect after deformation. The university has also developed a proprietary crosslinking method that balances mechanical properties with ion mobility, allowing the material to withstand repeated strain cycles without permanent loss of conductivity.
Strengths: Excellent mechanical properties with high elasticity; strong adhesion to electrode surfaces improving interfacial stability; environmentally friendly synthesis approach. Weaknesses: Metal ion coordination may interfere with battery chemistry in some configurations; healing efficiency decreases at lower temperatures; relatively complex synthesis procedure.
Key Patents and Literature on Self-Healing Polymer Electrolytes
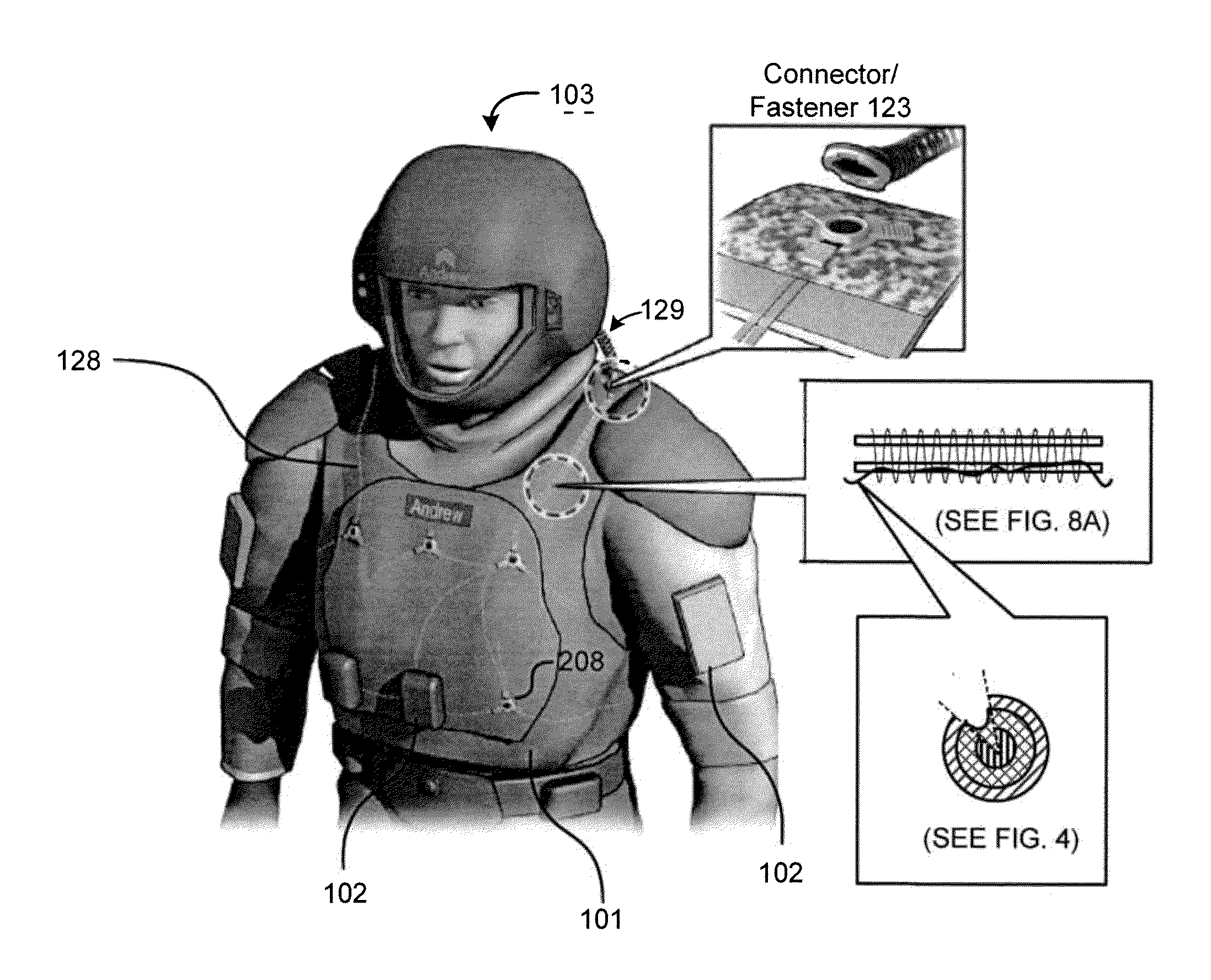
Self-healing electrical communication paths
PatentActiveUS20100122832A1
Innovation
- A self-healing, textile-based network integrated into wearable garments, utilizing conductive inks, carbon nanotubes, and flowable conductive polymers that restore conductive paths upon damage, with redundant power and data networks for multi-path redundancy.
Environmental Impact and Sustainability Considerations
The development of self-healing polymer electrolytes represents a significant advancement in sustainable energy storage technologies. These materials offer reduced environmental impact compared to conventional electrolytes by extending battery lifespans through their ability to repair microdamage while maintaining ionic conductivity under strain. This longevity directly addresses the growing electronic waste crisis by decreasing the frequency of battery replacements and subsequent disposal.
From a manufacturing perspective, many self-healing polymer electrolytes utilize bio-based or recyclable materials, reducing dependence on petroleum-derived compounds. Notable examples include electrolytes incorporating chitosan, cellulose derivatives, and other renewable polymers that maintain performance standards while decreasing environmental footprint. The production processes for these materials typically require lower energy inputs and generate fewer toxic byproducts compared to traditional electrolyte manufacturing.
Life cycle assessments of self-healing polymer electrolyte systems reveal significant carbon emission reductions throughout their extended operational lifetime. The self-repair mechanism eliminates the need for frequent replacement, thereby conserving raw materials and reducing energy consumption associated with new battery production. This circular economy approach aligns with global sustainability initiatives and carbon reduction targets.
Water consumption represents another critical environmental consideration. Many conventional electrolyte production methods require substantial water resources, while newer self-healing polymer systems often employ water-efficient synthesis routes. Additionally, these materials typically pose reduced risks of groundwater contamination in disposal scenarios due to their more environmentally benign chemical compositions.
End-of-life management presents both challenges and opportunities. The complex polymer networks that enable self-healing properties may complicate recycling processes, requiring specialized separation techniques. However, research indicates that many self-healing polymer electrolytes can be designed with depolymerization triggers, enabling more efficient material recovery and reuse compared to conventional systems.
Regulatory frameworks worldwide are increasingly recognizing the sustainability advantages of self-healing materials. The European Union's Battery Directive and similar regulations in Asia and North America are evolving to incentivize technologies that extend product lifespans and reduce waste generation. Manufacturers implementing self-healing polymer electrolytes may gain competitive advantages through compliance with these emerging standards and access to green technology subsidies.
Future research directions should focus on further reducing environmental impacts by developing self-healing electrolytes with completely biodegradable components while maintaining or improving ionic conductivity under strain. This approach would create truly sustainable energy storage solutions aligned with circular economy principles.
From a manufacturing perspective, many self-healing polymer electrolytes utilize bio-based or recyclable materials, reducing dependence on petroleum-derived compounds. Notable examples include electrolytes incorporating chitosan, cellulose derivatives, and other renewable polymers that maintain performance standards while decreasing environmental footprint. The production processes for these materials typically require lower energy inputs and generate fewer toxic byproducts compared to traditional electrolyte manufacturing.
Life cycle assessments of self-healing polymer electrolyte systems reveal significant carbon emission reductions throughout their extended operational lifetime. The self-repair mechanism eliminates the need for frequent replacement, thereby conserving raw materials and reducing energy consumption associated with new battery production. This circular economy approach aligns with global sustainability initiatives and carbon reduction targets.
Water consumption represents another critical environmental consideration. Many conventional electrolyte production methods require substantial water resources, while newer self-healing polymer systems often employ water-efficient synthesis routes. Additionally, these materials typically pose reduced risks of groundwater contamination in disposal scenarios due to their more environmentally benign chemical compositions.
End-of-life management presents both challenges and opportunities. The complex polymer networks that enable self-healing properties may complicate recycling processes, requiring specialized separation techniques. However, research indicates that many self-healing polymer electrolytes can be designed with depolymerization triggers, enabling more efficient material recovery and reuse compared to conventional systems.
Regulatory frameworks worldwide are increasingly recognizing the sustainability advantages of self-healing materials. The European Union's Battery Directive and similar regulations in Asia and North America are evolving to incentivize technologies that extend product lifespans and reduce waste generation. Manufacturers implementing self-healing polymer electrolytes may gain competitive advantages through compliance with these emerging standards and access to green technology subsidies.
Future research directions should focus on further reducing environmental impacts by developing self-healing electrolytes with completely biodegradable components while maintaining or improving ionic conductivity under strain. This approach would create truly sustainable energy storage solutions aligned with circular economy principles.
Safety Standards and Performance Metrics for Self-Healing Electrolytes
The development of safety standards and performance metrics for self-healing electrolytes represents a critical frontier in battery technology advancement. Current industry standards primarily focus on conventional electrolytes, creating a significant gap in regulatory frameworks for these innovative materials. Organizations including the International Electrotechnical Commission (IEC), Underwriters Laboratories (UL), and the IEEE are actively working to establish comprehensive safety protocols specifically addressing the unique properties of self-healing polymer electrolytes.
Key safety parameters under consideration include thermal stability thresholds, mechanical integrity under various strain conditions, and chemical compatibility with electrode materials. The thermal runaway resistance of self-healing electrolytes must be quantified through standardized testing procedures that account for their dynamic nature. Particularly important is the establishment of minimum recovery efficiency metrics after mechanical damage, with industry consensus building around a threshold of at least 85% conductivity restoration within specified timeframes.
Performance metrics for self-healing electrolytes are increasingly focusing on quantifiable recovery characteristics. The ionic conductivity retention ratio (ICRR) has emerged as a critical parameter, measuring the percentage of original conductivity maintained after experiencing defined strain conditions. Leading research groups have proposed standardized testing protocols involving controlled deformation cycles followed by recovery periods, with measurements taken at predetermined intervals to generate recovery profiles.
Cycle life performance under repeated strain-recovery events represents another essential metric, with current benchmarks suggesting self-healing electrolytes should maintain at least 80% of their initial conductivity after 100 mechanical strain cycles. The healing efficiency index (HEI), which quantifies the ratio between post-healing and original conductivity values, provides a standardized comparison method across different self-healing electrolyte formulations.
Environmental safety considerations have also gained prominence, with new metrics being developed to assess biodegradability, toxicity profiles, and end-of-life management for these advanced materials. The Environmental Impact Factor (EIF) framework specifically designed for self-healing electrolytes evaluates their ecological footprint throughout the complete lifecycle, from raw material sourcing to disposal.
Accelerated aging tests that simulate years of operational conditions within compressed timeframes are being standardized to predict long-term performance reliability. These protocols typically involve exposure to elevated temperatures, humidity variations, and mechanical stress cycles to evaluate how self-healing capabilities evolve over extended periods. The resulting data informs predictive models for electrolyte performance degradation under real-world conditions.
Key safety parameters under consideration include thermal stability thresholds, mechanical integrity under various strain conditions, and chemical compatibility with electrode materials. The thermal runaway resistance of self-healing electrolytes must be quantified through standardized testing procedures that account for their dynamic nature. Particularly important is the establishment of minimum recovery efficiency metrics after mechanical damage, with industry consensus building around a threshold of at least 85% conductivity restoration within specified timeframes.
Performance metrics for self-healing electrolytes are increasingly focusing on quantifiable recovery characteristics. The ionic conductivity retention ratio (ICRR) has emerged as a critical parameter, measuring the percentage of original conductivity maintained after experiencing defined strain conditions. Leading research groups have proposed standardized testing protocols involving controlled deformation cycles followed by recovery periods, with measurements taken at predetermined intervals to generate recovery profiles.
Cycle life performance under repeated strain-recovery events represents another essential metric, with current benchmarks suggesting self-healing electrolytes should maintain at least 80% of their initial conductivity after 100 mechanical strain cycles. The healing efficiency index (HEI), which quantifies the ratio between post-healing and original conductivity values, provides a standardized comparison method across different self-healing electrolyte formulations.
Environmental safety considerations have also gained prominence, with new metrics being developed to assess biodegradability, toxicity profiles, and end-of-life management for these advanced materials. The Environmental Impact Factor (EIF) framework specifically designed for self-healing electrolytes evaluates their ecological footprint throughout the complete lifecycle, from raw material sourcing to disposal.
Accelerated aging tests that simulate years of operational conditions within compressed timeframes are being standardized to predict long-term performance reliability. These protocols typically involve exposure to elevated temperatures, humidity variations, and mechanical stress cycles to evaluate how self-healing capabilities evolve over extended periods. The resulting data informs predictive models for electrolyte performance degradation under real-world conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!