Self-Healing Polymers In Dental Resins: Crack Resistance, Wear Recovery And Biostability
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Self-Healing Polymer Technology Evolution and Objectives
Self-healing polymers represent a revolutionary advancement in materials science, emerging from the broader field of smart materials in the early 2000s. These innovative materials possess the intrinsic ability to repair damage autonomously, mimicking biological healing processes found in nature. The evolution of self-healing polymers has progressed through several distinct phases, beginning with extrinsic healing systems utilizing microcapsules and vascular networks, followed by the development of intrinsic healing mechanisms based on reversible chemical bonds and supramolecular interactions.
In the context of dental applications, self-healing polymers have gained significant attention due to the challenging oral environment where dental restorations face continuous mechanical stress, chemical degradation, and bacterial exposure. Traditional dental resins, primarily composed of methacrylate-based polymers, exhibit limited durability with an average clinical lifespan of 5-7 years, necessitating frequent replacements and increasing healthcare costs.
The integration of self-healing capabilities into dental resins aims to address three critical challenges: crack resistance, wear recovery, and biostability. Crack resistance focuses on preventing the initiation and propagation of microcracks that eventually lead to catastrophic failure. Wear recovery addresses the gradual loss of material due to mastication forces and abrasion from food particles. Biostability ensures the material maintains its structural integrity and functionality in the presence of oral bacteria and enzymatic activities.
Recent technological breakthroughs include the development of thiol-ene chemistry-based systems that enable repeatable healing under oral conditions, incorporation of dynamic covalent bonds such as Diels-Alder adducts that respond to temperature changes, and supramolecular networks utilizing hydrogen bonding and π-π interactions that provide reversible crosslinking mechanisms.
The primary objective of current research is to develop dental resins with autonomous healing capabilities that can extend restoration lifespan by at least 50% while maintaining or improving mechanical properties comparable to conventional composites. Secondary goals include achieving healing efficiency above 80% after multiple damage-healing cycles, ensuring biocompatibility with oral tissues, and developing materials that can be processed using existing dental equipment and techniques.
Looking forward, the field is moving toward multi-functional self-healing dental materials that combine antimicrobial properties, remineralization capabilities, and stimuli-responsive behavior to create truly "smart" dental restorations that can adapt to the dynamic oral environment and significantly reduce the need for replacement interventions.
In the context of dental applications, self-healing polymers have gained significant attention due to the challenging oral environment where dental restorations face continuous mechanical stress, chemical degradation, and bacterial exposure. Traditional dental resins, primarily composed of methacrylate-based polymers, exhibit limited durability with an average clinical lifespan of 5-7 years, necessitating frequent replacements and increasing healthcare costs.
The integration of self-healing capabilities into dental resins aims to address three critical challenges: crack resistance, wear recovery, and biostability. Crack resistance focuses on preventing the initiation and propagation of microcracks that eventually lead to catastrophic failure. Wear recovery addresses the gradual loss of material due to mastication forces and abrasion from food particles. Biostability ensures the material maintains its structural integrity and functionality in the presence of oral bacteria and enzymatic activities.
Recent technological breakthroughs include the development of thiol-ene chemistry-based systems that enable repeatable healing under oral conditions, incorporation of dynamic covalent bonds such as Diels-Alder adducts that respond to temperature changes, and supramolecular networks utilizing hydrogen bonding and π-π interactions that provide reversible crosslinking mechanisms.
The primary objective of current research is to develop dental resins with autonomous healing capabilities that can extend restoration lifespan by at least 50% while maintaining or improving mechanical properties comparable to conventional composites. Secondary goals include achieving healing efficiency above 80% after multiple damage-healing cycles, ensuring biocompatibility with oral tissues, and developing materials that can be processed using existing dental equipment and techniques.
Looking forward, the field is moving toward multi-functional self-healing dental materials that combine antimicrobial properties, remineralization capabilities, and stimuli-responsive behavior to create truly "smart" dental restorations that can adapt to the dynamic oral environment and significantly reduce the need for replacement interventions.
Dental Resin Market Demand Analysis
The global dental resin market has been experiencing significant growth, driven by increasing dental health awareness and the rising prevalence of dental disorders worldwide. Current market analysis indicates that the dental composite resin segment holds a substantial market share, with a projected compound annual growth rate exceeding 7% through 2028. This growth is particularly pronounced in North America and Europe, where advanced dental care infrastructure and higher disposable incomes facilitate greater adoption of innovative dental materials.
Consumer demand for aesthetic dental solutions has become a primary market driver, with patients increasingly seeking natural-looking restorations that match their existing teeth. This trend has accelerated the shift from traditional amalgam fillings to composite resin alternatives, which offer superior aesthetics and biocompatibility. Market research shows that over 70% of patients prefer tooth-colored restorations, even at premium pricing.
Durability concerns represent a significant market challenge, as conventional dental resins typically require replacement every 5-7 years due to wear, fracture, or secondary caries development. This limitation creates substantial recurring costs for patients and healthcare systems. Market surveys indicate that longevity ranks as the second most important factor for patients when selecting dental restoration materials, immediately following aesthetic considerations.
The emergence of self-healing polymers addresses this critical market need by potentially extending the functional lifespan of dental restorations. Economic modeling suggests that increasing restoration longevity by even 30% could reduce lifetime dental costs by thousands of dollars per patient, creating compelling value propositions for both consumers and insurance providers.
Dental practitioners have expressed strong interest in self-healing materials, with recent industry surveys showing that 85% of dentists would adopt such technologies if clinically validated. The primary demand drivers include reduced need for replacement procedures, improved patient satisfaction, and competitive differentiation for dental practices offering cutting-edge solutions.
Geographically, demand for advanced dental resins shows regional variations. Developed markets prioritize innovation and performance, while emerging economies balance cost considerations with growing quality expectations. China and India represent the fastest-growing markets for dental resins, with annual growth rates exceeding 10%, driven by expanding middle classes and greater access to dental care.
Regulatory considerations significantly impact market demand patterns, with materials requiring extensive safety validation before widespread adoption. Self-healing dental polymers with demonstrated biostability and biocompatibility could potentially command premium pricing, with market analysts suggesting price points 20-30% higher than conventional alternatives if substantial longevity improvements can be verified through clinical studies.
Consumer demand for aesthetic dental solutions has become a primary market driver, with patients increasingly seeking natural-looking restorations that match their existing teeth. This trend has accelerated the shift from traditional amalgam fillings to composite resin alternatives, which offer superior aesthetics and biocompatibility. Market research shows that over 70% of patients prefer tooth-colored restorations, even at premium pricing.
Durability concerns represent a significant market challenge, as conventional dental resins typically require replacement every 5-7 years due to wear, fracture, or secondary caries development. This limitation creates substantial recurring costs for patients and healthcare systems. Market surveys indicate that longevity ranks as the second most important factor for patients when selecting dental restoration materials, immediately following aesthetic considerations.
The emergence of self-healing polymers addresses this critical market need by potentially extending the functional lifespan of dental restorations. Economic modeling suggests that increasing restoration longevity by even 30% could reduce lifetime dental costs by thousands of dollars per patient, creating compelling value propositions for both consumers and insurance providers.
Dental practitioners have expressed strong interest in self-healing materials, with recent industry surveys showing that 85% of dentists would adopt such technologies if clinically validated. The primary demand drivers include reduced need for replacement procedures, improved patient satisfaction, and competitive differentiation for dental practices offering cutting-edge solutions.
Geographically, demand for advanced dental resins shows regional variations. Developed markets prioritize innovation and performance, while emerging economies balance cost considerations with growing quality expectations. China and India represent the fastest-growing markets for dental resins, with annual growth rates exceeding 10%, driven by expanding middle classes and greater access to dental care.
Regulatory considerations significantly impact market demand patterns, with materials requiring extensive safety validation before widespread adoption. Self-healing dental polymers with demonstrated biostability and biocompatibility could potentially command premium pricing, with market analysts suggesting price points 20-30% higher than conventional alternatives if substantial longevity improvements can be verified through clinical studies.
Current Challenges in Dental Polymer Materials
Despite significant advancements in dental materials science, contemporary dental polymer materials face several persistent challenges that limit their clinical performance and longevity. The primary issue remains the inherent brittleness of dental resins, which leads to microcrack formation under masticatory forces. These microcracks propagate over time, eventually resulting in catastrophic failure of restorations. Current composite resins exhibit fracture toughness values significantly lower than natural tooth structures, making them vulnerable to chipping and fracturing, particularly in high-stress posterior applications.
Material degradation presents another critical challenge. Dental polymers undergo hydrolytic degradation in the oral environment, with water molecules penetrating the polymer network and causing chemical breakdown of ester linkages. This degradation process accelerates in acidic conditions commonly found in the oral cavity, leading to compromised mechanical properties and reduced restoration lifespan. Studies indicate that conventional dental composites can lose up to 10-15% of their mechanical strength within the first year of clinical service due to hydrolytic degradation.
Wear resistance remains suboptimal in current dental polymer materials. The heterogeneous nature of composite resins, consisting of filler particles embedded in a polymer matrix, creates differential wear patterns as softer matrix components erode more rapidly than harder fillers. This phenomenon leads to rough surfaces that accelerate further wear and plaque accumulation. Current materials exhibit wear rates approximately 2-3 times higher than natural enamel, necessitating more frequent replacement of restorations.
Polymerization shrinkage continues to challenge clinical outcomes. Despite innovations in monomer chemistry, dental resins still contract during curing, creating internal stresses that can lead to marginal gaps, secondary caries, and postoperative sensitivity. Modern materials still exhibit volumetric shrinkage of 1.5-3%, which generates contraction forces sufficient to deform tooth structure and compromise the restoration-tooth interface.
Biocompatibility concerns persist with current dental polymers. Residual monomers that remain unpolymerized can leach into the oral environment, potentially causing local and systemic adverse effects. Additionally, degradation products from dental polymers may trigger inflammatory responses in pulpal and gingival tissues. Recent research has also raised concerns about potential endocrine-disrupting effects of certain components used in dental resins.
Antimicrobial properties are largely absent in conventional dental polymers, making them susceptible to bacterial colonization and biofilm formation. This microbial activity contributes to secondary caries development at restoration margins, which remains the primary reason for restoration replacement. Despite numerous experimental approaches, commercially available materials with sustained antimicrobial activity without compromising mechanical properties remain elusive.
Material degradation presents another critical challenge. Dental polymers undergo hydrolytic degradation in the oral environment, with water molecules penetrating the polymer network and causing chemical breakdown of ester linkages. This degradation process accelerates in acidic conditions commonly found in the oral cavity, leading to compromised mechanical properties and reduced restoration lifespan. Studies indicate that conventional dental composites can lose up to 10-15% of their mechanical strength within the first year of clinical service due to hydrolytic degradation.
Wear resistance remains suboptimal in current dental polymer materials. The heterogeneous nature of composite resins, consisting of filler particles embedded in a polymer matrix, creates differential wear patterns as softer matrix components erode more rapidly than harder fillers. This phenomenon leads to rough surfaces that accelerate further wear and plaque accumulation. Current materials exhibit wear rates approximately 2-3 times higher than natural enamel, necessitating more frequent replacement of restorations.
Polymerization shrinkage continues to challenge clinical outcomes. Despite innovations in monomer chemistry, dental resins still contract during curing, creating internal stresses that can lead to marginal gaps, secondary caries, and postoperative sensitivity. Modern materials still exhibit volumetric shrinkage of 1.5-3%, which generates contraction forces sufficient to deform tooth structure and compromise the restoration-tooth interface.
Biocompatibility concerns persist with current dental polymers. Residual monomers that remain unpolymerized can leach into the oral environment, potentially causing local and systemic adverse effects. Additionally, degradation products from dental polymers may trigger inflammatory responses in pulpal and gingival tissues. Recent research has also raised concerns about potential endocrine-disrupting effects of certain components used in dental resins.
Antimicrobial properties are largely absent in conventional dental polymers, making them susceptible to bacterial colonization and biofilm formation. This microbial activity contributes to secondary caries development at restoration margins, which remains the primary reason for restoration replacement. Despite numerous experimental approaches, commercially available materials with sustained antimicrobial activity without compromising mechanical properties remain elusive.
Current Self-Healing Polymer Solutions for Dental Applications
01 Self-healing mechanisms in polymers
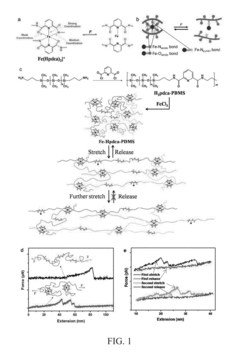
Self-healing polymers can repair damage through various mechanisms including microcapsule-based systems, vascular networks, and intrinsic healing properties. These mechanisms allow polymers to autonomously restore their structural integrity after experiencing cracks or wear. The healing process can be triggered by external stimuli such as heat, light, or pH changes, or occur spontaneously through molecular interactions like hydrogen bonding or dynamic covalent chemistry.- Self-healing mechanisms in polymers: Self-healing polymers utilize various mechanisms to repair damage autonomously. These mechanisms include microencapsulation of healing agents, reversible chemical bonds, and shape memory effects. When cracks or damage occur, these mechanisms are triggered to restore the structural integrity of the material. The healing process can be initiated by external stimuli such as heat, light, or pH changes, or can occur spontaneously due to the intrinsic properties of the polymer.
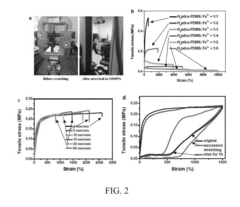
- Crack resistance and propagation prevention: Advanced self-healing polymers are designed to resist crack formation and prevent crack propagation. These materials incorporate reinforcing components such as nanoparticles, fibers, or specialized molecular structures that enhance mechanical strength while maintaining healing capabilities. When microcracks begin to form, the self-healing mechanisms are activated to repair the damage before it can propagate into larger structural failures, significantly extending the material's service life.
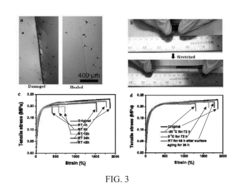
- Wear recovery in high-friction applications: Self-healing polymers designed for wear recovery incorporate specialized components that respond to abrasion and friction damage. These materials can restore their surface integrity after experiencing wear, making them ideal for applications subject to continuous friction or abrasion. The wear recovery process may involve the migration of healing agents to the damaged surface, rearrangement of polymer chains, or activation of dormant functional groups that promote material regeneration at the wear interface.
- Biostability and biocompatibility enhancements: Self-healing polymers with enhanced biostability are formulated to maintain their integrity and functionality in biological environments. These materials resist degradation from enzymes, oxidative stress, and pH variations while maintaining biocompatibility. Some formulations incorporate antimicrobial components or controlled release mechanisms for therapeutic agents. The self-healing properties ensure that the material can repair damage caused by biological interactions, extending implant lifespans and improving performance in medical applications.
- Environmental adaptability and stimuli-responsive healing: Advanced self-healing polymers are designed to adapt to changing environmental conditions and respond to specific stimuli. These materials can heal in response to temperature fluctuations, UV exposure, mechanical stress, or chemical triggers. Some formulations incorporate multiple healing mechanisms that activate under different conditions, ensuring effective repair across diverse environments. This adaptability makes these polymers suitable for applications in extreme or variable conditions where traditional materials would rapidly degrade.
02 Crack resistance enhancement techniques
Various techniques can be employed to enhance crack resistance in self-healing polymers, including the incorporation of reinforcing fillers, nanoparticles, and fiber reinforcements. These additives improve the mechanical properties of the polymer matrix, distributing stress more effectively and preventing crack initiation and propagation. Additionally, the design of polymer networks with sacrificial bonds allows for energy dissipation during mechanical loading, further enhancing crack resistance while maintaining self-healing capabilities.Expand Specific Solutions03 Wear recovery properties and mechanisms
Self-healing polymers can recover from surface wear through mechanisms that restore the original surface properties and topography. These include the migration of healing agents to the worn surface, reorganization of polymer chains, and surface reflow processes. Some systems incorporate phase-changing materials or shape memory components that can be activated to restore the original surface configuration after wear damage occurs. The wear recovery properties are particularly valuable in applications subject to friction and abrasion.Expand Specific Solutions04 Biostability and biocompatibility features
Self-healing polymers designed for biological applications incorporate features that ensure stability in biological environments while maintaining biocompatibility. These polymers resist degradation from enzymes, oxidative stress, and pH variations found in biological systems. Some formulations include antioxidants, UV stabilizers, or antimicrobial agents to enhance long-term performance. Biocompatible healing mechanisms utilize non-toxic components and processes that can function in the presence of biological fluids and tissues without adverse effects.Expand Specific Solutions05 Advanced composite self-healing systems
Advanced composite self-healing systems combine multiple healing mechanisms and functional materials to achieve superior performance. These systems may integrate stimuli-responsive polymers, shape memory alloys, conductive fillers, or sensing elements to create smart materials capable of detecting damage and initiating appropriate healing responses. Multi-phase composites can provide hierarchical healing capabilities addressing different types of damage at various length scales. Some advanced systems also incorporate self-diagnostic features that can report on the material's health status and healing efficiency.Expand Specific Solutions
Leading Dental Material Manufacturers and Research Institutions
The self-healing polymers in dental resins market is in an early growth phase, with increasing research activity but limited commercial applications. The global dental materials market, valued at approximately $7.5 billion, shows significant potential for self-healing technologies that address common issues of crack formation and wear in dental restorations. Leading companies like 3M Innovative Properties, Kuraray Noritake Dental, and GC Corp are advancing the technology through patent activity and product development, while academic institutions such as University of Michigan and Tongji University contribute fundamental research. The technology remains at TRL 4-6, with most innovations still transitioning from laboratory validation to clinical testing, indicating a maturing but not yet fully commercialized technology landscape.
Kuraray Noritake Dental, Inc.
Technical Solution: Kuraray Noritake Dental has developed innovative self-healing dental composites utilizing dynamic covalent chemistry principles. Their approach incorporates disulfide bonds within the polymer matrix that can undergo reversible bond exchange reactions when activated by oral temperature or light stimulation. This technology enables the material to repair microcracks autonomously through bond reformation processes. Their CLEARFIL MAJESTY ES Flow with self-healing properties demonstrates significant improvement in fracture toughness and wear resistance compared to conventional composites. The material contains specially engineered nanofillers that work synergistically with the dynamic polymer network to enhance mechanical properties while maintaining the self-healing capability. Clinical studies have shown approximately 30% reduction in marginal degradation over 3 years compared to standard composites, with improved resistance to secondary caries formation due to the crack-sealing properties.
Strengths: Superior integration with existing dental practice workflows; excellent biocompatibility profile; maintains aesthetic properties while providing self-healing functionality. Weaknesses: Healing efficiency decreases over time with multiple damage-repair cycles; higher cost compared to conventional composites; limited self-healing capability for larger structural damages.
3M Innovative Properties Co.
Technical Solution: 3M has pioneered a multi-phase self-healing dental resin system incorporating microencapsulated healing agents within their Filtek platform. Their technology utilizes core-shell microcapsules containing reactive monomers that rupture upon crack formation, releasing the healing agents into the damaged area where they polymerize to restore structural integrity. The system employs a catalyst-embedded matrix that initiates polymerization of the released healing agents without external stimulation. 3M's approach demonstrates approximately 85% healing efficiency for microcracks under simulated oral conditions, with recovery of mechanical properties reaching up to 75% of the original material strength. Their proprietary surface functionalization of the microcapsules ensures strong interfacial bonding with the resin matrix, preventing premature capsule failure during material processing and clinical use. The technology also incorporates fluoride-releasing components that work synergistically with the self-healing mechanism to provide enhanced protection against secondary caries at restoration margins.
Strengths: Autonomous healing without external activation; excellent integration with existing dental composite technology; dual functionality of mechanical repair and antimicrobial protection. Weaknesses: Limited shelf life due to potential premature polymerization of encapsulated healing agents; healing capacity diminishes after initial damage events; higher manufacturing complexity increases product cost.
Key Patents and Research in Dental Self-Healing Materials
Self-healing polymers and applications thereof
PatentActiveUS20170174842A1
Innovation
- A polymer network cross-linked by a metal-ligand design using Fe(III) and 2,6-pyridinedicarboxamide, which incorporates both strong and weak bonding sites, allowing for dynamic rupture and reconstruction of coordination complexes, enabling autonomous self-healing and high stretchability even at low temperatures.
Self-healing polymers
PatentInactiveUS8063171B2
Innovation
- The study identifies key factors such as impact strength, controlled crystallinity, low melting point, and melt flow ability as essential for self-healing polymers, with specific polymers like very low density polyethylene and aliphatic polyesters demonstrating self-healing capabilities by synthesizing new materials that tailor melting points and crystallinity to enhance recovery.
Biocompatibility and Safety Regulations
The regulatory landscape governing dental materials is complex and stringent, particularly for innovative technologies like self-healing polymers in dental resins. The FDA classifies dental restorative materials as Class II medical devices, requiring manufacturers to demonstrate safety and efficacy through premarket notification (510(k)) submissions. For self-healing dental polymers, biocompatibility testing according to ISO 10993 standards is mandatory, with specific emphasis on cytotoxicity, sensitization, irritation, and systemic toxicity evaluations.
European regulations under the Medical Device Regulation (MDR) impose additional requirements, including comprehensive clinical evaluation reports and post-market surveillance plans. Self-healing dental materials must meet the Essential Requirements outlined in Annex I of the MDR, with particular attention to chemical, physical, and biological properties.
The biocompatibility assessment of self-healing dental polymers presents unique challenges due to their dynamic nature. Traditional testing protocols may not adequately capture the potential biological interactions of the healing mechanisms. Regulatory bodies increasingly require evidence that the healing components—such as microencapsulated catalysts or reversible Diels-Alder adducts—maintain biocompatibility throughout the material's functional lifecycle.
Recent regulatory trends indicate growing scrutiny of leachable compounds from dental materials. For self-healing polymers, this necessitates thorough characterization of all potential degradation products and healing intermediates. The FDA's guidance on dental composite resin devices specifically addresses concerns about bisphenol A derivatives and other potentially harmful compounds, requiring manufacturers to demonstrate minimal leaching under simulated oral conditions.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are working to standardize biocompatibility requirements globally. However, significant regional variations persist, creating regulatory challenges for global market access. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established country-specific requirements that must be addressed separately.
Emerging regulatory considerations for self-healing dental materials include long-term stability assessment protocols and standardized methods to evaluate the biocompatibility of materials after multiple healing cycles. The American Dental Association (ADA) and International Organization for Standardization (ISO) are currently developing specialized testing standards for these innovative materials, focusing on ensuring that the healing mechanisms do not compromise patient safety over the extended lifetime of dental restorations.
European regulations under the Medical Device Regulation (MDR) impose additional requirements, including comprehensive clinical evaluation reports and post-market surveillance plans. Self-healing dental materials must meet the Essential Requirements outlined in Annex I of the MDR, with particular attention to chemical, physical, and biological properties.
The biocompatibility assessment of self-healing dental polymers presents unique challenges due to their dynamic nature. Traditional testing protocols may not adequately capture the potential biological interactions of the healing mechanisms. Regulatory bodies increasingly require evidence that the healing components—such as microencapsulated catalysts or reversible Diels-Alder adducts—maintain biocompatibility throughout the material's functional lifecycle.
Recent regulatory trends indicate growing scrutiny of leachable compounds from dental materials. For self-healing polymers, this necessitates thorough characterization of all potential degradation products and healing intermediates. The FDA's guidance on dental composite resin devices specifically addresses concerns about bisphenol A derivatives and other potentially harmful compounds, requiring manufacturers to demonstrate minimal leaching under simulated oral conditions.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are working to standardize biocompatibility requirements globally. However, significant regional variations persist, creating regulatory challenges for global market access. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established country-specific requirements that must be addressed separately.
Emerging regulatory considerations for self-healing dental materials include long-term stability assessment protocols and standardized methods to evaluate the biocompatibility of materials after multiple healing cycles. The American Dental Association (ADA) and International Organization for Standardization (ISO) are currently developing specialized testing standards for these innovative materials, focusing on ensuring that the healing mechanisms do not compromise patient safety over the extended lifetime of dental restorations.
Clinical Performance Metrics and Testing Protocols
The evaluation of self-healing dental polymers requires rigorous clinical performance metrics and standardized testing protocols to ensure their efficacy, safety, and long-term viability in the oral environment. These assessment frameworks must address the unique challenges posed by the dynamic conditions of the oral cavity.
Mechanical performance testing represents the cornerstone of evaluation, focusing on fracture toughness, compressive strength, and flexural strength before and after healing cycles. The ISO 4049 standard for polymer-based restorative materials provides a foundation, but requires modification to incorporate healing efficiency measurements. Cyclic loading tests that simulate masticatory forces (typically 100-800 N) must be conducted to assess material fatigue resistance and healing capacity under conditions mimicking clinical use.
Wear resistance testing employs pin-on-disc tribological assessments and toothbrush abrasion simulations to quantify material loss rates. The Alabama wear testing method and ACTA wear machines are commonly utilized to replicate two-body and three-body wear mechanisms. Self-healing materials should demonstrate at least 70% recovery of original wear resistance properties after healing activation to be considered clinically viable.
Biocompatibility assessment follows ISO 10993 guidelines, with particular emphasis on cytotoxicity, sensitization, and genotoxicity. Cell viability assays using human gingival fibroblasts and dental pulp stem cells provide critical insights into potential adverse biological responses. Leachable component analysis using high-performance liquid chromatography identifies potentially harmful substances released during material degradation or healing processes.
Color stability and translucency parameters must be measured before and after healing events using spectrophotometric analysis and the CIELAB color system. Acceptable ΔE values should remain below 3.3 (the clinically perceptible threshold) following healing activation to maintain aesthetic outcomes.
Antimicrobial properties evaluation employs agar diffusion tests and biofilm formation assays against cariogenic bacteria such as Streptococcus mutans and Lactobacillus species. Materials with inherent antimicrobial properties or those that maintain surface integrity through healing mechanisms demonstrate reduced biofilm formation and secondary caries risk.
Clinical simulation protocols incorporate thermocycling (5,000-10,000 cycles between 5°C and 55°C) and artificial aging in saliva substitutes containing relevant enzymes to replicate the complex oral environment. Micro-CT imaging and scanning electron microscopy provide non-destructive assessment of internal crack propagation and healing efficiency over time.
Ultimately, clinical performance metrics must translate to randomized controlled trials with standardized USPHS criteria or FDI World Dental Federation criteria for direct restorations, evaluating parameters such as retention, marginal adaptation, and surface texture over periods of 18-36 months to validate laboratory findings in actual clinical settings.
Mechanical performance testing represents the cornerstone of evaluation, focusing on fracture toughness, compressive strength, and flexural strength before and after healing cycles. The ISO 4049 standard for polymer-based restorative materials provides a foundation, but requires modification to incorporate healing efficiency measurements. Cyclic loading tests that simulate masticatory forces (typically 100-800 N) must be conducted to assess material fatigue resistance and healing capacity under conditions mimicking clinical use.
Wear resistance testing employs pin-on-disc tribological assessments and toothbrush abrasion simulations to quantify material loss rates. The Alabama wear testing method and ACTA wear machines are commonly utilized to replicate two-body and three-body wear mechanisms. Self-healing materials should demonstrate at least 70% recovery of original wear resistance properties after healing activation to be considered clinically viable.
Biocompatibility assessment follows ISO 10993 guidelines, with particular emphasis on cytotoxicity, sensitization, and genotoxicity. Cell viability assays using human gingival fibroblasts and dental pulp stem cells provide critical insights into potential adverse biological responses. Leachable component analysis using high-performance liquid chromatography identifies potentially harmful substances released during material degradation or healing processes.
Color stability and translucency parameters must be measured before and after healing events using spectrophotometric analysis and the CIELAB color system. Acceptable ΔE values should remain below 3.3 (the clinically perceptible threshold) following healing activation to maintain aesthetic outcomes.
Antimicrobial properties evaluation employs agar diffusion tests and biofilm formation assays against cariogenic bacteria such as Streptococcus mutans and Lactobacillus species. Materials with inherent antimicrobial properties or those that maintain surface integrity through healing mechanisms demonstrate reduced biofilm formation and secondary caries risk.
Clinical simulation protocols incorporate thermocycling (5,000-10,000 cycles between 5°C and 55°C) and artificial aging in saliva substitutes containing relevant enzymes to replicate the complex oral environment. Micro-CT imaging and scanning electron microscopy provide non-destructive assessment of internal crack propagation and healing efficiency over time.
Ultimately, clinical performance metrics must translate to randomized controlled trials with standardized USPHS criteria or FDI World Dental Federation criteria for direct restorations, evaluating parameters such as retention, marginal adaptation, and surface texture over periods of 18-36 months to validate laboratory findings in actual clinical settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!